
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 05 March 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1358070
The fibroblast activating protein (FAP) is expressed by some fibroblasts found in healthy tissues. However, FAP is overexpressed in more than 90% of epithelial tumors, including breast and gynecological tumors. As a result, the FAP ligand could be used as a target for diagnosis and treatment purposes. Positron emission tomography/computed tomography (PET/CT) is a hybrid imaging technique commonly used to locate and assess the tumor’s molecular and metabolic functions. PET imaging involves the injection of a radiotracer that tends to accumulate more in metabolically active lesions such as cancer. Several radiotracers have been developed to target FAP in PET/CT imaging, such as the fibroblast-activation protein inhibitor (FAPI). These tracers bind to FAP with high specificity and affinity, allowing for the non-invasive detection and quantification of FAP expression in tumors. In this review, we discussed the applications of FAPI PET/CT in the diagnosis and treatment of breast and the most common gynecologic malignancies. Radiolabeled FAPI can improve the detection, staging, and assessment of treatment response in breast and the most common gynecologic malignancies, but the problem with normal hormone-responsive organs remains insurmountable. Compared to the diagnostic applications of FAPI, further research is needed for future therapeutic applications.
Positron emission tomography/computed tomography (PET/CT) is an imaging technique used to obtain anatomical, functional, and molecular information about the tumor. It plays an important role in the diagnosis of malignant tumors and is widely used to detect and stage cancer (1). 18-Fluorodeoxyglucose (18F-FDG) is the most commonly used radiotracer for PET/CT imaging. 18F-FDG has a high sensitivity and specificity to detect, stage, and assess treatment response in cancer patients. However, 18F-FDG also has a number of limitations. This radiotracer has a relatively low sensitivity for the detection of micrometastases and lymph nodes less than 1cm and it can not accurately distinguish between acute inflammatory infections from tumor growth (2, 3). Moreover, patients need to fast before the examination, and the whole examination can take up to a longer scanning time. As a result, new radiotracers are being developed to improve the diagnostic accuracy and facilitate the examination process of PET/CT.
The fibroblast activation protein (FAP) is a membrane-bound serine protease that is expressed by activated fibroblasts and other cell types in the tumor microenvironment. FAP has both gelatinase and dipeptidyl peptidase activities, allowing it to cleave a variety of substrates, including type I collagen and dipeptides. Therefore, FAP plays a key role in the remodeling of the extracellular matrix and in the formation of fibrous tissues, which are critical components of wound healing and tissue repair processes (4–6). FAP has also been found to be overexpressed in cancer-associated fibroblasts (CAF) found in the tumor microenvironment of various malignant epithelial tumors such as breast, colon, and pancreatic cancer. However, FAP is generally not expressed or expressed at very low levels in normal tissue and benign tumors. Activated CAFs can secrete various factors such as cytokines, growth factors, extracellular matrix proteins, and enzymes. These factors can promote angiogenesis, immune evasion, tumor growth, and progression. As a result, elevated FAP levels are usually linked with a poor prognosis (7).
The fibroblast activation protein inhibitor (FAPI) is a small molecule that can bind with the FAP enzyme domain found on the surface of the CAFs. As a result, FAPI is increasingly being used to diagnose and target cancers with high FAP expression (8). FAPI can be coupled with the chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and the radiotracer gallium-68 (68Ga) in PET/CT imaging. FAPI PET/CT imaging shows promising results in cancer imaging, particularly for the most common gynecologic malignancies (9, 10). The FAPI PET/CT examination can be performed in 10 minutes to 1 hour without the need for fasting and resting time. As a result, when compared with 18F-FDG PET/CT, FAPI PET/CT has a higher patient throughput (5). 68Ga-FAPI is also being used as a radiotracer for PET/CT imaging. 68Ga-FAPI has low uptake in normal tissue and high uptake in many cancers, including cancers with low 18F-FDG affinity. Compared with 18F-FDG PET/CT, 68Ga-FAPI has a higher tumor-to-background ratio (TBR) and can therefore facilitate the distinction between normal and cancerous tissue (11–13). On the other hand, FAPI can also be combined with beta-emitting isotopes such as lutetium-177 (177Lu) or yttrium-90 (90Y) and used to treat malignant tumors with high fibroblastic activity, such as breast cancer and pancreatic cancer (14).
The normal physiological distribution of FAPI in the human body is similar to that of 18F-FDG. However, the uptake of radiolabeled FAPI is lower in areas with high physiological 18F-FDG uptake, such as the brain. Giesel et al. (5) compared the physiological uptake of two radiolabeled FAPI, 68Ga-FAPI-02 and 68Ga-FAPI-04, to acquire the PET/CT images in two cancer patients. PET/CT imaging was performed at 0.2 hours, 1 hour, and 3 hours after the radiopharmaceutical injection. The biological distribution of the radiopharmaceutical in the patients was quantified by measuring the mean standard uptake value (SUVmean) and maximum standard uptake value (SUVmax). These FAPI ligands had a lower SUVmax than 18F-FDG in the brain (0.32 versus 11.01), liver (1.69 versus 2.77), and oropharyngeal mucosa (2.57 versus 4.88). The TBR of the two radiopharmaceuticals was similar at 1 hour after injection. Meyer et al. (15) showed that 68Ga-FAPI-46 PET/CT imaging had a good dose distribution, favorable tracer kinetics, and high diagnostic efficacy in six cancer patients. Other studies have also shown that since the kidney rapidly clears the various radiolabeled FAPI ligands, its uptake in normal organs remains low and only changes slightly over time, thus facilitating the imaging procedure (16, 17). Normal organs and inflamed tissue will have some physiological uptake of FAPI. Some healthy patients will also have high physiological uptake in the breast 60 minutes after the FAPI injection, with an average SUV of 4.5 ± 1.5 (18). In healthy adult tissue FAP is scarcely present, with some exceptions such as during embryogenesis, human placenta and in uterine stroma, particularly during the proliferative phase (19, 20). In addition, some case reports have shown that hormones can stimulate FAP expression in hormone-sensitive organs such as the breast or uterus (21). Therefore, we aimed to explore the application of using radiolabeled FAPI for diagnosing and treating breast and the most common gynecologic malignancies.
Breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases, making it the most prominent cancer and the leading cause of cancer-related death among women worldwide (22). Breast cancer often has bone metastasis, which can lead to pain and severe deterioration in the patient’s quality of life (23). Breast cancer can be divided into 4 molecular subtypes, including luminal-A, luminal-B, human epidermal growth factor-2 overexpressed (HER2-OE), and basal-like (Triple-negative breast cancer; TNBC) based on the expression of the Kiel-67 (Ki-67) protein, and the estrogen (ER), progesterone (PR), and HER-2 receptors (24). Luminal A-like cancers highly express the ER and PR receptors (>20%), have low expression levels of Ki-67 (<20%), and do not express the HER-2 receptor. Luminal B-like cancers also express the ER receptor, but they have low expression levels of the PR receptor (<20%). These tumors may also express the HER-2 receptor and have high expression levels of Ki-67 (>20%). The HER2-OE tumors are characterized by overexpressed HER-2 and negative hormone receptors and the basal-like cancers do not express any receptors.
The uptake of 18F-FDG varies between the different molecular subtypes and could be used to facilitate differential diagnosis (25). Groheux D et al. (26) confirmed it, showing significantly higher 18F-FDG uptake in ER− tumours than in ER+ tumours and a significantly higher 18F-FDG uptake in PR− tumours than in PR+ tumours. Besides, triple-negative breast cancer is usually highly 18F-FDG–avid. Although 18F-FDG PET/CT has great value in diagnosing and staging breast cancer, its high physiological background activity in multiple organs and inflamed tissued limits its specificity and leads to a high false positive rate (27).
