- 1Medical Imaging Key Laboratory of Sichuan Province, and Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Department of Oncology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 3Department of Radiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: To develop a CT-based nomogram to predict the response of advanced esophageal squamous cell carcinoma (ESCC) to neoadjuvant chemotherapy plus immunotherapy.
Methods: In this retrospective study, 158 consecutive patients with advanced ESCC receiving contrast-enhanced CT before neoadjuvant chemotherapy plus immunotherapy were randomized to a training cohort (TC, n = 121) and a validation cohort (VC, n = 37). Response to treatment was assessed with response evaluation criteria in solid tumors. Patients in the TC were divided into the responder (n = 69) and non-responder (n = 52) groups. For the TC, univariate analyses were performed to confirm factors associated with response prediction, and binary analyses were performed to identify independent variables to develop a nomogram. In both the TC and VC, the nomogram performance was assessed by area under the receiver operating characteristic curve (AUC), calibration slope, and decision curve analysis (DCA).
Results: In the TC, univariate analysis showed that cT stage, cN stage, gross tumor volume, gross volume of all enlarged lymph nodes, and tumor length were associated with the response (all P < 0.05). Binary analysis demonstrated that cT stage, cN stage, and tumor length were independent predictors. The independent factors were imported into the R software to construct a nomogram, showing the discriminatory ability with an AUC of 0.813 (95% confidence interval: 0.735–0.890), and the calibration curve and DCA showed that the predictive ability of the nomogram was in good agreement with the actual observation.
Conclusion: This study provides an accurate nomogram to predict the response of advanced ESCC to neoadjuvant chemotherapy plus immunotherapy.
Introduction
Esophageal cancer is the seventh most common malignancy and the sixth most common cause of cancer-related deaths worldwide (1, 2). Esophageal squamous cell carcinoma (ESCC) is the predominant histological type (3). Surgery is the main element of treatments for resectable esophageal cancer (1), but the 3-, 5-, and 10-year survival rates were 35%, 25%, and 18%, respectively (4). Most patients with ESCC had already reached the locally advanced stage at the time of diagnosis because of subtle symptoms at the early stage, and their prognoses were usually unsatisfactory. Neoadjuvant therapy (radiotherapy, chemotherapy, or a combination) before surgery has the advantage of targeting micrometastases and increasing complete resection rates (5), and for locally advanced ESCC, neoadjuvant therapy has already become one of the standard treatment options.
Immunotherapy has been recognized as an exciting therapeutic strategy for the treatment of various types of cancer in recent years, which uses the patient’s own immune system to fight malignant cells by suppressing the immune checkpoint pathway (6, 7). After a long debate about whether the immune system can recognize and kill tumor cells specifically, a growing body of evidence indicates that immune cells do indeed play an important role in controlling tumor cells (8). An immune checkpoint inhibitor, such as programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, rescues the antitumor effect of T cells by blocking the immune checkpoint pathway to fight cancer (9). Anti-PD-1 antibody plus chemotherapy may extend the survival time of ESCC patients and has already become the standard treatment (10). According to the results from randomized phase III trials, immune checkpoint inhibitors in combination with chemotherapy have been recommended as the first-line treatment instead of chemotherapy alone for patients with advanced esophageal cancer (11–15). Hence, neoadjuvant chemotherapy plus immunotherapy has become one of the most important treatment regimens for advanced ESCC. Preoperative neoadjuvant chemotherapy plus immunotherapy can further improve the survival rate and reduce the risk of distant metastasis and local recurrence for advanced ESCC patients. However, some patients may not benefit from it and may miss valuable surgical opportunities.
CT plays a key role in initial diagnosis, guidance of treatment, and subsequent follow-up for patients with esophageal cancer (16, 17). CT has been applied to predict PD-L1 and CD8+ TIL expression levels in ESCC patients and differentiate between immune checkpoint inhibitor-related pneumonitis and radiation pneumonitis for patients with unresected locally advanced stage non-small cell lung cancer (18, 19). To our knowledge, there exist no reports on the response prediction in patients with advanced ESCC who were treated with neoadjuvant chemotherapy plus immunotherapy. Therefore, our study aimed to develop and validate a CT-based nomogram to predict the response to neoadjuvant chemotherapy plus immunotherapy in patients with advanced ESCC, in order to assess the patients’ response to neoadjuvant chemotherapy plus immunotherapy based on pretherapeutic CT images and recommend neoadjuvant treatments to the “responders.”
Materials and methods
Patients
Our institutional ethics committee approved this study, and an informed consent was signed by each patient before partaking in this study.
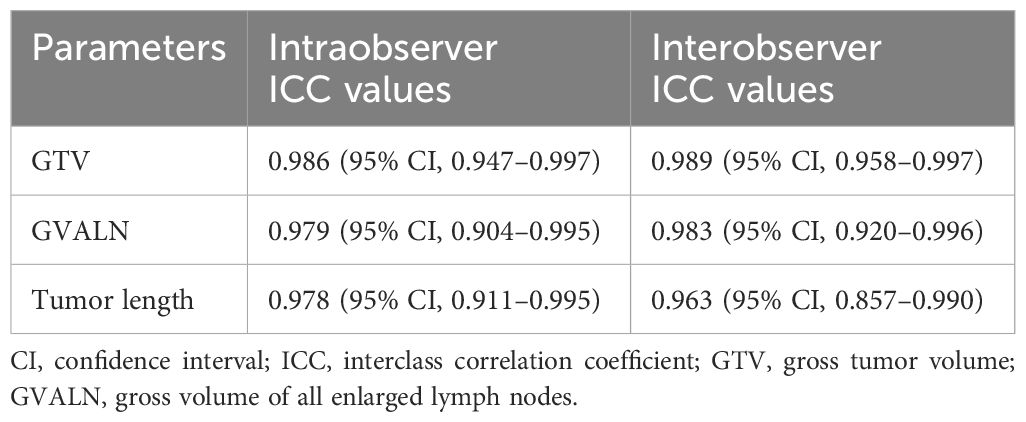
We retrospectively collected and analyzed patients with advanced ESCC who received neoadjuvant chemotherapy plus immunotherapy in our hospital from January 2020 to September 2022. The inclusion criteria were as follows: 1) diagnosis of ESCC based on preoperative endoscopic pathological examination, 2) patients underwent CT before neoadjuvant therapy, and 3) patients’ clinical TNM stage was cT3-4aN0-2M0 as depicted on CT. According to the inclusion criteria, we collected 175 consecutive cases. The exclusion criteria were any of the following: 1) patients received any tumor-related treatments (e.g., chemotherapy or radiotherapy) before undergoing CT (n = 10), 2) patients had concomitant malignant tumors of another type (n = 2), or 3) the quality of the images was not good enough (n = 5). Therefore, 17 cases were excluded from our study, and 158 patients were ultimately included in the study. All participants were randomly assigned to a training cohort (TC, n = 121) and a validation cohort (VC, n = 37) with SPSS (version 25, Chicago, IL, USA) (20). All the patients underwent CT examinations before and after two cycles of neoadjuvant chemotherapy plus immunotherapy. The age, gender, and anatomic distribution of the tumor in the TC and VC are depicted in Table 1.
