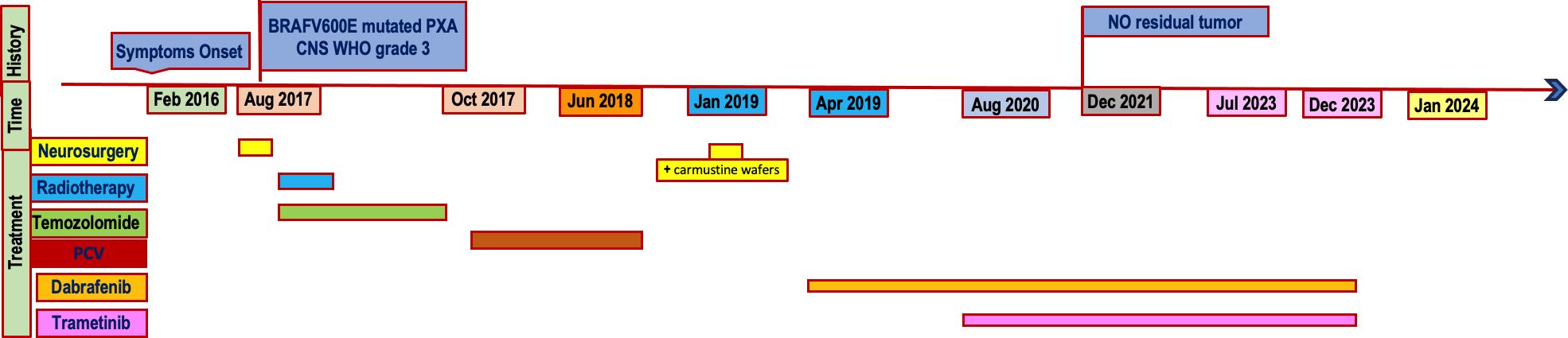
- 1Neuro-oncology Department, Meyer Children’s Hospital IRCCS, Florence, Italy
- 2Pathology Department, Meyer Children’s Hospital IRCCS, Florence, Italy
- 3Radiology Department, Meyer Children’s Hospital IRCCS, Florence, Italy
- 4Radiotherapy Department, Careggi Hospital, Florence, Italy
- 5Neurosurgery Department, Meyer Children’s Hospital IRCCS, Florence, Italy
Dabrafenib plus trametinib is a promising new therapy for patients affected by BRAFV600E-mutant glioma, with high overall response and manageable toxicity. We described a complete and long-lasting response in a case of recurrent anaplastic pleomorphic xanthoastrocytoma CNS WHO-grade 3 BRAFV600E mutated. Due to very poor prognosis, there are a few described cases of high-grade glioma (HGG) patients treated with the combined target therapy as third-line treatment. The emergence of optimized sequencing strategies and targeted agents, including multimodal and systemic therapy with dabrafenib plus trametinib, will continue to broaden personalized therapy in HGG improving patient outcomes.
1 Introduction
High-grade gliomas (HGGs), tumors of neuroepithelial origin (1), represent the most common primary intracranial tumor in adults (2, 3). Differently, low- grade gliomas (LGGs) predominate in children (4, 5).
HGGs display a dismal prognosis despite surgical and chemo radiotherapeutic advances (1) and standard of care is commonly not curative. Throughout the understanding of molecular basis of tumors and recent insights, survival outcomes modestly increased, however, remaining limited and challenging. Therefore, worldwide researches are moving towards new frontiers and ongoing trials are investigating novel targeted agents (1). In the last years, important advances in the field of molecular biology and pathology have been accomplished (6).
MAPK (mitogen-activated protein kinase) pathway, implicated in carcinogenesis, has been found altered in most glial tumors (7, 8), promoting cellular overgrowth and overcoming metabolic stress (9). The pathway includes a small G protein (RAS) and three protein kinases in a downstream signaling pathway (respectively RAF – composed of A-RAF, B-RAF and RAF-1 or C-RAF kinases, MEK – composed of MEK1 and MEK2, ERK – composed of ERK1 and ERK2) (10, 11). ERK (extracellular signal-regulated kinase) is a MAPK that functions as the major effector of the RAS oncoprotein, translocating to the nucleus to activate transcription factors (10). Driving oncogenic mutations should develop upstream of the MAPK pathway (11).