On the other hand, the background uptake of the radiolabeled FAPI in the brain, liver, and oral mucosa is significantly lower than that of 18F-FDG, thus providing a more accurate staging tool for breast cancer staging (17). 68Ga-FAPI showed an overall high tumor uptake in breast cancer and had a higher metastatic detection rate than 18F-FDG, especially for bone and peritoneal cancer metastasis (28, 29). Ding et al. (13) found in a mouse model (n=40) that compared with 18F-FDG, 68Ga-FAPI-04 was more sensitive in detecting multiple distant metastases in early-stage breast cancer, but less sensitive than 18F-FDG in advanced stage disease. Elboga et al. (30) compared the diagnostic accuracy of 68Ga-FAPI-04 and 18F-FDG PET/CT in detecting breast cancer based on the SUVmax, and TBRs measurement in 48 breast cancer patients and found that 68Ga-FAPI-04 performed better than 18F-FDG. Similarly, Komek et al. (31) compared the diagnostic accuracy of 68Ga-FAPI-04 with 18F-FDG using pathological results as a gold standard in 20 women diagnosed with breast cancer and found that 68Ga-FAPI-04 had a superior detection rate for primary breast cancer and metastasis in the lymph node, liver, bone, and brain. However, a retrospective dual-center analysis conducted by Dendl K et al. (11) showed that the SUVmax of the radiolabeled FAPI in the breast of premenopausal women was significantly higher than that of postmenopausal patients. It has certain guiding significance for the differential diagnosis of breast cancer and physiologic uptake in young women. 68Ga-FAPI PET/CT still has high false positive and false negative rates caused by the non-tumor-specific uptake in scars, normal breast tissue, inflammatory tissue, and postoperative wound healing (18). Besides, 68Ga-FAPI could also improve the detection of metastasis in latent breast cancer due to its favorable physical properties (32, 33).
Although most studies have shown that 68Ga-FAPI PET/CT has an overall advantage in the diagnosis of breast cancer, it has a number of limitations. Moreover, the 68Ga has a short half-life and thus its production requires an onsite cyclotron. This problem can be overcome by using fluorine-18, aluminum fluoride, and 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) to label the FAPI (18F-ALF-NOTA-FAPI-04). Compared with 68Ga, the longer half-life of 18F allows for more flexible imaging protocols and better time management for clinical facilities. The 18F-labeled FAPI also exhibits better in vivo stability and pharmacokinetics, which can improve imaging quality and accuracy. In addition, the production and transportation of 18F-labeled tracers are generally more convenient than those of 68Ga, since it does not require an onsite cyclotron for production (34–36).
There are various treatment options for breast cancer, where surgery remains the main option. However, this treatment is often combined with adjuvant and neoadjuvant systemic therapies, including chemotherapy, HER2-directed therapy, and endocrine therapy (37, 38). In recent years, radionuclide therapy has shown promising results in managing breast cancer. Lindner et al. (39) marked FAPI-04 with 90Y and used it to treat two patients with metastatic breast cancer. Both patients experienced a significant reduction in pain after administering low doses of the drug. The authors anticipated that higher activities can be given resulting in better tumoricidal effects. These findings indicated that this treatment could effectively manage malignant tumors with highly active fibroblasts, such as breast cancer. In addition, other new FAPI reagents 177Lu-DOTA.SA.FAPi and 177Lu-DOTAGA.(SA.FAPi)2 also show promising results in the treatment of advanced refractory breast cancer (40–42). The histopathology of a breast cancer patient revealed atypical cells arranged cords and tubules in the fibrotic stroma suggestive of invasive ductal carcinoma of the breast and the immunohistochemistry reported cells immunopositive for Her2neu, while negative for ER and PR. As the patient had depleted all approved therapy options, she was counselled for 177Lu-DOTA.SA.FAPi therapy. Post-treatment, the patient experienced a decrease in the intensity of headaches. Post-treatment 4-week laboratory parameters were well within the normal range and no treatment-related adverse events were observed. The result showed that 177Lu-DOTA.SA.FAPi therapy may open up a new opportunity in breast cancer therapy, particularly for the patients refractory to conventional treatment options (40). Novel radiopharmaceuticals such as 90Y-FAPI-46, 177Lu-FAP-2286, and 177Lu-FAPI-46 are currently being explored as potential new treatments for breast cancer (43–46).
Ovarian cancer is the fifth most common cause of cancer death in women, behind only to lung, breast, colorectal, and pancreatic cancer. Its incidence and mortality increase with age. Most patients with ovarian cancer are older than 50, but patients can be diagnosed with ovarian cancer at any age (22, 47, 48). The early diagnosis of ovarian cancer is beneficial to the detection of metastases and preoperative staging. The metastasis of ovarian cancer usually includes peritoneal, lymphatic or hematogenous dissemination, which is widely spread through ascites circulation. 18F-FDG PET/CT is superior to conventional imaging in detecting small metastases of ovarian cancer, but it also has some limitations. Despite its limitations, 18F-FDG remains the most commonly used radiotracer for diagnosing ovarian cancer. However, the accumulation of this radiotracer in normal ovarian tissue often leads to a false positive diagnosis, particularly in premenopausal women (18, 49, 50).
FAP is often highly expressed in the stroma of ovarian cancers and has an important role in the proliferation, invasion, and migration of ovarian cancer cells (51, 52). Therefore, the radiolabeled FAPI could be used to facilitate the diagnosis of ovarian cancer. As opposed to 18F-FDG, 68Ga-DOTA-FAPI-04 has no uptake in benign ovarian lesions, and its uptake is not affected by the menstrual cycle (53–55). As a result, studies have shown that the 68Ga-DOTA-FAPI-04 can accurately distinguish between benign and early-stage malignant ovarian lesions (53). A case report by Siripongsatian D et al. (56) also showed a negative 18F-FDG uptake and strong 68Ga-FAPI uptake in a recurrent ovarian clear cell carcinoma. In addition, a retrospective study by Zheng et al. (57) in 27 patients with pathologically confirmed ovarian cancer showed that compared with 18F-FDG, 68Ga-FAPI PET/CT could more accurately differentiate between the different types of malignant ovarian tumors, including high-grade serous carcinomas (HGSC), low-grade serous carcinomas (LGSC), and ovarian mucinous carcinoma. Moreover, Zheng et al. (57) found that 68Ga-FAPI PET/CT was more sensitive than 18F-FDG PET/CT in the detection of primary ovarian tumors (14/14 (100%) versus 11/14 (78%)), lymph node metastasis (75/75 (100%) versus 60/75 (80%)), and peritoneal and pleural metastasis (9/9 (100%) versus 5/9 (56%). These findings suggest that 68Ga-FAPI PET/CT could supplement the diagnostic information provided by 18F-FDG PET/CT.
The peritoneum is the most common metastatic site and the main clinical challenge in ovarian cancer. Liu et al. (58) evaluated the diagnostic performance of the SUVmax and TBR of 18F-FDG and 68Ga-FAPI PET/CT in 29 patients with suspected platinum-sensitive recurrent ovarian cancer. Compared with the TBR of 18F-FDG, the TBR of 68Ga-FAPI PET/CT had superior sensitivity (95.83% versus 47.83%) and specificity (90.32% and 53.57%) for recurrent ovarian lesions and could therefore be used to assess the treatment response in platinum-sensitive recurrent ovarian cancer. The 68Ga-FAPI PET/CT displayed a huge advantage in peritoneal metastasis examination than 18F-FDG PET/CT. And in a study of 49 patients with epithelial ovarian cancer, Chen et al. (59) used surgical pathology to evaluate and compare the diagnostic performance of 18F-FDG and 68Ga-FAPI-04 PET/CT. They concluded that 68Ga-FAPI-04 PET/CT achieved higher sensitivity than 18F-FDG PET/CT in the detection and diagnosis of lymph node and peritoneal metastases, suggesting advantages regarding the preoperative staging of patients with EOC and, thereby, improving treatment decision-making.