Contrast-enhanced CT scans
All image data were acquired using a 64-section multidetector computed tomography (MDCT) (LightSpeed VCT, GE Medical Systems, USA). Patients were orally given 100 ml to 200 ml of water as a negative contrast agent before CT data collection. The CT examinations were performed in a supine position. First, the routine unenhanced scans were performed. Subsequently, a 70–100-ml contrast agent (Omnipaque, Iohexol, GE Healthcare, USA), calculated based on a ratio of 1.5 ml/kg body weight, was injected into an antecubital vein with a 20-G needle at the rate of 3.0 ml/s, and then, 20 ml of saline was rinsed with a pump syringe (Vistron CT Injection System, Medrad, USA). Twenty to 30 s after the contrast injection, contrast-enhanced CT data were obtained. The CT scanning parameters were as follows: peak voltage 120 kV, tube current 200 mA (using automatic exposure control), rotation time 0.5 s, collimation 64 × 0.6 mm, pitch 0.9, section thickness 5 mm, and matrix 512 × 512 mm. The CT scan covered the area from the neck to the middle of the left kidney. During one breath-hold with a full-held inspiration for 10–15 s, each examination was performed. Finally, CT data were transmitted directly to the General Electric Advantage Workstation 4.4 at the mediastinal window settings, and the window width was 400 HU with a window level of 40 HU.
Treatment
All enrolled patients received two cycles of neoadjuvant chemotherapy plus immunotherapy. The concrete treatment drugs were as follows (21, 22): taxel (docetaxel or paclitaxel) and platinum doublet (cisplatin or carboplatin) plus immunotherapy (sintilimab, pembrolizumab, or camrelizumab). The details of the above chemotherapy were as follows: during each cycle of the 21-day duration, cisplatin or carboplatin (75 mg/m2) on day 1, docetaxel or paclitaxel (135 mg/m2) on days 1 and 8, and sintilimab, pembrolizumab, or camrelizumab (200 mg) on day 1 were administered intravenously. All patients were re-evaluated with a CT scan of the chest and upper abdomen approximately 4 to 6 weeks after neoadjuvant chemotherapy plus immunotherapy. The neoadjuvant regimens of the patients in the TC and VC are depicted in Table 1.
Assessment of response
Due to the absence of the phenomenon of pseudoprogression in this study, tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as follows (23): complete response (CR) was the disappearance of all target lesions; partial response (PR) was at minimum a 30% reduction in the sum of the longest target lesion diameters using the sum of the longest target lesion diameters at baseline as reference; progressive disease (PD) was at minimum a 20% increase in the sum of the longest target lesion diameters using the minimum sum of the longest target lesion diameters recorded since the start of treatment (rock bottom) as reference, or the appearance of at least one new lesion; and stable disease (SD) was neither PR nor PD. The patients with CR or PR according to RECIST (v.1.1) within two treatment cycles were considered “responders,” while those who suffered from PD or SD were considered “non-responders.” As reported (24), the application of iRECIST had no impact on response-related endpoints, compared to RECIST 1.1, and we used RECIST 1.1 to assess the tumoral treatment response.
Pretherapeutic CT features assessment
The pretherapeutic CT features of advanced ESCC were assessed on the 3D-slicer 4.11. The esophageal wall thickness that exceeded 5 mm on axial contrast-enhanced CT was considered abnormal thickness caused by the tumor (25). The pretherapeutic CT features included cT stage, cN stage, anatomic distribution, gross tumor volume (GTV), gross volume of all enlarged lymph nodes (GVALN), and tumor length. The shape of the esophageal tumor was manually outlined around the abnormal tissue on enhanced CT (Figure 1) independently by two radiologists (observer 1 with 3 years of expertise in radiology and observer 2 with 4 years of expertise in radiology), and then the above software automatically calculated the GTV. The volume of each enlarged lymph node was also obtained by the previous two radiologists in a way similar to the previous GTV, and GVALN was obtained by the sum of the volume of each enlarged lymph node. The tumor lengths were independently assessed by observers 1 and 2 on axial and sagittal contrast-enhanced CT images as follows: the upper and lower edges of the tumor on axial contrast-enhanced CT images were determined and marked, and then we measured the tumor length on sagittal contrast-enhanced CT images through the markers with the referring standards of double contrast barium examinations. The measurements of GTV, GVALN, and tumor length of ESCC independently by the above two radiologists were used to test the interobserver reproducibility. Observer 1 remeasured the GTV, GVALN, and tumor length of all target lesions 1 month later to test the intraobserver reproducibility. Before their measurements, a radiology professor with 25 years of radiology experience taught them how to assess quantitative CT tumor features randomly in 20 patients.