Most BRAF variants are missense mutations at amino acid position 600, resulting in an exchange of valine for glutamate (referred to as BRAFV600E) (12). Activating BRAFV600E kinase mutations occur in ~7% of human malignancies (13). Initially described in melanoma, colon and papillary thyroid carcinoma, these alterations have also been observed in primary nervous system tumors (14). High mutation frequencies have been detected in pleomorphic xanthoastrocytomas (PXA), gangliogliomas and extra-cerebellar pilocytic astrocytomas (14), but the mutation has also been found in others HGGs (12), in particular in epithelioid glioblastoma (15).
The BRAF inhibitors vemurafenib, dabrafenib and encorafenib selectively target BRAF kinase, interfering with MAPK signaling pathway (16). Selumetinib and trametinib are MEK inhibitors (MEKi) (7). The combination of BRAF and MEK inhibitor have been approved in various cancers by the US Food and Drugs Administration (FDA) (17) and the European Medicines Agency (EMA). It is known that the blockage of two downstream pathway components with dual BRAF/MEK inhibition may improve tumor control and patient survival (18).
Recently, MEK inhibitors and BRAF inhibitors have been successfully used in pediatric LGG patients (19), with a relatively well-tolerated side effect profile (1). Few data are available on their efficacy in relapsing refractory HGGs.
Herein we report a case of complete long-lasting response to combined dabrafenib/trametinib as third-line therapy in a patient with frontal HGG.
2 Case report
In February 2016, a 21-year-old white female presented her first seizure episode. In August 2016 she was admitted to Anna Meyer Children’s Hospital IRCCS in Florence for recurrent episodes. Imaging revealed a left frontal lesion (Figure 1). A partial resection was performed. The histological examination diagnosed anaplastic PXA BRAFV600E mutated CNS WHO-grade 