Lymph node staging is a key step in diagnosing and treating patients with malignant ovarian tumors. Primary detumescence surgery and platinum-based adjuvant chemotherapy are the two main treatment options for ovarian cancer. However, about 80% of ovarian cancer patients relapse after treatment (60). Lindner T et al. (61) have developed a novel variant of FAPI by chelating it with technetium-99m (99mTc-FAPI-34) for single photon emission computed tomography (SPECT) imaging of ovarian cancer. The resulting 99mTc-labeled FAPI tracers revealed excellent binding properties (up to 45% binding, above 95% internalization), high affinity, and significant tumor uptake in biodistribution studies. This study also reported on a patient with metastasized ovarian cancer and a patient with pancreatic cancer that both received 6GBq 90Y-FAPI-46 as a last-line treatment. Significant tumor uptake was seen, with low uptake in healthy organs. The chelating agent used in this compound could also be labeled with the radionuclide rhenium-188(188Re). However, further research is required to evaluate this new radioisotope’s efficacy in treating ovarian cancer. In 2021, Kuyumcu et al. (62) published a dosimetry study where a low dose of 177Lu-FAPI-04 (267.5 ± 8.6 MBq) was given to four patients with metastatic advanced-stage cancer (breast cancer, thymic carcinoma, thyroid cancer, and ovarian cancer). Data acquisition was obtained using whole-body SPECT/CT imaging, and blood samples were collected for bone marrow dosimetry. The study shows that the estimated absorbed radiation dose to critical organs is significantly low with 177Lu-FAPI-04 compared with clinically established peptide-based radionuclide therapies. However, the authors concluded that employing different radioisotopes may be preferred due to the short tumor retention time of FAPI-04. Moreover, development of tracers with better tumor retention was warranted. Baum RP et al. (44) performed 177Lu-FAP-2286 for peptide-targeted radionuclide therapy (PTRT) in 11 patients with advanced adenocarcinomas of pancreas, breast, rectum, and ovary after prior confirmation of uptake on 68Ga-FAP-2286 or 68Ga-FAPI-04 PET/CT. Administration of 177Lu-FAP-2286 was well tolerated, with no adverse symptoms or clinically detectable pharmacologic effects being noticed or reported in any of the patients. It provided evidence of the feasibility of treating different aggressive adenocarcinomas with 177Lu-FAP-2286. These findings still strongly encourage additional research regarding FAPI and ovarian cancer, since ovarian cancer still lacks sufficient early diagnostic and therapeutic options.
Cervical cancer is the fourth most commonly diagnosed cancer and the fourth leading cause of cancer death in women all over the world. In 2020, there were an estimated 604,000 new cases of cervical cancer and 324,000 deaths worldwide, of which nearly 90% occurred in low- and middle-income countries (22, 63). The human papillomavirus (HPV) is one of the main causes of cervical cancer (22). From 2012 to 2019, the incidence of cervical cancer in women over 20 years old who received the HPV vaccine decreased by 65%, indicating a significant decline in the number of cervical cancer cases caused by HPV (63). However, 15–61% patients diagnosed with cervical cancer will subsequently go on to develop metastatic diseases (64). Therefore, early diagnosis and staging of cervical cancer are necessary. Squamous cell carcinoma is the most common histological cervical cancer. Other histological subtypes include adenocarcinoma, adenosquamous carcinoma, undifferentiated carcinoma, and neuroendocrine carcinoma. A biopsy is necessary to obtain a definitive diagnosis of cervical cancer. However, 18F-FDG PET/CT is often used to monitor disease progression and lymph node involvement after chemotherapy (65). Since cervical cancer often expresses FAP, radiolabeled FAPI is increasingly being used to diagnose and stage the disease. Wegen et al. (66) retrospectively reviewed 7 patients with histologically proven cervical cancer. All 7 patients had focal uptake above background in their tumor lesions in 68Ga-FAPI-46 PET/CT. The results showed that 68Ga-FAPI-46 PET/CT showed a higher TBR than 18F-FDG PET/CT in primary tumor as well as in lymph nodes metastasis. The higher TBR eventually improved the detection of both the primary tumor and lymph node metastasis in cervical cancer patients.
Surgical resection and radiotherapy have an important role in the management of early-stage cervical cancer. However, patients with more advanced disease are treated with palliative chemotherapy. Although no studies were found evaluating the efficacy of radiolabeled FAPI in the treatment of cervical cancer, radioimmunotherapy drugs for HPV-positive cervical cancer show promising results in the management of cervical cancer. Phaeton R et al. (67) reported on the efficacy of using 188Re and 177Lu labeled monoclonal antibodies C1P5-E6 in CasKi cervical cancer xenograft nude mice. The findings of these studies have shown that these radioimmunotherapy drugs can inhibit tumor growth. However, clinical trials are required to confirm the efficacy of this treatment in improving tumor control and survival in patients diagnosed with advanced, recurrent, and metastatic cervical cancer.
Endometrial cancer is the sixth most common cancer in women worldwide. In 2020, the global incidence of endometrial cancer was 417,337 cases (22). Approximately 70% of patients with endometrial cancer are confined to the uterine body at the time of diagnosis, with early stage and good prognosis. Although the overall treatment effect of endometrial cancer is good, up to 15-20% of patients have relapsed (68). Endometrial cancer is categorized into type I and type II. Type I mostly consists of grade I or grade II endometrioid adenocarcinomas, which are sensitive to estrogen and often accompanied by endometrial hyperplasia in the early stages. Patients with this type cancer have favorable prognoses. Type II, on the other hand, comprises grade III endometrioid adenocarcinoma, clear serous carcinoma, undifferentiated carcinoma, and carcinosarcoma. These tumors are not influenced by estrogen or obesity and are associated with a worse prognosis (69). However, the pathological classification of endometrial cancer based on grading and histotype might varies for different observers (70, 71). 18F-FDG PET/CT is commonly used to evaluate the stage of endometrial carcinoma and has shown high performance in diagnosing preoperative lymph node metastasis and postoperative recurrence (72). However, the use of radiolabeled FAPI in the diagnosis and staging of endometrial cancer remains controversial. Studies have shown that the high uptake of the radiolabeled FAPI within normal uterine tissue and the low uptake in metastatic endometrial lesions limit its diagnostic efficacy (73). A high uptake of 18F-FDG within the normal uterus was reported in most women (66.7%) and was negatively correlated with age (18). Conversely, the uptake of 68Ga-FAPI-04 PET/CT was higher than that of 18F-FDG PET/CT in the metastatic lesions of some patients diagnosed with clear cell endometrial cancer (74). These findings suggest that FAPI PET/CT combined with 18F-FDG PET/CT may improve the diagnostic accuracy of primary and metastatic endometrial cancer.
The main treatment options for endometrial cancer include surgery with or without radiotherapy, brachytherapy and chemotherapy (75). The recent identification of the molecular endometrial cancer subtypes has led to the development of targeted therapies, particularly for early recurrent endometrial cancer subtypes (76). However, there are relatively few studies on the use of radiolabeled FAPI in the treatment of endometrial cancer, and further research is required to evaluate its role in the management of endometrial cancer.