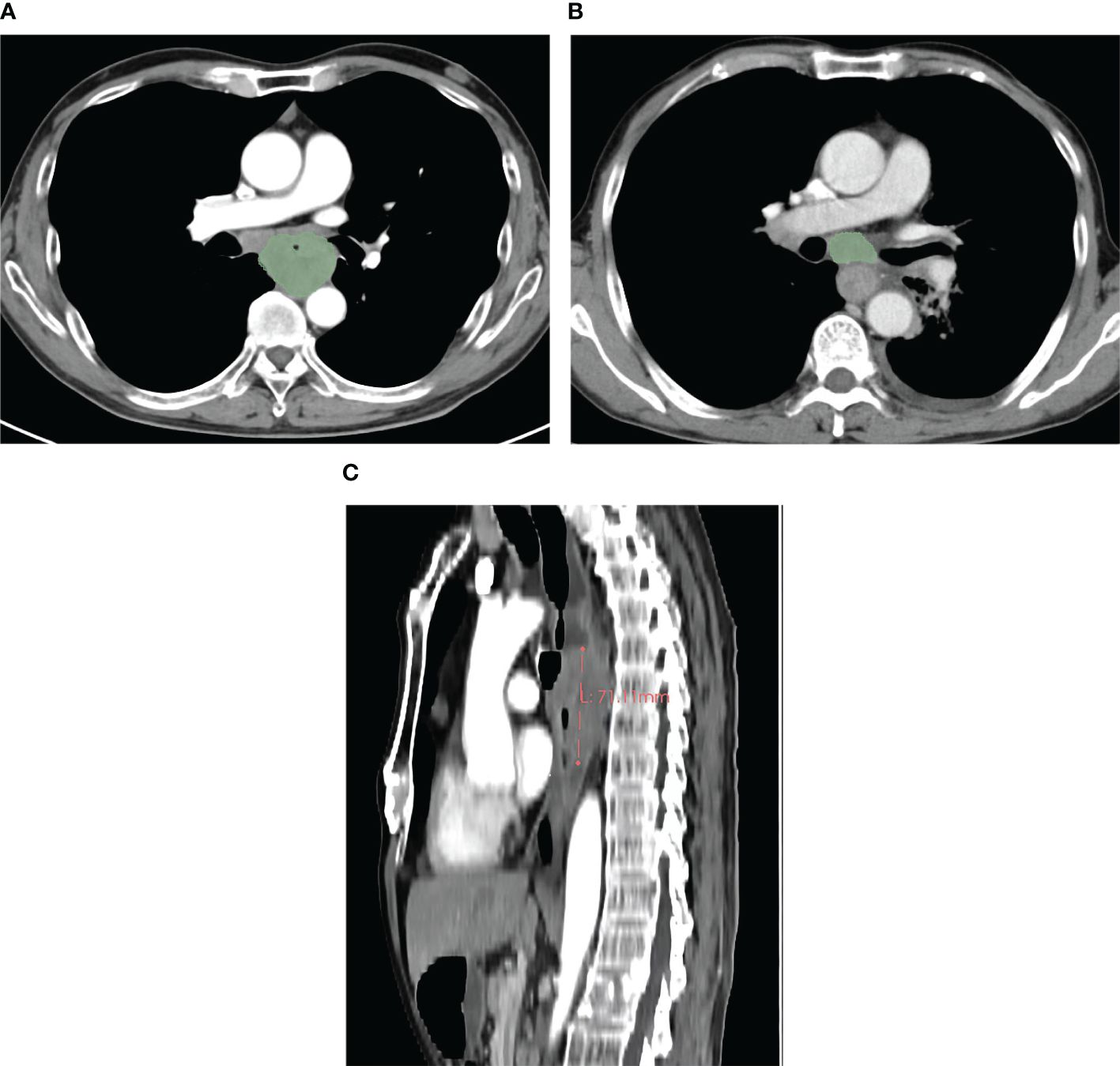
Figure 1 In a 59-year-old male patient with cT4N1M0 esophageal squamous cell carcinoma, the pretherapeutic contrast-enhanced CT scans show the gross tumor volume obtained by slice-by-slice manual delineation of the tumor (A), and the gross tumor volume is 57.66 cm3. In a 64-year-old male patient with esophageal squamous cell carcinoma at cT3N1M0, the gross volume of the enlarged lymph nodes was obtained in a way similar to that of the tumor (B), and the gross volume of all enlarged lymph nodes is 21.28 cm3. In a 59-year-old male patient with cT4N2M0 esophageal squamous cell carcinoma, the tumor length was obtained with the value of 7.11 cm (C).
For assessing the pretherapeutic categorical CT features including cT stages, cN stages, and anatomic distribution, observer 1 discussed with observer 2 to reach a consensus. When observer 1 and observer 2 disagreed with each other, they could consult the above professor. The cT and cN stages were assessed using the pretherapeutic CT data based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging of cancers of the esophagus and esophagogastric junction (26). For the cT stage, cT3 of the advanced tumor infiltrated the adventitia, and the cT4 tumor infiltrated adjacent structures. For the cN stage, the number of involved nodes determines the N stage: the N0 stage involves no node, the N1 stage involves one to two nodes, the N2 stage involves three to six regional nodes, and the N3 stage involves seven or more regional nodes. Based on the AJCC staging of cancers of the esophagus and esophagogastric junction, the short axis of nodes can be easily measured on CT, and the intrathoracic and abdominal lymph nodes >1 cm can be considered enlarged. All evaluations were performed without knowledge of the histological results.
Statistical analysis
Statistical analyses were performed by SPSS (version 25, Chicago, IL, USA). According to the published study on pancreatitis using similar statistical analysis (27), univariate analysis was performed to identify the pretherapeutic CT features associated with the response prediction. Chi-squared test or Fisher’s exact test was used to compare categorical variables, and the independent-sample t-test or Mann–Whitney U test was used to compare continuous variables. Binary logistic analysis was performed for statistically significant CT features from the univariate analysis and showed no multicollinearity (27). A P <0.05 was considered statistically significant for all statistical tests.
Nomogram development and validation
The R software (version 4.2.2) was used to develop and validate the nomogram. For the development of the nomogram, only independent predictive factors determined by binary logistic analysis were selected. In our nomogram, the regression coefficient of each independent predictive factor in binary logistic regression was proportionally transformed into a specific number on a scale of 0 to 100 points. Moreover, the accuracy of the predictive nomogram was assessed using the concordance index (C-index) and the calibration curves. The receiver-operating characteristic (ROC) curve and the largest area under the receiver-operating characteristic curve (AUC) were obtained with the optimal cutoff point in the nomogram. Additionally, we used decision curve analysis (DCA) to validate the clinical application value of our model because DCA is a novel algorithm for evaluating the net benefit value of a model under different thresholds (28).