3. The lesion was composed of pleomorphic, xanthomatous and oligodendrocyte-like cells. Perivascular lymphocytic cuffing and numerous granular bodies were present. Mitoses (more than 5 X 10 HPF) and necrosis were seen (Figure 2). At immunohistochemistry GFAP, CD34 and BRAF p.V600E resulted positive; rare cells expressed synaptophysin. Molecular study confirmed BRAF p.V600E mutation (c. 1799T>A) whereas FISH analysis documented homozygous deletion of CDKN2A.
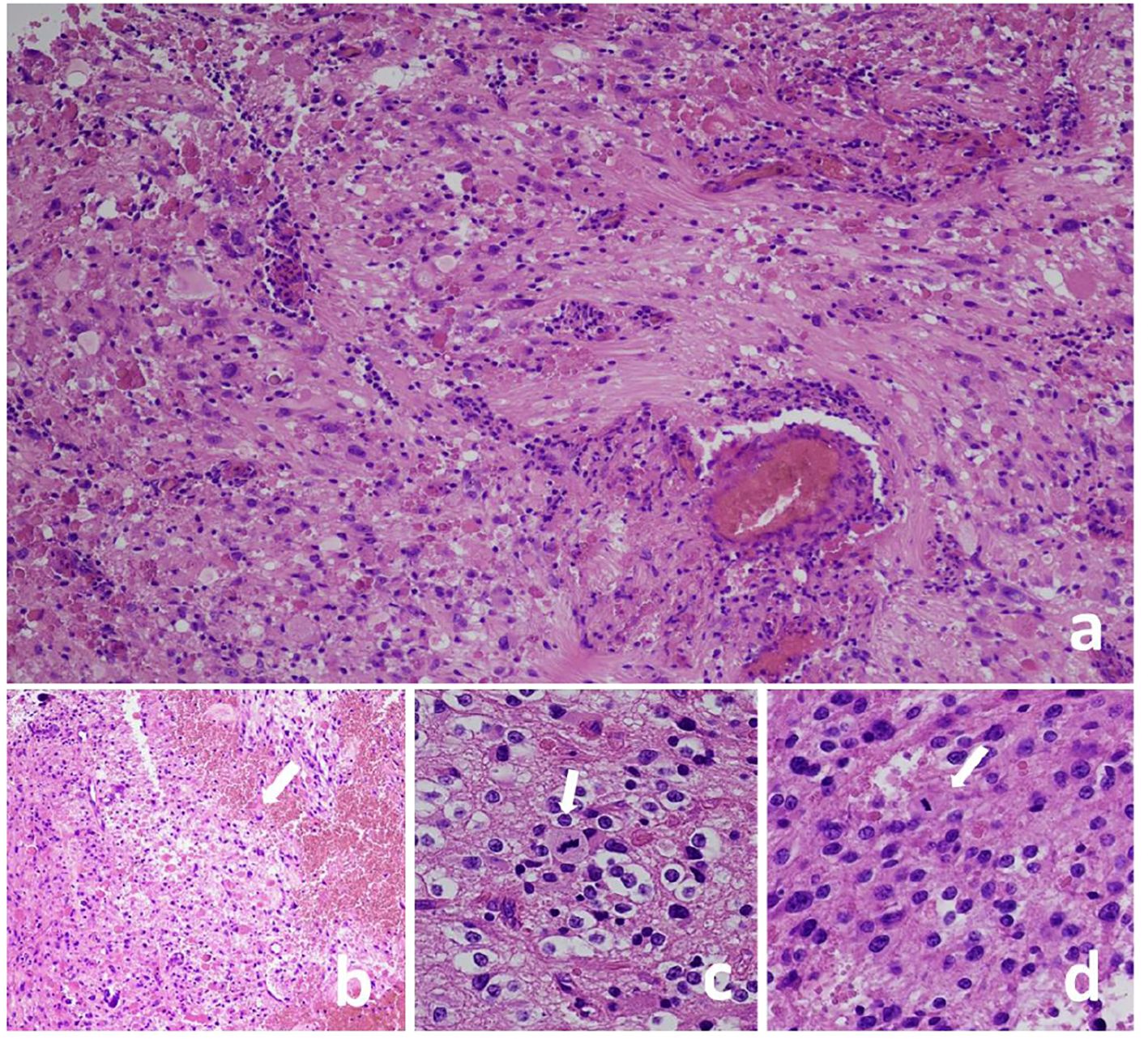
Figure 2 Pleomorphic xanthoastrocytoma, CNS WHO grade 3, lesion composed of pleomorphic cells (A) and oligodendrocyte-like cells (C). Perivascular lymphocyte cuffing and granular bodies are present (A) as well as necrosis [(B), arrow] and mitoses [(C, D), arrows]. Hematoxylin and eosin stain (A-D); Original magnification: a-b 10 X, c 40 X, d 20 X.
From October 2016 to December 2016 a volumetric modulated radiotherapy course was delivered for a total dose of 59,4 Gy in 33 fractions with concomitant and adjuvant temozolomide therapy (Stupp regimen). However, O6-methylguanine-DNA methyltransferase (MGMT) promoter was not methylated. Brain MRI at the end of radiotherapy revealed residual disease (Figure 3A).
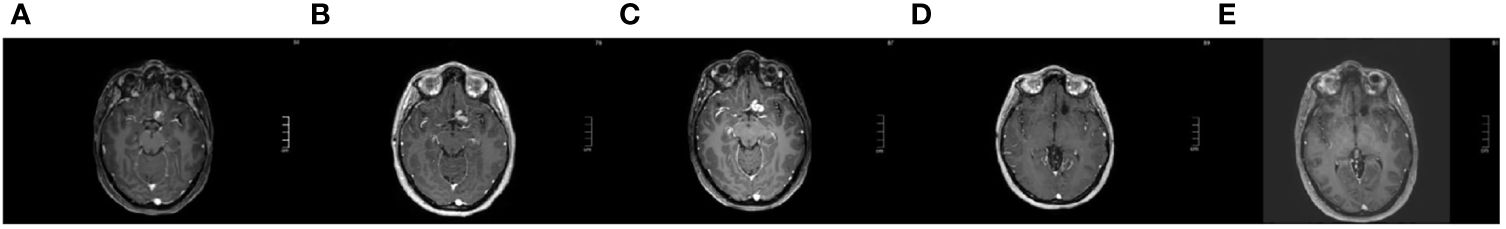
Figure 3 Axial T1 contrast-enhanced brain MRI [(A): after first line therapy, January 2017; (B): at first progression, October 2017; (C): at second progression, presurgical, December 2018; (D): complete response during target therapy, December 2021; (E): persistence complete response one month after target therapy interruption, January 2024].
In October 2017 (14 months after first surgical resection), a brain MRI showed progressive disease next to the resected area (first progression, Figure 3B), therefore six courses of chemotherapy with procarbazine, lomustine and vincristine (PCV) were administered (the last in June 2018) with disease control.