This narrative review is focused on the role of radiolabeled FAPI in patients with breast cancer, ovarian cancer, cervical cancer and endometrial cancer, aiming to summarize the main evidence on those specific cancer. FAPI uptake in normal hormone-responsive organs determined significant differences in terms of pre- and postmenopausal status. Previously conducted studies indicated that differences in breast parenchyma are based on dynamic physiologic processes of the inner environment in response to endogenous and exogenous factors such as increased or decreased hormonal stimulations (77). The literature review demonstrates that ovary has no physiological FAPI uptake and there is no association with menstrual cycle. And changes in hormone response will influence FAPI uptake to a different extent in breast, endometrium, and uterus (11, 21). A series of histopathologic analyses demonstrated strong-to-moderate FAP expression is present in the stroma of breast carcinomas (78, 79). Differential uptake in hormone-sensitive organs may be a challenging fact as it may also be induced by physiological or benign conditions. Whether FAPI can overcome the assessment of potential cancers in hormone-sensitive organs, which is severely limited on FDG PET/CT, is unknown.
Besides, early response evaluation during and after therapy is possible with 68Ga-FAPI PET/CT scans, as FAP is part of the tumor microenvironment and molecular changes in the tumor stroma can subsequently be depicted (80). Consequently, these scans may allow precise monitoring, for example, after radiotherapy, which improves staging and further radiotherapy planning (81). As a visualization tool, FAPI has its own limitations compared to FDG, especially the accumulation observed in inflammatory and other non-tumorigenic conditions, which can further affect the diagnostic efficacy of FAPI. Table 1 shows the comparison studies on the diagnostic performance of 68Ga-FAPI PET/CT and 18F-FDG PET/CT. For FAPI image interpretation, there are several pitfalls include non–tumor-specific 68Ga-FAPI uptake in the mammary glands, or the uterus. Pitfalls may originate from specific uptake through an increased FAP expression level and mechanisms of unspecific uptake, including edema, tracer extravasation, and some inflammatory disorders (18). Remarkably, FAPI, a single molecule, is both a diagnostic and possibly therapeutic agent, enabling additional theranostic application. However, literature evidence about the diagnosis of FAPI in breast cancer patients and the most common gynecologic malignancies has rapidly grown recently, to a major extent in the diagnostic use of 68Ga-labeled FAPI PET/CT at staging, restaging or differential diagnosis, but with few reports in the therapeutic perspective. Table 2 shows the comparison studies on the theranostic performance of FAPI. Data was reported on breast cancer and the most common gynecologic malignancies patients that were treated with different FAP targeted radionuclide therapies shows that FAP targeted radionuclide therapy has resulted in objective responses in difficult to treat end stage cancer patients with manageable adverse events. Although no prospective data is yet available, these early data encourages further research (82). However, as a theranostic tool, FAPI’s relatively low blood retention necessitates modifications for enhancing therapeutic efficacy (14, 83). This is an important part of the issues that need to be addressed in future research on FAPI. More structural variants of FAPI will be needed in the future to ameliorate a number of problems with existing FAPI structures.
In this review, we discussed the applications of FAPI PET/CT in the diagnosis and treatment of breast and the most common gynecologic malignancies. Radiolabelled FAPI may improve the detection, staging, and assessment of treatment response in breast and the most common gynecologic malignancies, particularly when combined with 18F-FDG. In addition, it can also facilitate the differentiation between malignant and other benign lesions caused by inflammatory, infectious, and fibrotic diseases. For normal hormone-responsive organs, changes in hormone response will influence FAPI uptake to a different extent in breast, endometrium, and uterus. Therefore, hormonal effects are also an important factor that should be emphasized in FAPI applications. While the use of radiolabeled FAPI treatment for breast cancer has been extensively researched, investigations into its efficacy for the most common gynecologic malignancies remain in the preliminary stages. Therefore further research is required to confirm the clinical efficacy of this treatment (Figures 1–4).
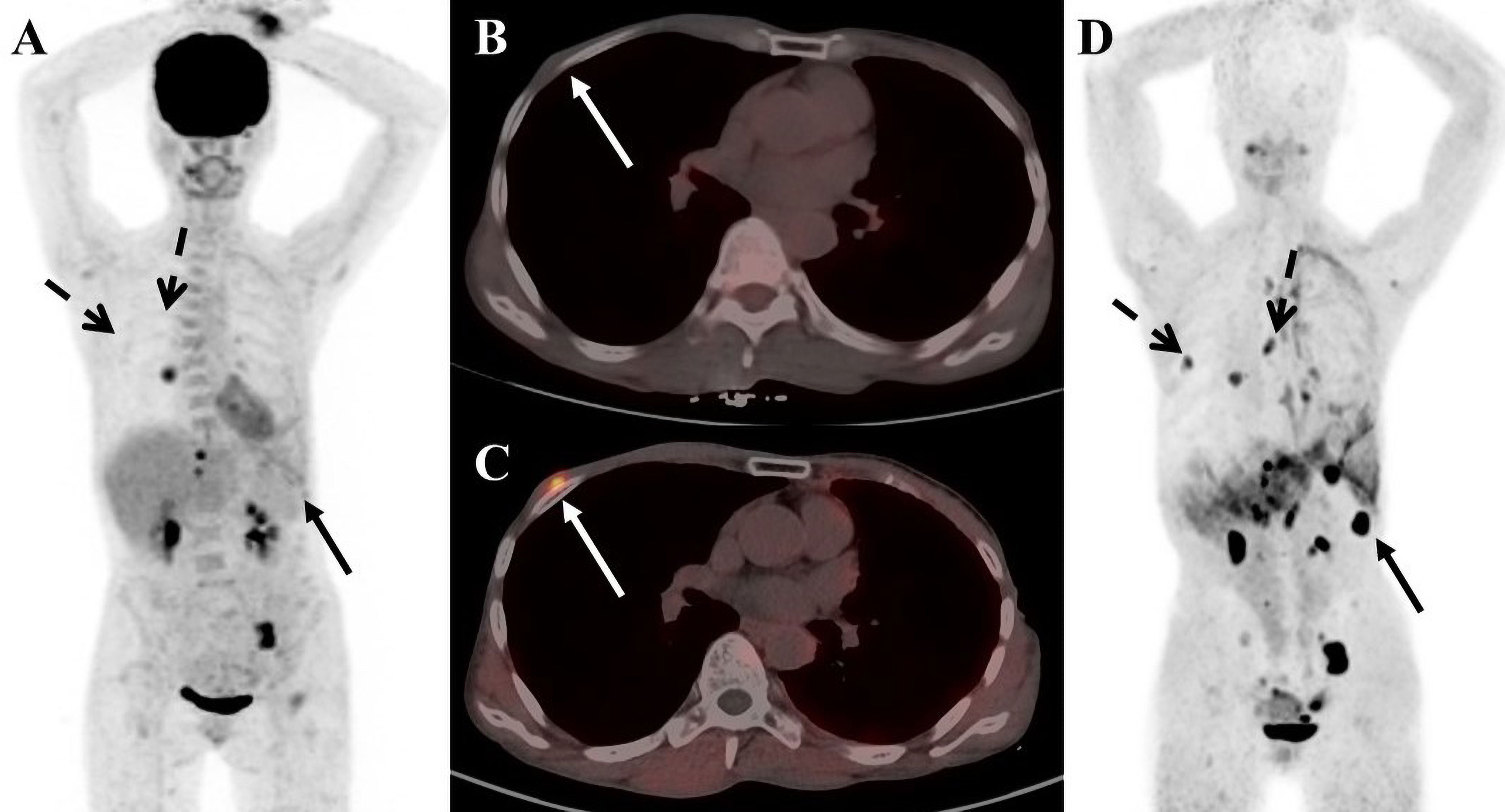
Figure 1 A 56-year-old female patient with breast cancer. (A) 18F-FDG PET/CT maximum-intensity-projection (MIP) image shows increased 18F-FDG uptake in the middle lobe of the right lung, the left ilium, the left pubic bone, and the left 11 ribs. (B) 18F-FDG PET/CT axial fusion chest image shows a slight thickening of the right chest wall, and no increase in 18F-FDG uptake. (C) FAPI PET/CT chest axial fusion image shows an increase in FAPI uptake in the right chest wall(SUVmax 7.3; white arrow). (D) FAPI PET/CT MIP image reveals a significant increase in imaging agent uptake in the in multiple lymph nodes, left lobe of liver and spleen parenchyma, without an increase in FDG uptake at the corresponding sites (black solid arrow and black dashed arrow).