Results
Response in patients
In the TC, 9 (7.4%), 60 (49.6%), 41 (33.9%), and 11 (9.1%) patients demonstrated CR, PR, PD, and SD, respectively. In the VC, 2 (5.4%), 20 (54.1%), 11 (29.7%), and 4 (10.8%) patients demonstrated CR, PR, PD, and SD, respectively. Hence, there were 69 responders and 52 non-responders in the TC and 22 responders and 15 non-responders in the VC.
Intra- and interobserver measurement agreement of quantitative features
Intraobserver and interobserver agreements of GTV, GVALN, and tumor length are shown in Table 2. Both intra- and interobserver ICC values of the above measurements were >0.90 (P < 0.001 for all). Thus, the GTV, GVALN, and tumor length obtained by the first measurements from the first observer were used for the subsequent analyses.
Univariate analysis of pretherapeutic CT and clinical features in the TC: correlation with response
The pretherapeutic CT and clinical features are listed in Table 1. According to our univariate analysis, the cT stage, cN stage, GTV, GVALN, and tumor length were associated with response in patients with ESCC after neoadjuvant chemotherapy plus immunotherapy (P < 0.05 for all). In detail, patients with lower cT stage, lower cN stage, lower GTV, lower GVALN, and lower tumor length were more likely to be responders (P = 0.001, < 0.001, = 0.001, = 0.002, and < 0.001, respectively). However, there were no statistically significant differences in gender, age, neoadjuvant regimen, and anatomic distribution between responders and non-responders (P = 0.376, 0.717, 0.617, and 0.094, respectively).
Binary analysis of CT features with response in the TC
As for potential independent predictive factors for therapeutic response after neoadjuvant chemotherapy plus immunotherapy including cT stage, cN stage, GTV, GVALN, and tumor length, the binary logistic regression analysis was used for identifying the independent predictive factors. The results of the binary logistic regression analysis indicated that cT stage, cN stage, and tumor length were independent predictive factors in patients with advanced ESCC (P = 0.039, 0.001, and 0.002, respectively).
CT-based nomogram for prediction of therapeutic response in the TC
Based on the above binary analysis in the TC, a nomogram was constructed to predict the response of patients with ESCC to neoadjuvant chemotherapy plus immunotherapy that incorporated the three independent predictive factors: cT stage, cN stage, and tumor length (Figure 2). A total score was calculated with the use of cT stage, cN stage, and tumor length which were reachable on CT. In our nomogram, the value of each variable was scored on the axis of the score scale. By adding each score, the total score could be easily calculated, and we were able to predict patients’ response after the neoadjuvant treatment by projecting the total score onto the total point scale.
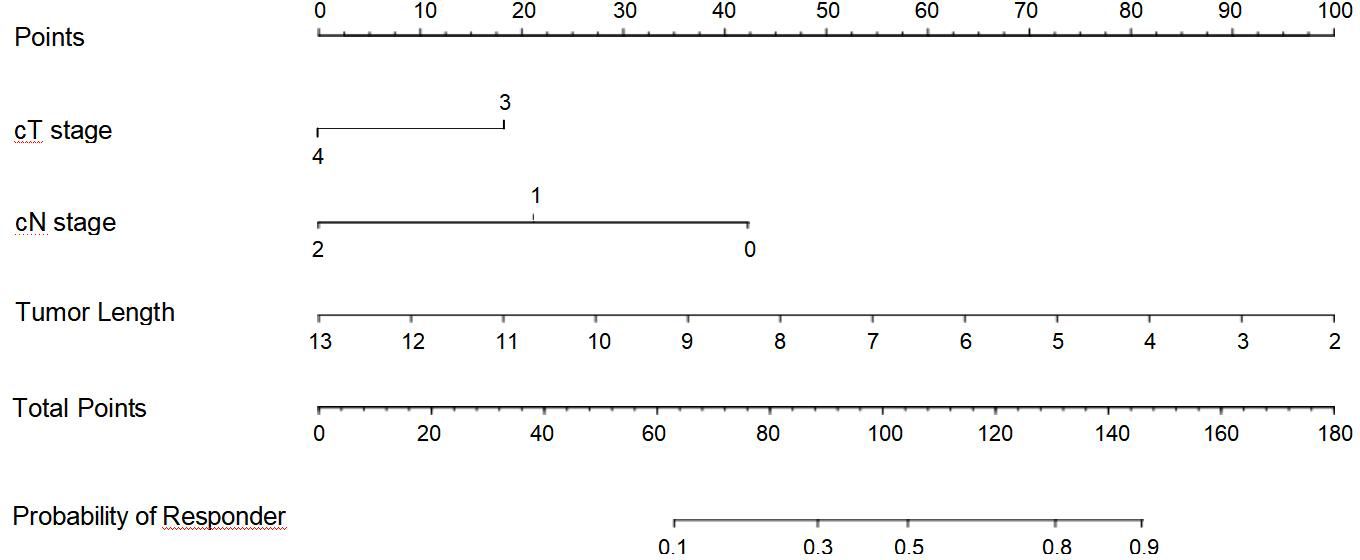
Figure 2 Nomogram for predicting the response of advanced esophageal squamous cell carcinoma after neoadjuvant chemotherapy plus immunotherapy.
Performance of the CT-based nomogram in the TC and VC
In the TC and VC, an individual calibration curve was used to confirm the accuracy of the nomogram based on the predictive nomogram, showing that the predicted values of our model coincided with the actual values (Figure 3). The AUC values of 0.813 [95% confidence interval (CI), 0.735–0.890] in the TC and of 0.797 (95%CI, 0.643–0.950) in the VC suggested excellent predictive power, respectively (Figure 4). DCA demonstrated good clinical application of this nomogram in the TC and VC (Figure 5).