In January 2019 (7 months after the end of second line treatment) a cranial MRI showed progression of disease (second progression, Figure 3C) and another neurosurgical partial resection with carmustine wafers implantation was performed. The histological analysis confirmed the previous diagnosis. Considering the residual disease, in April 2019 the 24-year-old female patient with BRAF mutated anaplastic PXA started third-line therapy with dabrafenib. In August 2019 she suffered from Herpes Zoster reactivation, leading to temporary target drug suspension. The well-known tumor residue was less evident on the subsequent MRIs performed every three/four months. Given the literature data of the most effectiveness with better tolerability and the reduced possibility of resistance (13, 20–22), in August 2020 the patient started combination treatment with dabrafenib plus trametinib. Temporary interruption was required for pyrexia and in September 2020 for the occurrence of erythema nodosum grade 3 Common Terminology Criteria for Adverse Events (CTCAE) v.4. Dabrafenib and trametinib were then continued at a reduced dose (25%-50% reduction). The combined therapy was overall well tolerated. Since December 2021 the residual tumor has not been longer visible (Figure 3D). MRI evaluation, performed on July 27th,2023, showed no recurrence of the disease, three years after BRAF/MEK inhibitor combination treatment beginning. In December 2023, considering the optimal response and the reported toxicity, the dual target treatment was interrupted. Last MRI, performed on January 29th, 2024 (one month after drug cessation, 5 years after second progression) revealed persistent complete response (Figure 3E).
3 Discussion and conclusion
PXA is a tumor with a wide range of morphology (19). Two WHO grades (CNS WHO 2 or 3) are assigned, based on a mitotic count of more than 5 mitoses per 10 microscopic high power fields (19). Grade 3 includes the anaplastic variant (23). Anaplastic PXA is associated with poorer clinical outcomes compared with PXA CNS WHO 2 (24). Anaplastic variant of PXA shows histological characteristics as well as clinical course comparable with Grade 3 astrocytoma (25). Gross total resection should be the goal of initial treatment and it remains unclear whether adjuvant radiation and chemotherapy are able to prevent progression or dissemination (24). Early disease recurrence in anaplastic PXA is associated with fatal outcomes (25). BRAFV600E mutation can be detected in up to 70% of these tumors, combined with CDKN2A homozygous deletion in greater than 90% (19). Considering the emerging molecular landscape and the frequent failure of conventional therapies, novel therapeutic strategies are under investigation in the treatment of HGGs.
Targeted therapies, including mutant BRAF inhibitors (dabrafenib) and MEK inhibitors (trametinib), have yet shown promising results in other cancers refractory to conventional chemotherapy (26). The safety and effectiveness of MEKi treatment have also been established in improving symptomatology and quality of life in patients affected by plexiform neurofibromas in Neurofibromatosis Type I (7). Considering brain tumors, MAPK inhibitors have shown encouraging results in LGG showing alterations of this pathway. Dabrafenib demonstrated meaningful clinical activity and acceptable tolerability in patients with BRAFV600-mutant LGG (27). Trametinib was an active and feasible treatment for progressive pediatric MAPK-aberrant LGGs, leading to disease control (28). Recently, the Food and Drug Administration (FDA) approved dabrafenib in combination with trametinib for the treatment of pediatric BRAFV600E LGG (29). Instead, data are still limited on their efficacy in BRAFV600E mutated HGGs. In 2014 Robinson et al. described the first known case of complete response in a BRAFV600E-mutated HGG to vemurafenib (BRAF inhibitor) therapy (20). In 2022 Arbour et al. reported an 18-year-old female with a grade 3 PXA treated upfront with dabrafenib and trametinib and conducted a systematic literature review of patients with HGG and BRAFV600E mutations treated with BRAF inhibitors (30).
In a phase 2 Rare Oncology Agnostic Research (ROAR) basket trial (NCT02034110) Dabrafenib plus trametinib showed clinically meaningful activity in patients with BRAFV600E mutation-positive recurrent or refractory HGG: 15 (33%; 95% CI 20-49) of 45 patients had an objective response by investigator assessment, including three complete responses and 12 partial responses (31). Further ongoing studies are evaluating MEK inhibition also in HGG patients. An Open Label, multi-center Roll-over Study is assessing Long-term effect of BRAFV600E and MEK inhibition with dabrafenib and trametinib in a subset of HGG (NCT03975829) (1). A phase I/II Trial is designed to study the combination of Dabrafenib, Trametinib and Hydroxychloroquine for Patients with Recurrent LGG or HGG with a BRAF aberration (NCT04201457). Another phase II trial studies how well the combination of dabrafenib and trametinib after radiation therapy in children and young adults with BRAF V600 mutated HGG (NCT03919071).