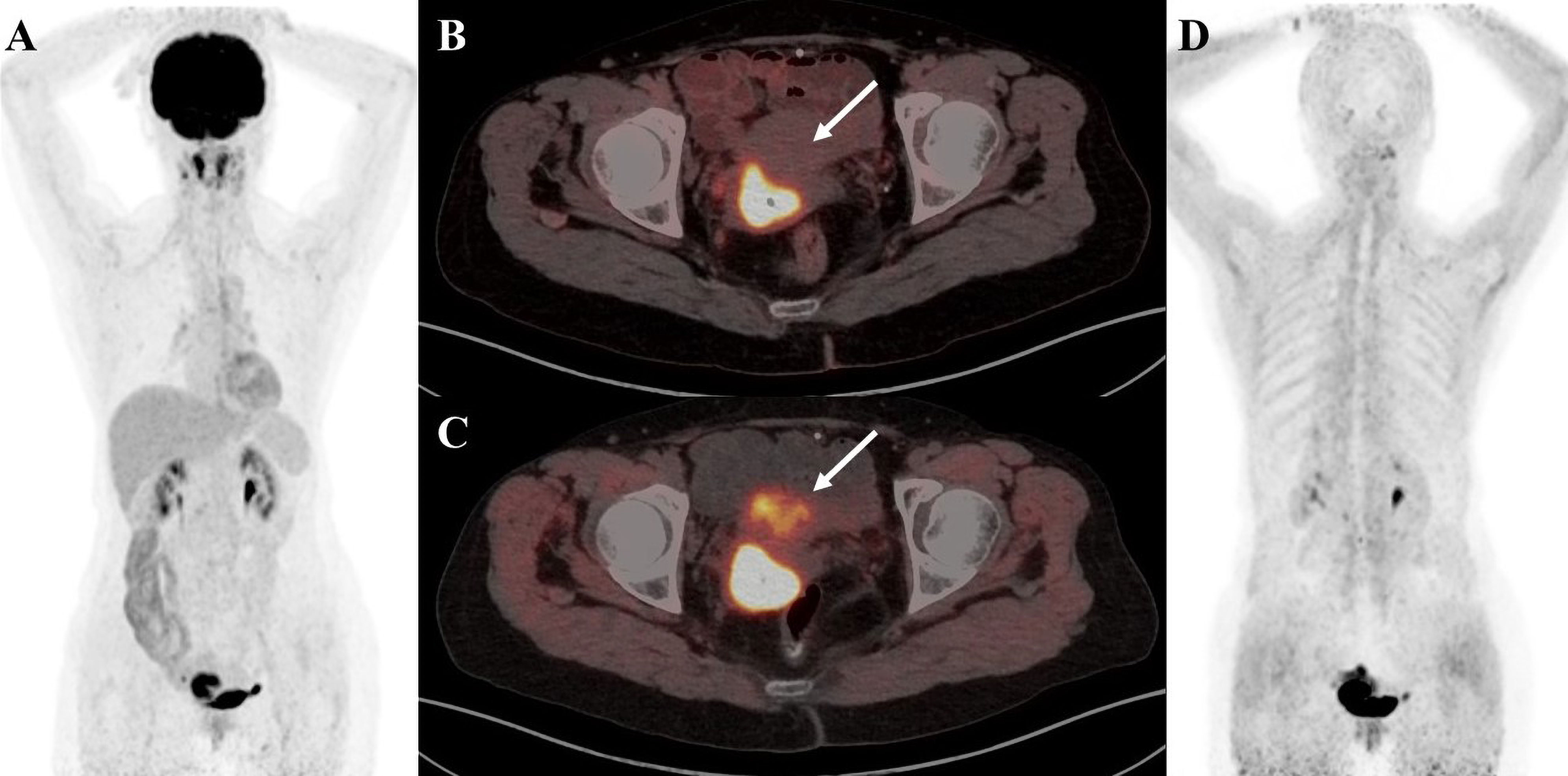
Figure 2 A 58-year-old female patient with cervical cancer was diagnosed as high-grade squamous intraepithelial lesion by cervical biopsy. (A) 18F-FDG PET/CT MIP image shows increased 18F-FDG uptake in the nasopharyngeal wall of the right pharyngeal recess and the bilateral palatine tonsils. (B) 18F-FDG PET/CT axial fusion image and (C) FAPI PET/CT axial fusion image show irregular thickening of the cervical wall, a mass-like shape, about 4.4cm × 2.3cm × 3.2cm in size, and increased uptake of imaging agent (SUVmax-FDG 14.3 vs SUVmax-FAPI 20.1, white arrow). (D) FAPI PET/CT MIP image reveals a significant increase in imaging agent uptake in the multiple lymph nodes, left lobe of liver and spleen parenchyma, without an increase in FDG uptake at the corresponding sites (black arrow).