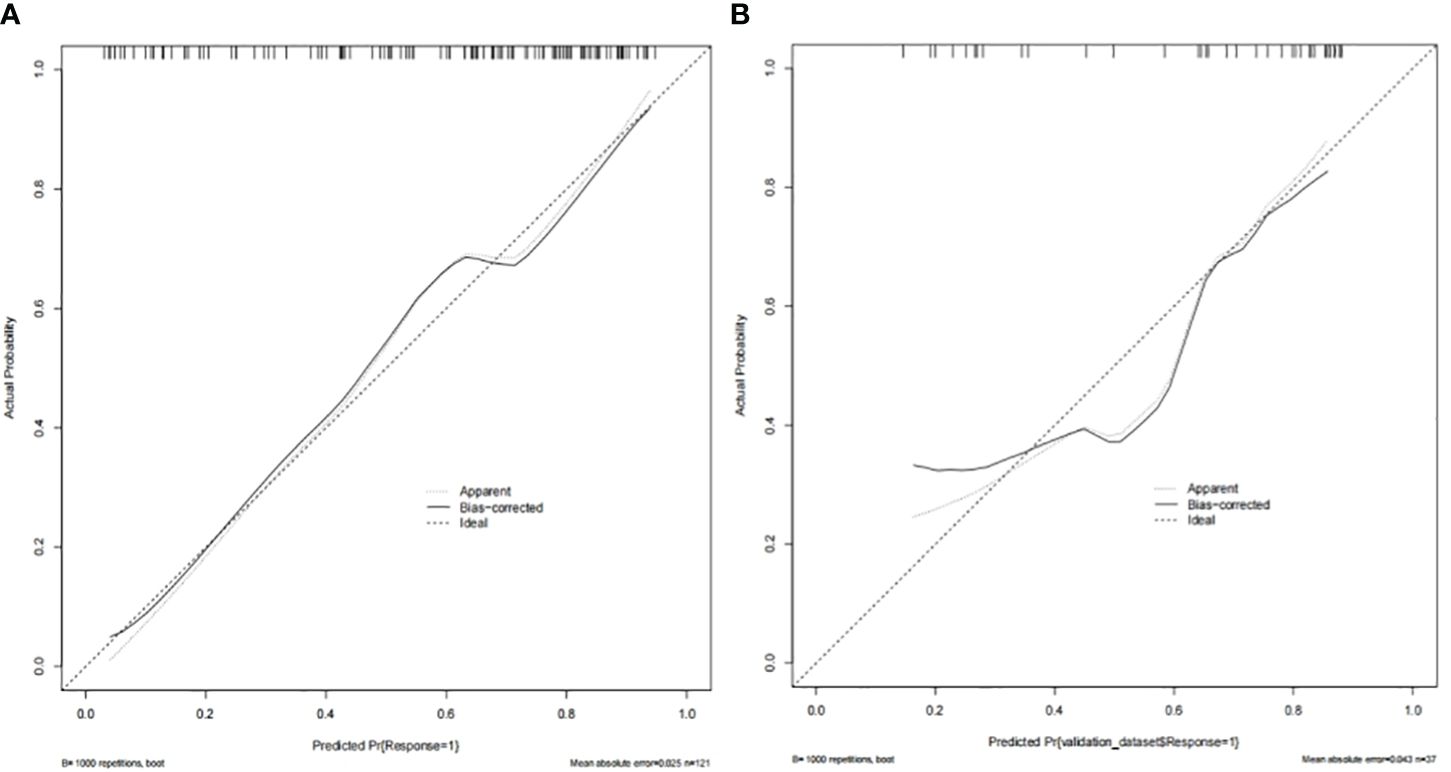
Figure 3 The nomogram’s calibration curves in the training cohort (A) and the validation cohort (B).
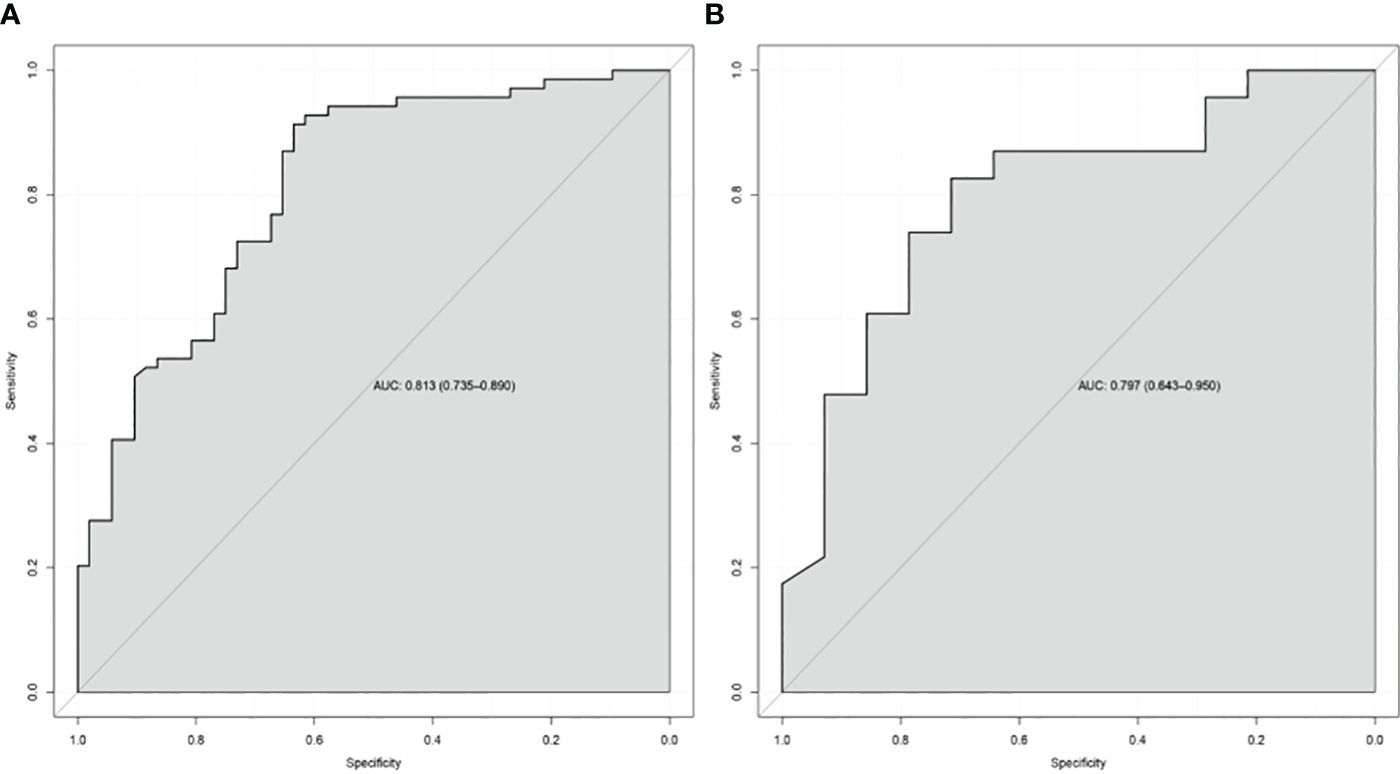
Figure 4 The receiver-operating characteristic curve of the nomogram of the training cohort (A) and the validation cohort (B). AUC, the area under the receiver-operating characteristic curve.