Our case report suggests that BRAF/MEK inhibition is a potential promising strategy also in the treatment of recurrent and refractory HGG, non-stable responsive to surgery, radiotherapy, first and second line chemotherapy. The patient on the third-line combined target therapy achieved even a complete extraordinary response, with disappearance of residual disease.
The patient started a therapy with BRAF and MEK inhibitors on the basis that previous studies on melanoma suggested the possibility of resistance (13, 20), Moreover, Hargrave et al. in a phase II trial in pediatric relapsed/refractory BRAFV600–mutant HGG assessed tolerable safety and durable responses of the combined therapy, compared to traditional chemotherapy (32). Hypotheses for mechanisms of acquired resistance to BRAF inhibition include secondary mutations in BRAF, MAPK reactivation, and activation of alternative survival pathways (13). Reports in colorectal cancer suggest BRAF-mutant tumors may escape inhibition by amplifying receptor tyrosine kinases (20, 33), Additionally, combination of MEK and BRAF inhibitors reduces squamous cell carcinoma risk observed with BRAF inhibitors monotherapy (1). Combined treatment is reported to be well tolerated with mostly moderate and reversible side effects (21). In an open-label study involving patients with metastatic melanoma with BRAFV600 mutations, dabrafenib and trametinib were safety combined at full monotherapy doses, with significatively improvement of progression-free survival (22). In our case in combined therapy temporary interruption was required in two events: pyrexia and for the occurrence of erythema nodosum, recurred some months later. Dabrafenib was then continued at a reduced dose (25% reduction) and the combined therapy was overall well tolerated.
Data on long-term response are still poor. Our case report describes an extremely great 3-year persistent response on combined target therapy. We must take into account that the combined target therapy was a component of a multimodal approach including neurosurgery and carmustine wafers implantation (CW). Approved to treat newly or recurrent HGG, CW efficacy was reported doubtful: CW may provide a therapeutic coverage during the usual radiotherapy delay of 2 to 6 weeks (34). In our case, the optimal neuro radiological response was observed at almost two years since CW implantation, therefore it was most likely related to the dual target treatment. However, CW was a part of the third line therapy, thus composed of a multimodal approach.
Despite promising preclinical and clinical trials, several issues persist (1). Disease control after MEKi withdrawal was not sustained in a fraction of patients (28). Even on temporary effect, therapeutic goals could include extending survival and improving quality of life in patients with relapsed disease (20). CNS tumors with alternative BRAF alterations, such as alternate V600 mutations or BRAF fusions, may differently respond to target therapy (20): for example it is important to note that BRAF inhibitor therapy in patients with BRAF gene fusion or duplications activates the MAPK signaling pathway in cells with wild-type BRAF at V600 (27), therefore in this setting MEK inhibitors represent the strategy of choice (35).
Moreover, several studies are investigating the use of targeted therapy as a first-line treatment (26), which could open extraordinary perspectives.
Long term follow up would supply data on disease evolution after treatment discontinuation and further studies are expected to provide standardized treatment duration indications.
In conclusion, our case report suggests that BRAF/MEK inhibition may represent a potential therapeutic strategy also in patients with refractory relapsing HGGs BRAF mutated, not responsive to conventional therapies. The achieved complete response in a recurrent disease is an exceptional reached goal. The long-lasting response is also of great importance, giving long-term insights in combined target therapy. However, this is a limited study, reporting our favorable experience only in a single patient. Further studies are ongoing and more data on larger cohorts are needed to clarify present issues. Despite this exciting result, ongoing prospective studies will determine whether dabrafenib and trametinib combination can improve relapsed HGGs BRAF mutated outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Comitato Etico Regione Toscana, Azienda Ospedaliera Universitaria Meyer IRCCS. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BC: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Validation, Writing – original draft. MT: Writing – original draft. MG: Validation, Visualization, Writing – review & editing. MD: Validation, Visualization, Writing – review & editing. LaG: Resources, Validation, Writing – review & editing. AB: Resources, Validation, Writing – review & editing. MC: Visualization, Writing – review & editing. AI: Resources, Validation, Writing – review & editing. ID: Resources, Validation, Writing – review & editing. LG: Supervision, Validation, Visualization, Writing – review & editing. IS: Conceptualization, Data curation, Supervision, Validation, Visualization, Writing – review & editing. CF: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Combined target therapy granted by “Agenzia italiana del farmaco” (AIFA) 5% fund. This work was supported by a grant from Fondazione Anna Meyer, Florence, Italy.