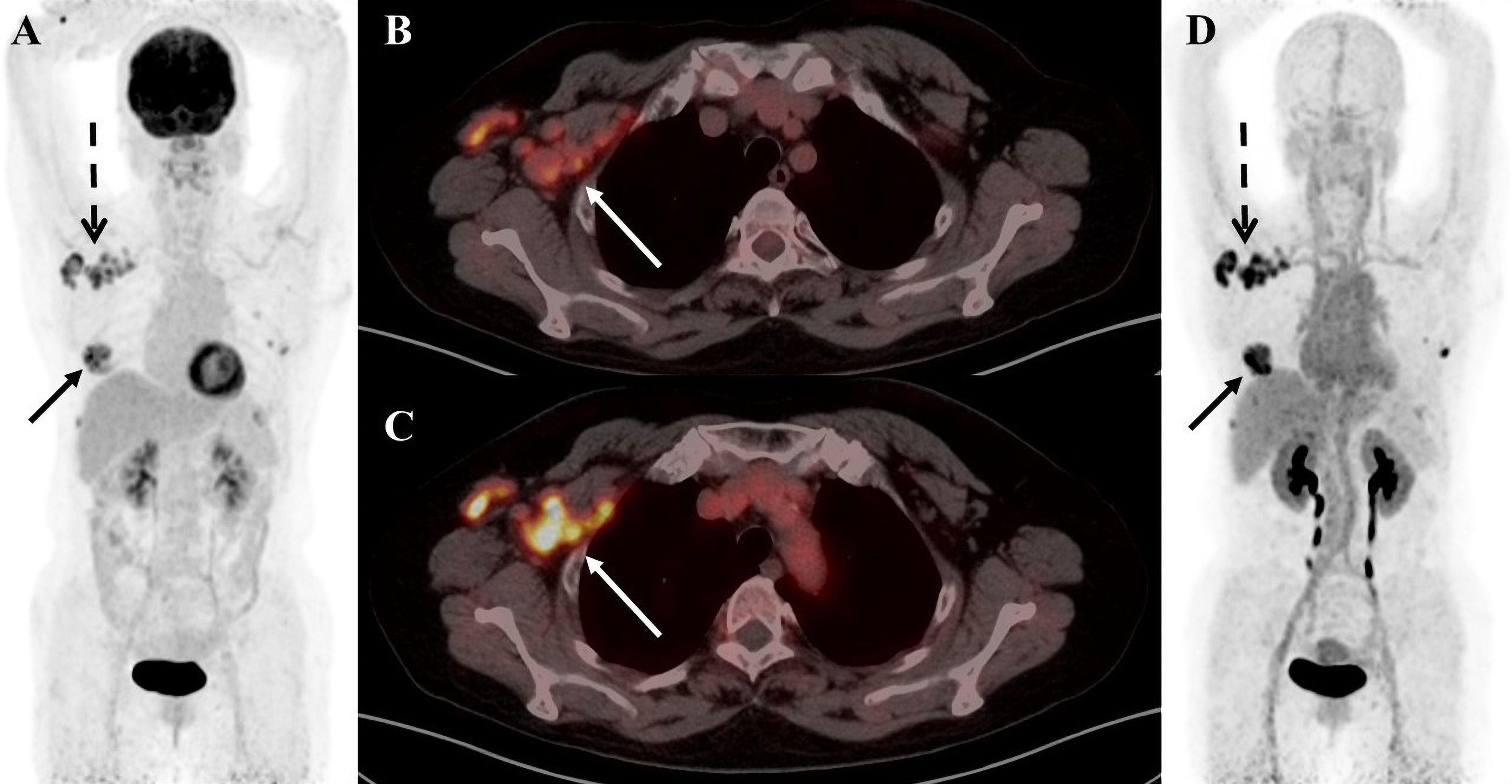
Figure 3 A 55-year-old female patient was diagnosed with breast carcinoma. (A) 18F-FDG PET/CT MIP image reveals a significant increase in imaging agent uptake in the right breast and multiple lymph nodes around the right axilla. (B) 18F-FDG PET/CT axial fusion image and (C) FAPI PET/CT axial fusion image show multiple enlarged lymph nodes around the right axilla and right pectoralis minor muscle, the larger measuring approximately 2.1 cm × 1.9 cm(SUVmax-FDG 4.5 vs SUVmax-FAPI 8.4, white arrow). (D) FAPI PET/CT MIP image reveals a significant FAPI uptake increase than FDG in multiple lymph nodes behind the right papilla, in the right axilla and around the right pectoralis minor(black solid arrow and black dashed arrow).
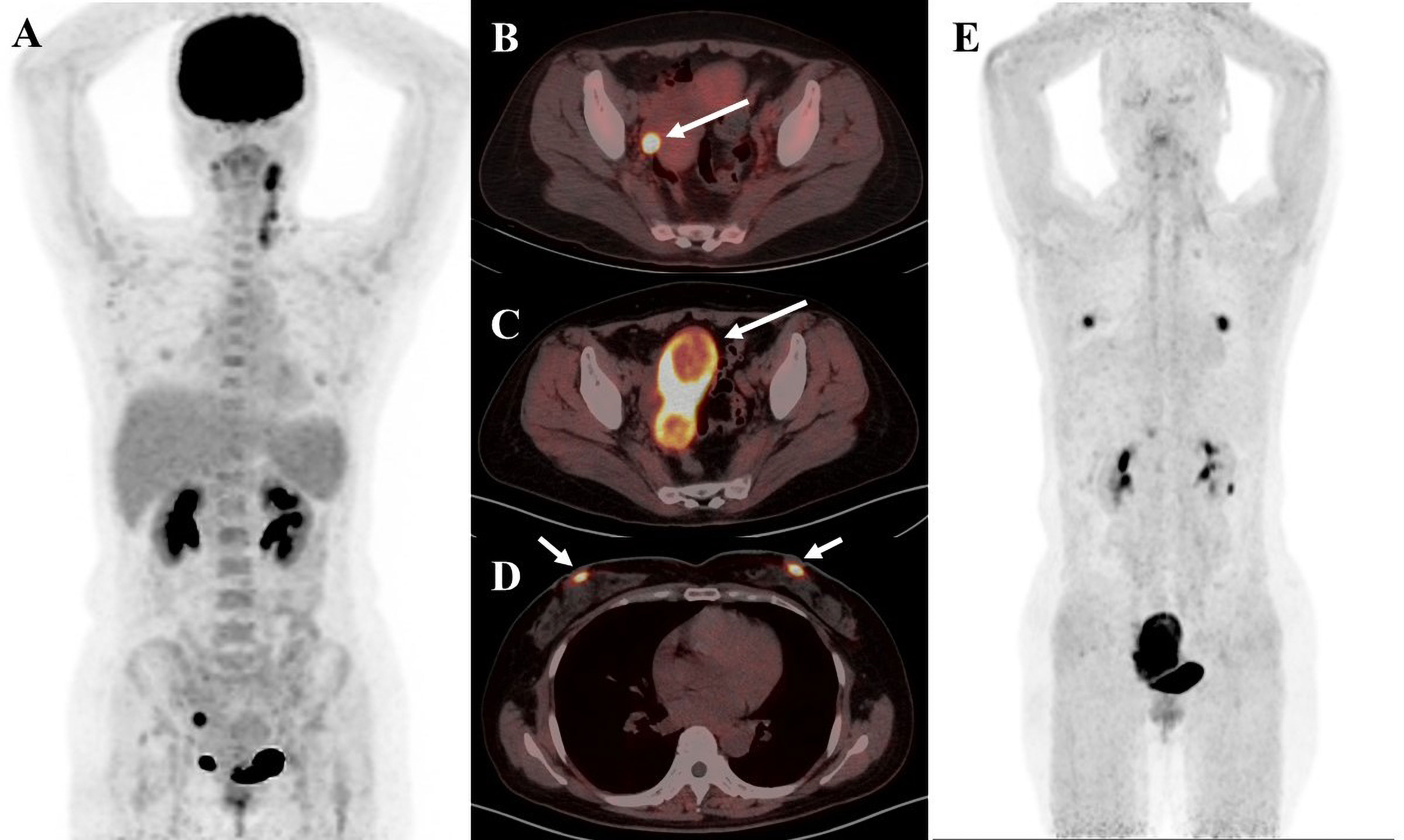
Figure 4 A 41-year-old female patient diagnosed with cervical cancer. (A) 18F-FDG PET/CT MIP image shows increased uptake of enlarged multiple lymph nodes in the left posterior cervical triangle and left supraclavicular region as well as nodular foci of increased glucose metabolism in the right adnexal region (B) 18F-FDG PET/CT axial fusion image and (C) FAPI PET/CT axial fusion image show nodular foci of increased contrast uptake are seen in the right adnexal region of the uterus(SUVmax-FDG 12.8 vs SUVmax-FAPI 14.5, white arrow). (D) FAPI PET/CT axial fusion image shows physiologic FAPI uptake exists in the mammary gland.