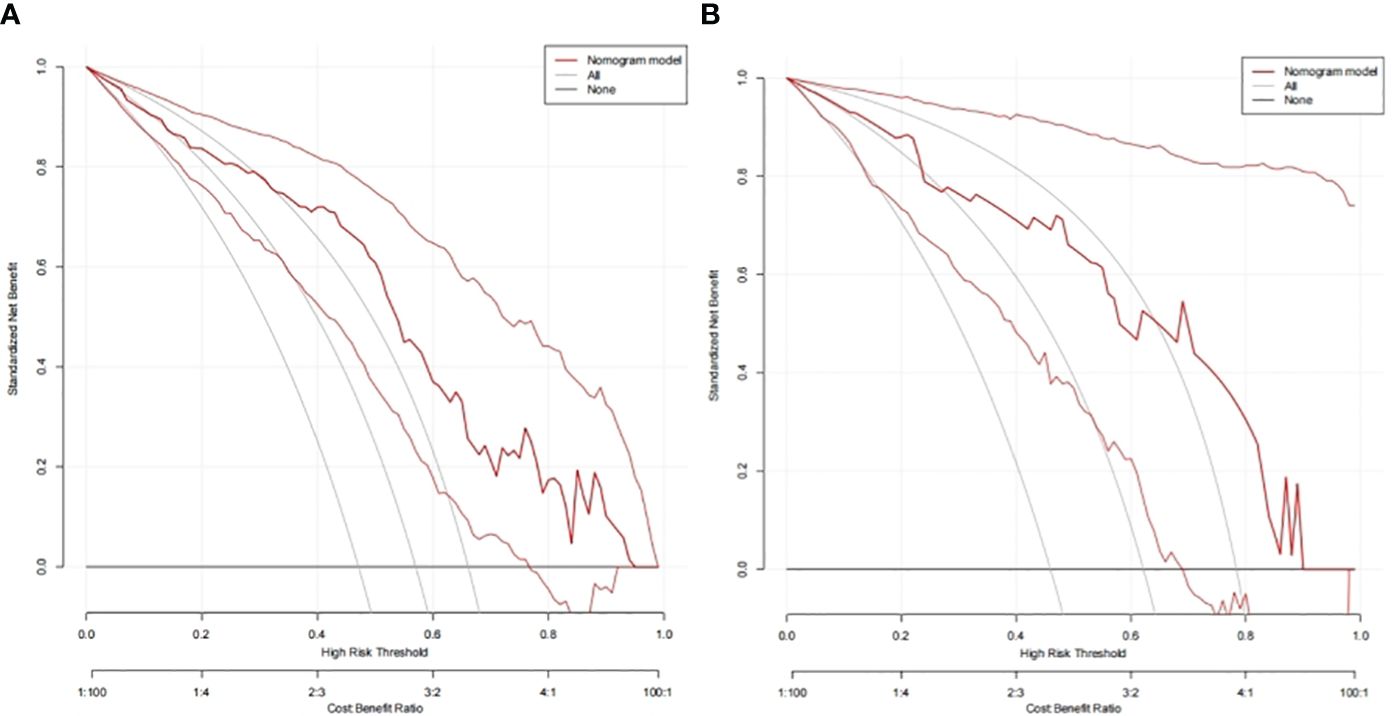
Figure 5 Decision curves of the nomogram to predict response in the training cohort (A) and the validation cohort (B).
Discussion
Previous research studies (29, 30) have explored the treatment response to chemoradiotherapy in patients with esophageal cancer based on CT. Immunotherapy has become increasingly important in patients’ treatments, but there is no literature on the prediction of response to this therapy. The current study shows that cT stage, cN stage, and tumor length depicted on CT are independent predictive factors in patients with advanced ESCC who have received neoadjuvant chemotherapy plus immunotherapy for the first time. We investigated the feasibility of a nomogram developed with independent predictive factors to predict the response.
As shown in this study, the cT stage and cN stage of the tumor could be independent factors in predicting response to neoadjuvant chemotherapy plus immunotherapy. Previous studies manifested that the clinical stage could predict the response to neoadjuvant treatment (31–33). The higher the cT stage, the higher the probability of non-responder could be. This is due to the invasion of blood vessels, cancer cells entering the bloodstream and causing metastasis, which leads to adverse results. The lack of metastatic lymph nodes indicates that the disease is still at an early stage, suggesting a superior response. Qiao et al. reported that the pathologic CR is more likely to appear in patients without lymph node metastases (34). Several published papers have shown the appearance of metastatic lymph nodes is a major predictive factor contributing to high recurrence rate in esophageal cancer (35, 36).
This study revealed that tumor length could be another independent predictive factor. Tumor length of esophageal cancer is considered an important factor related to the degree of peripheral invasiveness of the tumor by researchers (37–39). Song et al. found that tumor length was still an independent predictive factor even in patients with esophageal cancer at the same TNM stages (40). Wang et al. reported that primary tumor length could be used to predictively stratify patients and is a predictive indicator for clinical treatment decisions regarding esophageal cancer treatment (41). Based on previously published literature in terms of predicting response in patients with advanced ESCC, we took these possible predictive factors into consideration and predicted the response in patients after neoadjuvant chemotherapy plus immunotherapy for the first time.