Conflict of interest
CF took part in the Advisory Board financed by Novartis on September 5th 2022 and she took part as principal investigator in CDRB436G2201 NCT02684058 study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rallis KS, George AM, Wozniak AM, Bigogno CM, Chow B, Hanrahan JG, et al. Molecular genetics and targeted therapies for pediatric high-grade glioma. Cancer Genomics Proteomics. (2022) 19:390–414. doi: 10.21873/cgp.20328
2. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology. (2014) 16:896–913. doi: 10.1093/neuonc/nou087
3. Aggarwal P, Luo W, Pehlivan KC, Hoang H, Rajappa P, Cripe TP, et al. Pediatric versus adult high grade glioma: Immunotherapeutic and genomic considerations. Front Immunol. (2022) 13:1038096. doi: 10.3389/fimmu.2022.1038096
4. Buccoliero AM, Giunti L, Moscardi S, Castiglione F, Provenzano A, Sardi I, et al. Pediatric high grade glioma classification criteria and molecular features of a case series. Genes. (2022) 13:624. doi: 10.3390/genes13040624
5. Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. JCO. (2017) 35:2370–7. doi: 10.1200/JCO.2017.73.0242
6. Zhang ZH, Lin MT, Chen L. Editorial: molecular advances in diagnosis and treatment of CNS tumors. Front Oncol. (2020) 10:590293. doi: 10.3389/fonc.2020.590293
7. Cacchione A, Fabozzi F, Carai A, Colafati GS, del Baldo G, Rossi S, et al. Safety and efficacy of mek inhibitors in the treatment of plexiform neurofibromas: A retrospective study. Cancer Control. (2023) 30:107327482211449. doi: 10.1177/10732748221144930
8. Grave N, Scheffel TB, Cruz FF, Rockenbach L, Goettert MI, Laufer S, et al. The functional role of p38 MAPK pathway in Malignant brain tumors. Front Pharmacol. (2022) 13:975197. doi: 10.3389/fphar.2022.975197
9. Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. (2020) 13:113. doi: 10.1186/s13045-020-00949-4
10. McCain J, The MAPK. (ERK) pathway: investigational combinations for the treatment of BRAF-mutated metastatic melanoma. P T. (2013) 38:96–108.
11. Burotto M, Chiou VL, Lee J, Kohn EC. The MAPK pathway across different Malignancies: A new perspective. Cancer. (2014) 120:3446–56. doi: 10.1002/cncr.28864
12. Kyung Myung J, Cho H, Park CK, Kim SK, Lee SH, Park SH. Analysis of the BRAFV600E mutation in central nervous system tumors. Trans Oncol. (2012) 5:430–6. doi: 10.1593/tlo.12328
13. Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. (2010) 468:973–7. doi: 10.1038/nature09626
14. Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. (2011) 121:397–405. doi: 10.1007/s00401-011-0802-6
15. Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. (2013) 37:685–98. doi: 10.1097/PAS.0b013e31827f9c5e
16. Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, et al. BRAF inhibitors: molecular targeting and immunomodulatory actions. Cancers. (2020) 12:1823. doi: 10.3390/cancers12071823
17. Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. (2020) 6:797–810. doi: 10.1016/j.trecan.2020.05.009
18. Kata K, Rodriguez-Quintero JC, Arevalo OD, Zhang JJ, Bhattacharjee MB, Ware C, et al. BRAF/MEK dual inhibitors therapy in progressive and anaplastic pleomorphic xanthoastrocytoma: case series and literature review. J Natl Compr Cancer Network. (2022) 20:1193–202. doi: 10.6004/jnccn.2022.7046
19. Rudà R, Capper D, Waldman AD, Pallud J, Minniti G, Kaley TJ, et al. EANO - EURACAN - SNO Guidelines on circumscribed astrocytic gliomas, glioneuronal, and neuronal tumors. Neuro-Oncology. (2022) 24:2015–34. doi: 10.1093/neuonc/noac188
20. Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. (2014) 14:258. doi: 10.1186/1471-2407-14-258