TL: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JZ: Visualization, Writing – review & editing. YY: Visualization, Writing – review & editing. MT: Visualization, Writing – review & editing. YC: Conceptualization, Writing – original draft, Writing – review & editing, Visualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are grateful to the members of Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University and Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province for their guidance, cooperation, and assistance in completing this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jacobson FL, Van den Abbeele AD. Importance of (68)Ga-FAPI PET/CT for detection of cancer. Radiology. (2022) 303:200–1. doi: 10.1148/radiol.212884
2. Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging. (2004) 31 Suppl 1:S125–34. doi: 10.1007/s00259-004-1535-8
3. Hess S, Scholtens AM, Gormsen LC. Patient preparation and patient-related challenges with FDG-PET/CT in infectious and inflammatory disease. PET Clin. (2020) 15:125–34. doi: 10.1016/j.cpet.2019.11.001
4. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. (2019) 60:801–5. doi: 10.2967/jnumed.119.227967
5. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. (2019) 60:386–92. doi: 10.2967/jnumed.118.215913
6. Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jager D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. (2018) 59:1423–9. doi: 10.2967/jnumed.118.210435
7. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. (2019) 18:99–115. doi: 10.1038/s41573-018-0004-1
8. Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. (2021) 62:160–7. doi: 10.2967/jnumed.120.244806
9. Taralli S, Lorusso M, Perrone E, Perotti G, Zagaria L, Calcagni ML. PET/CT with fibroblast activation protein inhibitors in breast cancer: diagnostic and theranostic application-A literature review. Cancers (Basel). (2023) 15(3):908. doi: 10.3390/cancers15030908
10. Dendl K, Koerber SA, Tamburini K, Mori Y, Cardinale J, Haberkorn U, et al. Advancement and future perspective of FAPI PET/CT in gynecological Malignancies. Semin Nucl Med. (2022) 52:628–34. doi: 10.1053/j.semnuclmed.2022.04.002
11. Dendl K, Koerber SA, Finck R, Mokoala KMG, Staudinger F, Schillings L, et al. (68)Ga-FAPI-PET/CT in patients with various gynecological Malignancies. Eur J Nucl Med Mol Imaging. (2021) 48:4089–100. doi: 10.1007/s00259-021-05378-0
12. Lan L, Liu H, Wang Y, Deng J, Peng D, Feng Y, et al. The potential utility of [(68) Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [(18)F]FDG. Eur J Nucl Med Mol Imaging. (2022) 49:963–79. doi: 10.1007/s00259-021-05522-w
13. Ding F, Huang C, Liang C, Wang C, Liu J, Tang D. (68)Ga-FAPI-04 vs. (18)F-FDG in a longitudinal preclinical PET imaging of metastatic breast cancer. Eur J Nucl Med Mol Imaging. (2021) 49:290–300. doi: 10.1007/s00259-021-05442-9
14. Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. (2019) 60:1421–9. doi: 10.2967/jnumed.118.224469
15. Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of (68)Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. (2020) 61:1171–7. doi: 10.2967/jnumed.119.236786
16. Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. Biodistribution, pharmacokinetics, dosimetry of [(68)Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [(18)F]F-FDG PET/CT in patients with various cancers. Eur J Nucl Med Mol Imaging. (2021) 48:1915–31. doi: 10.1007/s00259-020-05132-y
17. Giesel FL, Kratochwil C, Schlittenhardt J, Dendl K, Eiber M, Staudinger F, et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. (2021) 48:4377–85. doi: 10.1007/s00259-021-05307-1
18. Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D, et al. Pitfalls and common findings in (68)Ga-FAPI PET: A pictorial analysis. J Nucl Med. (2022) 63:890–6. doi: 10.2967/jnumed.121.262808
19. Simkova A, Busek P, Sedo A, Konvalinka J. Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications. Biochim Biophys Acta Proteins Proteom. (2020) 1868:140409. doi: 10.1016/j.bbapap.2020.140409
20. Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, et al. Expression of the fibroblast activation protein during mouse embryo development. Int J Dev Biol. (2001) 45:445–7.
21. Sonni I, Lee-Felker S, Memarzadeh S, Quinn MM, Mona CE, Luckerath K, et al. (68)Ga-FAPi-46 diffuse bilateral breast uptake in a patient with cervical cancer after hormonal stimulation. Eur J Nucl Med Mol Imaging. (2021) 48:924–6. doi: 10.1007/s00259-020-04947-z
22. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
23. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. (2017) 69:313–7. doi: 10.1007/s13304-017-0424-1
24. Tsang JYS, Tse GM. Molecular classification of breast cancer. Adv Anat Pathol. (2020) 27:27–35. doi: 10.1097/PAP.0000000000000232
25. Groheux D, Cochet A, Humbert O, Alberini JL, Hindie E, Mankoff D. (1)(8)F-FDG PET/CT for staging and restaging of breast cancer. J Nucl Med. (2016) 57 Suppl 1:17S–26S. doi: 10.2967/jnumed.115.157859
26. Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. (2011) 38:426–35. doi: 10.1007/s00259-010-1640-9
27. Lim HS, Yoon W, Chung TW, Kim JK, Park JG, Kang HK, et al. FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics. (2007) 27 Suppl 1:S197–213. doi: 10.1148/rg.27si075507
28. Pang Y, Zhao L, Chen H. 68Ga-FAPI outperforms 18F-FDG PET/CT in identifying bone metastasis and peritoneal carcinomatosis in a patient with metastatic breast cancer. Clin Nucl Med. (2020) 45:913–5. doi: 10.1097/RLU.0000000000003263
29. Li T, Jiang X, Zhang Z, Chen X, Wang J, Zhao X, et al. Case Report: (68)Ga-FAPI PET/CT, a more advantageous detection mean of gastric, peritoneal, and ovarian metastases from breast cancer. Front Oncol. (2022) 12:1013066. doi: 10.3389/fonc.2022.1013066
30. Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Uzun E, et al. Superiority of (68)Ga-FAPI PET/CT scan in detecting additional lesions compared to (18)FDG PET/CT scan in breast cancer. Ann Nucl Med. (2021) 35:1321–31. doi: 10.1007/s12149-021-01672-x
31. Komek H, Can C, Guzel Y, Oruc Z, Gundogan C, Yildirim OA, et al. (68)Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: a comparative pilot study with the (18)F-FDG PET/CT. Ann Nucl Med. (2021) 35:744–52. doi: 10.1007/s12149-021-01616-5
32. Xu W, Meng T, Shang Q, Pang Y, Chen H. Uncommon metastases from occult breast cancer revealed by 18 F-FDG and 68 ga-FAPI PET/CT. Clin Nucl Med. (2022) 47:751–3. doi: 10.1097/RLU.0000000000004193
33. Shang Q, Hao B, Xu W, Meng T, Pang Y, Sun L, et al. (68)Ga-FAPI PET/CT detected non-FDG-avid bone metastases in breast cancer. Eur J Nucl Med Mol Imaging. (2022) 49:2096–7. doi: 10.1007/s00259-021-05664-x
34. Jiang X, Wang X, Shen T, Yao Y, Chen M, Li Z, et al. FAPI-04 PET/CT using [(18)F]AlF labeling strategy: automatic synthesis, quality control, and in vivo assessment in patient. Front Oncol. (2021) 11:649148. doi: 10.3389/fonc.2021.649148
35. Wei Y, Zheng J, Ma L, Liu X, Xu S, Wang S, et al. [(18)F]AlF-NOTA-FAPI-04: FAP-targeting specificity, biodistribution, and PET/CT imaging of various cancers. Eur J Nucl Med Mol Imaging. (2022) 49:2761–73. doi: 10.1007/s00259-022-05758-0