Because the cT stage, cN stage, and tumor length of ESCC obtained on CT were independently associated with the therapeutic response after neoadjuvant chemotherapy plus immunotherapy, the novel nomogram was subsequently developed with the three independent predictors to predict the response. After internal validation of this novel nomogram, the C-index was 0.813, and the calibration curve indicated the perfect accuracy of this model. This model may be a practical and theoretical basis for the clinical pretherapeutic decision-making regarding whether the ESCC patient can benefit from neoadjuvant chemotherapy plus immunotherapy and posttherapeutic follow-up. In clinical practice, clinicians can incorporate cT stage, cN stage, and tumor length based on the pretherapeutic CT features into our nomogram in order to obtain the probability that the patient may be a “responder.” Based on the obtained probability, they can comprehensively make the appropriate treatment decision for the patient. Patients who cannot benefit from neoadjuvant chemotherapy plus immunotherapy even suffer side effects like anemia, decreased white blood cell count, asthenia, vomiting, decreased weight, and rash (12). To improve the quality of life and survival rate of patients with ESCC, we can distinguish the “responders” from “non-responders” before their treatment to recommend that the responders receive neoadjuvant chemotherapy plus immunotherapy while the non-responders do not receive this therapy, avoiding its side effects.
This study had several limitations. On one hand, our research was a single-center retrospective study with a small sample size. Therefore, future work will involve collecting data from multiple centers and large samples to confirm our findings. On the other hand, we did not apply the K-fold cross-validation but the SPSS (version 25, Chicago, IL, USA) for data grouping because the latter has been commonly used in similar research (20). We will compare the utility of K-fold cross-validation to perform the relevant study with the utility of SPSS in the future. In addition, since the conventional thickness of the chest CT scan was 5 mm rather than 1 mm in our hospital, we used 5-mm-thick sections for the retrospective study. We will conduct further research using 1-mm-thick slices to validate the findings in the future. Lastly, the CT images were used to predict the response to neoadjuvant chemotherapy plus immunotherapy rather than to assess the CT nomogram to guide surgery. We will conduct a relevant comprehensive study on the CT nomogram to guide surgery in the future.
In conclusion, we explored the factors for predicting the response of advanced ESCC to neoadjuvant chemotherapy plus immunotherapy and found that cT stage, cN stage, and tumor length are independent predictive factors for prediction. We constructed and validated a new nomogram based on the independent CT predictive factors to predict the response, with good accuracy and reliability. The novel nomogram may be able to help physicians and patients to make appropriate intervention decisions in a timely manner.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WG: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal Analysis, Data curation. CZ: Writing – original draft, Visualization, Investigation, Data curation. DG: Writing – original draft, Visualization, Validation, Investigation, Formal Analysis. MX: Writing – original draft, Visualization, Investigation, Formal Analysis, Data curation. YG: Writing – review & editing, Supervision, Resources, Methodology, Conceptualization. HZ: Writing – review & editing, Supervision, Software, Methodology, Conceptualization. TC: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. XZ: Writing – review & editing, Supervision, Software, Resources, Project administration, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors are grateful for the financial support given by the National Natural Science Foundation of China (grant no. 82271959) and the Nanchong-University Cooperative Research Project (grant no. 20SXQT0329) for the conduct of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ESCC, esophageal squamous cell carcinoma; CT, computed tomography; MDCT, multidetector computed tomography; GTV, gross tumor volume; GVALN, gross volume of all enlarged lymph nodes; AJCC, American Joint Committee on Cancer; ICC, intraclass correlation coefficient; CI, confidence interval.
References
1. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2019) 17:855–83. doi: 10.6004/jnccn.2019.0033
2. Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. (2015) 34:502–7. doi: 10.1186/s40880-015-0042-6
3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
4. Oezcelik A, Kaiser GM, Niebel W, Sleyman C, Treckmann JW, Sotiropoulos GC, et al. Ten-year survival of esophageal cancer after an en-bloc esophagectomy. J Surg Oncol. (2012) 105:284–7. doi: 10.1002/jso.22096
5. Lewis S, Lukovic J. Neoadjuvant therapy in esophageal cancer. Thorac Surg Clin. (2022) 32:447–56. doi: 10.1016/j.thorsurg.2022.06.003
6. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
7. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. (2015) 348:74–80. doi: 10.1126/science.aaa6204
8. Zhao Q, Yu J, Meng X. A good start of immunotherapy in esophageal cancer. Cancer Med. (2019) 8:4519–26. doi: 10.1002/cam4.2336
9. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. (2015) 33:1974–82. doi: 10.1200/JCO.2014.59.4358
10. Kato K, Sun J, Shah MA, Enzinger PC, Adenis A, Doi T, et al. LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 study. Ann Oncol. (2020) 31:S1192–3. doi: 10.1016/j.annonc.2020.08.2298
11. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
12. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. (2021) 326:916–25. doi: 10.1001/jama.2021.12836
13. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
14. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) 384:829–41. doi: 10.1056/NEJMoa2026982
15. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cel. (2022) 40:277–88. doi: 10.1016/j.ccell.2022.02.007
16. Ou J, Li R, Zeng R, Wu CQ, Chen Y, Chen TW, et al. CT radiomic features for predicting resectability of esophageal squamous cell carcinoma as given by feature analysis: A case control study. Cancer Imaging. (2019) 19:66. doi: 10.1186/s40644-019-0254-0