21. Heinzerling L, Eigentler TK, Fluck M, Hassel JC, Heller-Schenck D, Leipe J, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. (2019) 4:e000491. doi: 10.1136/esmoopen-2019-000491
22. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. (2012) 367:1694–703. doi: 10.1056/NEJMoa1210093
23. Shaikh N, Brahmbhatt N, Kruser TJ, Kam KL, Appin CL, Wadhwani N, et al. Pleomorphic xanthoastrocytoma: a brief review. CNS Oncol. (2019) 8:CNS39. doi: 10.2217/cns-2019-0009
24. Rutkowski MJ, Oh T, Niflioglu GG, Safaee M, Tihan T, Parsa AT. Pleomorphic xanthoastrocytoma with anaplastic features: retrospective case series. World Neurosurg. (2016) 95:368–74. doi: 10.1016/j.wneu.2016.07.068
25. Choudry UK, Khan SA, Qureshi A, Bari E. Primary anaplastic pleomorphic xanthoastrocytoma in adults. Case report and review of literature. Int J Surg Case Rep. (2016) 27:183–8. doi: 10.1016/j.ijscr.2016.08.022
26. Leclair NK, Lambert W, Roche K, Gillan E, Gell JJ, Lau CC, et al. Early experience with targeted therapy as a first-line adjuvant treatment for pediatric low-grade glioma. Neurosurg Focus. (2022) 53:E15. doi: 10.3171/2022.9.FOCUS22410
27. Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation–positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res. (2019) 25:7303–11. doi: 10.1158/1078-0432.CCR-19-2177
28. Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. (2020) 149:499–510. doi: 10.1007/s11060-020-03640-3
29. Barbato MI, Nashed J, Bradford D, Ren Y, Khasar S, Miller CP, et al. FDA approval summary: dabrafenib in combination with trametinib for BRAF V600E mutation–positive low-grade glioma. Clin Cancer Res. (2024) 30:263–8. doi: 10.1158/1078-0432.CCR-23-1503
30. Arbour G, Ellezam B, Weil AG, Cayrol R, Vanan MI, Coltin H, et al. Upfront BRAF/MEK inhibitors for treatment of high-grade glioma: A case report and review of the literature. Neuro-Oncol Adv. (2022) 4:vdac174. doi: 10.1093/noajnl/vdac174
31. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicenter, open-label, single-arm, phase 2, basket trial. Lancet Oncol. (2022) 23:53–64. doi: 10.1016/S1470-2045(21)00578-7
32. Hargrave DR, Terashima K, Hara J, Kordes UR, Upadhyaya SA, Sahm F, et al. Phase II trial of dabrafenib plus trametinib in relapsed/refractory BRAF V600–mutant pediatric high-grade glioma. JCO. (2023) 41:5174–83. doi: 10.1200/JCO.23.00558
33. Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. (2012) 483:100–3. doi: 10.1038/nature10868
34. Champeaux C, Weller J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J Neurooncol. (2020) 147:159–69. doi: 10.1007/s11060-020-03410-1
Keywords: high-grade glioma, MEK inhibitors, target therapy, dabrafenib, trametinib, pleomorphic xanthoastrocytoma, BRAFV600E, BRAF inhibitors
Citation: Castelli B, Tellini M, Guidi M, Di Nicola M, Giunti L, Buccoliero AM, Censullo ML, Iacono A, Desideri I, Genitori L, Sardi I and Fonte C (2024) Case report: complete long-lasting response to multimodal third line treatment with neurosurgical resection, carmustine wafer implantation and dabrafenib plus trametinib in a BRAFV600E mutated high-grade glioma. Front. Oncol. 14:1359093. doi: 10.3389/fonc.2024.1359093
Received: 20 December 2023; Accepted: 21 March 2024;
Published: 07 May 2024.
Edited by:
Gerardo Caruso, University Hospital of Policlinico G. Martino, ItalyReviewed by:
Byram Ozer, NCI Neuro-Oncology Branch, United StatesNicholas Gottardo, Perth Children’s Hospital, Australia
Copyright © 2024 Castelli, Tellini, Guidi, Di Nicola, Giunti, Buccoliero, Censullo, Iacono, Desideri, Genitori, Sardi and Fonte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Castelli, QmFyYmFyYS5jYXN0ZWxsaUBtZXllci5pdA==
 Barbara Castelli
Barbara Castelli Marco Tellini1
Marco Tellini1 Isacco Desideri
Isacco Desideri Iacopo Sardi
Iacopo Sardi