17. Umeoka S, Koyama T, Togashi K, Saga T, Watanabe G, Shimada Y, et al. Esophageal cancer: evaluation with triple-phase dynamic CT–initial experience. Radiology. (2006) 239:777–83. doi: 10.1148/radiol.2393050222
18. Wen Q, Yang Z, Zhu J, Qiu Q, Dai H, Feng A, et al. Pretreatment CT-based radiomics signature as a potential imaging biomarker for predicting the expression of PD-L1 and CD8+TILs in ESCC. Onco Targets Ther. (2020) 13:12003–13. doi: 10.2147/OTT.S261068
19. Qiu Q, Xing L, Wang Y, Feng A, Wen Q. Development and validation of a radiomics nomogram using computed tomography for differentiating immune checkpoint inhibitor-related pneumonitis from radiation pneumonitis for patients with non-small cell lung cancer. Front Immunol. (2022) 13:870842. doi: 10.3389/fimmu.2022.870842
20. Zou ZH, Liu XQ, Li WH, Zhou XT, Li XF. Development and validation of multiple linear regression models for predicting total hip arthroplasty acetabular prosthesis. J Orthop Surg Res. (2024) 19:73. doi: 10.1186/s13018-024-04526-0
21. Zhao J, Hao S, Tian J, Li Y, Han D. Comparison of neoadjuvant immunotherapy plus chemotherapy versus neoadjuvant chemoradiotherapy for patients with esophageal squamous cell carcinoma: A propensity score matching study. J Inflammation Res. (2023) 16:3351–63. doi: 10.2147/JIR.S424454
22. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. (2021) 13:3518–28. doi: 10.21037/jtd
23. Wang S, Xu G, Li M, Zheng J, Wang Y, Feng X, et al. M1 macrophage predicted efficacy of neoadjuvant camrelizumab combined with chemotherapy vs chemotherapy alone for locally advanced ESCC: A pilot study. Front Oncol. (2023) 13:1139990. doi: 10.3389/fonc.2023.1139990
24. Park HJ, Kim GH, Kim KW, Lee CW, Yoon S, Chae YK, et al. Comparison of RECIST 1.1 and iRECIST in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Cancers (Basel). (2021) 13:120. doi: 10.3390/cancers13010120
25. Moss AA, Schnyder P, Thoeni RF, Margulis AR. Esophageal carcinoma: pretherapy staging by computed tomography. AJR Am J Roentgenol. (1981) 136:1051–6. doi: 10.2214/ajr.136.6.1051
26. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. (2017) 6:119–30. doi: 10.21037/acs
27. Zver T, Calame P, Koch S, Aubry S, Vuitton L, Delabrousse E. Early prediction of acute biliary pancreatitis using clinical and abdominal CT features. Radiology. (2022) 302:118–26. doi: 10.1148/radiol.2021210607
28. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. (2008) 8:53. doi: 10.1186/1472-6947-8-53
29. Li C, Pan Y, Yang X, Jing D, Chen Y, Luo C, et al. CT-based radiomics for predicting radio-chemotherapy response and overall survival in nonsurgical esophageal carcinoma. Front Oncol. (2023) 13:1219106. doi: 10.3389/fonc.2023.1219106
30. Hou Z, Ren W, Li S, Liu J, Sun Y, Yan J, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget. (2017) 8:104444–54. doi: 10.18632/oncotarget.v8i61
31. Amini A, Ajani J, Komaki R, Allen PK, Minsky BD, Blum M, et al. Factors associated with local-regional failure after definitive chemoradiation for locally advanced esophageal cancer. Ann Surg Oncol. (2014) 21:306–14. doi: 10.1245/s10434-013-3303-0
32. Nomura M, Shitara K, Kodaira T, Kondoh C, Takahari D, Ura T, et al. Recursive partitioning analysis for new classification of patients with esophageal cancer treated by chemoradiotherapy. Int J Radiat Oncol Biol Phys. (2012) 84:786–92. doi: 10.1016/j.ijrobp.2011.12.069
33. Chao YK, Tseng CK, Wen YW, Liu YH, Wan YL, Chiu CT, et al. Using pretreatment tumor depth and length to select esophageal squamous cell carcinoma patients for nonoperative treatment after neoadjuvant chemoradiotherapy. Ann Surg Oncol. (2013) 20:3000–8. doi: 10.1245/s10434-013-2962-1
34. Qiao Y, Zhao C, Li X, Zhao J, Huang Q, Ding Z, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immunol. (2022) 13:953229. doi: 10.3389/fimmu.2022.953229
35. López-Sebastián J, Martí-Obiol R, López-Mozos F, Ortega-Serrano J. Recurrence of esophageal cancer after R0 surgery. Risk factors and evolution. Rev Esp Enferm Dig. (2013) 105:318–25. doi: 10.4321/s1130-01082013000600002
36. Hiyoshi Y, Yoshida N, Watanabe M, Kurashige J, Karashima R, Iwagami S, et al. Late recurrence after radical resection of esophageal cancer. World J Surg. (2016) 40:913–20. doi: 10.1007/s00268-015-3334-8
37. Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal Malignancy: univariate and multivariate survival analyses. J Surg Oncol. (2006) 93:258–67. doi: 10.1002/jso.20449
38. Ma MQ, Yu ZT, Tang P, Jiang HJ, Zhao XJ, Zhang JG, et al. Is tumor length a prognostic indicator for esophageal squamous cell carcinoma? A single larger study among Chinese patients. Int J Clin Exp Pathol. (2015) 8:5008–16.
39. Twine CP, Roberts SA, Lewis WG, Dave BV, Rawlinson CE, Chan D, et al. Prognostic significance of endoluminal ultrasound-defined disease length and tumor volume (EDTV) for patients with the diagnosis of esophageal cancer. Surg Endosc. (2010) 24:870–8. doi: 10.1007/s00464-009-0681-2
40. Song Z, Wang J, Lin B, Zhang Y. Analysis of the tumor length and other prognosis factors in pT1–2 node-negative esophageal squamous cell carcinoma in a Chinese population. World J Surg Oncol. (2012) 10:273. doi: 10.1186/1477-7819-10-273
Keywords: esophagus, squamous cell carcinoma, immunotherapy, chemotherapy, computed tomography, nomogram
Citation: Guo W-w, Zhou C, Gao D, Xu M, Gui Y, Zhou H-y, Chen T-w and Zhang X-m (2024) A computed tomography-based nomogram for neoadjuvant chemotherapy plus immunotherapy response prediction in patients with advanced esophageal squamous cell carcinoma. Front. Oncol. 14:1358947. doi: 10.3389/fonc.2024.1358947
Received: 20 December 2023; Accepted: 21 May 2024;
Published: 05 June 2024.
Edited by:
Venkatesan Renugopalakrishnan, Harvard University, United StatesReviewed by:
Zhengchao Zhang, Yantai Affiliated Hospital of Binzhou Medical University, ChinaQiang Wen, Shandong Provincial Hospital, China
Copyright © 2024 Guo, Zhou, Gao, Xu, Gui, Zhou, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-wu Chen, dGlhbnd1Y2hlbl9uc21jQDE2My5jb20=
 Wen-wen Guo1
Wen-wen Guo1 Hai-ying Zhou
Hai-ying Zhou Tian-wu Chen
Tian-wu Chen Xiao-ming Zhang
Xiao-ming Zhang