36. Lindner T, Altmann A, Giesel F, Kratochwil C, Kleist C, Kramer S, et al. (18)F-labeled tracers targeting fibroblast activation protein. EJNMMI Radiopharm Chem. (2021) 6:26. doi: 10.1186/s41181-021-00144-x
37. McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. (2016) 57 Suppl 1:9S–16S. doi: 10.2967/jnumed.115.157834
38. Yang F, Xiao Y, Ding JH, Jin X, Ma D, Li DQ, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. (2023) 35:84–100 e8. doi: 10.1016/j.cmet.2022.09.021
39. Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. (2018) 59:1415–22. doi: 10.2967/jnumed.118.210443
40. Ballal S, Yadav MP, Kramer V, Moon ES, Roesch F, Tripathi M, et al. A theranostic approach of [(68)Ga]Ga-DOTA.SA.FAPi PET/CT-guided [(177)Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. (2021) 48:942–4. doi: 10.1007/s00259-020-04990-w
41. Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. First-in-human results on the biodistribution, pharmacokinetics, and dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharm (Basel). (2021) 14(12):1212. doi: 10.3390/ph14121212
42. Moon ES, Elvas F, Vliegen G, De Lombaerde S, Vangestel C, De Bruycker S, et al. Targeting fibroblast activation protein (FAP): next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA(5m) chelators. EJNMMI Radiopharm Chem. (2020) 5:19. doi: 10.1186/s41181-020-00102-z
43. Ferdinandus J, Costa PF, Kessler L, Weber M, Hirmas N, Kostbade K, et al. Initial clinical experience with (90)Y-FAPI-46 radioligand therapy for advanced-stage solid tumors: A case series of 9 patients. J Nucl Med. (2022) 63:727–34. doi: 10.2967/jnumed.121.262468
44. Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using (177)Lu-FAP-2286: first-in-humans results. J Nucl Med. (2022) 63:415–23. doi: 10.2967/jnumed.120.259192
45. Assadi M, Rekabpour SJ, Jafari E, Divband G, Nikkholgh B, Amini H, et al. Feasibility and therapeutic potential of 177Lu-fibroblast activation protein inhibitor-46 for patients with relapsed or refractory cancers: A preliminary study. Clin Nucl Med. (2021) 46:e523–e30. doi: 10.1097/RLU.0000000000003810
46. Rathke H, Fuxius S, Giesel FL, Lindner T, Debus J, Haberkorn U, et al. Two tumors, one target: preliminary experience with 90Y-FAPI therapy in a patient with metastasized breast and colorectal cancer. Clin Nucl Med. (2021) 46:842–4. doi: 10.1097/RLU.0000000000003842
48. Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. (2018) 319:595–606. doi: 10.1001/jama.2017.21421
49. Marzola MC, Chondrogiannis S, Rubello D. Fludeoxyglucose F 18 PET/CT assessment of ovarian cancer. PET Clin. (2018) 13:179–202. doi: 10.1016/j.cpet.2017.11.005
50. Kitajima K, Murakami K, Sakamoto S, Kaji Y, Sugimura K. Present and future of FDG-PET/CT in ovarian cancer. Ann Nucl Med. (2011) 25:155–64. doi: 10.1007/s12149-010-0449-8
51. Sun L, Ke M, Wang X, Yin M, Wei J, Xu L, et al. FAP(high) alpha-SMA(low) cancer-associated fibroblast-derived SLPI protein encapsulated in extracellular vesicles promotes ovarian cancer development via activation of PI3K/AKT and downstream signaling pathways. Mol Carcinog. (2022) 61:910–23. doi: 10.1002/mc.23445
52. Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected]. Exp Mol Pathol. (2009) 87:189–94. doi: 10.1016/j.yexmp.2009.09.001
53. Wang Q, Yang S, Tang W, Liu L, Chen Y. (68)Ga-DOTA-FAPI-04 PET/CT as a promising tool for differentiating ovarian physiological uptake: preliminary experience of comparative analysis with (18)F-FDG. Front Med (Lausanne). (2021) 8:748683. doi: 10.3389/fmed.2021.748683
54. Nishizawa S, Inubushi M, Ozawa F, Kido A, Okada H. Physiological FDG uptake in the ovaries after hysterectomy. Ann Nucl Med. (2007) 21:345–8. doi: 10.1007/s12149-007-0029-8
55. Kim SK, Kang KW, Roh JW, Sim JS, Lee ES, Park SY. Incidental ovarian 18F-FDG accumulation on PET: correlation with the menstrual cycle. Eur J Nucl Med Mol Imaging. (2005) 32:757–63. doi: 10.1007/s00259-005-1771-6
56. Siripongsatian D, Promteangtrong C, Kunawudhi A, Kiatkittikul P, Chotipanich C. Intense 68Ga-FAPI-46 activity in lesions of recurrent ovarian clear cell carcinoma that were negative on FDG PET/CT study. Clin Nucl Med. (2022) 47:e210–e2. doi: 10.1097/RLU.0000000000003975
57. Zheng W, Liu L, Feng Y, Wang L, Chen Y. Comparison of 68 Ga-FAPI-04 and fluorine-18-fluorodeoxyglucose PET/computed tomography in the detection of ovarian Malignancies. Nucl Med Commun. (2023) 44:194–203. doi: 10.1097/MNM.0000000000001653
58. Liu S, Feng Z, Xu X, Ge H, Ju X, Wu X, et al. Head-to-head comparison of [18F]-FDG and [68 Ga]-DOTA-FAPI-04 PET/CT for radiological evaluation of platinum-sensitive recurrent ovarian cancer. Eur J Nucl Med Mol Imaging. (2023) 50(5):1521–31. doi: 10.1007/s00259-022-06096-x
59. Chen J, Xu K, Li C, Tian Y, Li L, Wen B, et al. [(68)Ga]Ga-FAPI-04 PET/CT in the evaluation of epithelial ovarian cancer: comparison with [(18)F]F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2023) 50:4064–76. doi: 10.1007/s00259-023-06369-z
60. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. (2009) 115:1234–44. doi: 10.1002/cncr.24149
61. Lindner T, Altmann A, Kramer S, Kleist C, Loktev A, Kratochwil C, et al. Design and development of (99m)Tc-labeled FAPI tracers for SPECT imaging and (188)Re therapy. J Nucl Med. (2020) 61:1507–13. doi: 10.2967/jnumed.119.239731
62. Kuyumcu S, Kovan B, Sanli Y, Buyukkaya F, Has Simsek D, Ozkan ZG, et al. Safety of fibroblast activation protein-targeted radionuclide therapy by a low-dose dosimetric approach using 177Lu-FAPI04. Clin Nucl Med. (2021) 46:641–6. doi: 10.1097/RLU.0000000000003667
63. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
64. Crowley FJ, O'Cearbhaill RE, Collins DC. Exploiting somatic alterations as therapeutic targets in advanced and metastatic cervical cancer. Cancer Treat Rev. (2021) 98:102225. doi: 10.1016/j.ctrv.2021.102225
65. Gandy N, Arshad MA, Park WE, Rockall AG, Barwick TD. FDG-PET imaging in cervical cancer. Semin Nucl Med. (2019) 49:461–70. doi: 10.1053/j.semnuclmed.2019.06.007
66. Wegen S, Roth KS, Weindler J, Claus K, Linde P, Trommer M, et al. First clinical experience with [68Ga]Ga-FAPI-46-PET/CT versus [18F]F-FDG PET/CT for nodal staging in cervical cancer. Clin Nucl Med. (2023) 48:150–5. doi: 10.1097/RLU.0000000000004505
67. Phaeton R, Jiang Z, Revskaya E, Fisher DR, Goldberg GL, Dadachova E. Beta emitters rhenium-188 and lutetium-177 are equally effective in radioimmunotherapy of HPV-positive experimental cervical cancer. Cancer Med. (2016) 5:9–16. doi: 10.1002/cam4.562
68. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. (2005) 366:491–505. doi: 10.1016/S0140-6736(05)67063-8
69. Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers. (2021) 7:88. doi: 10.1038/s41572-021-00324-8
70. Hoang LN, McConechy MK, Kobel M, Han G, Rouzbahman M, Davidson B, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. (2013) 37:1421–32. doi: 10.1097/PAS.0b013e31828c63ed
71. Han G, Sidhu D, Duggan MA, Arseneau J, Cesari M, Clement PB, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol. (2013) 26:1594–604. doi: 10.1038/modpathol.2013.102
72. Bollineni VR, Ytre-Hauge S, Bollineni-Balabay O, Salvesen HB, Haldorsen IS. High diagnostic value of 18F-FDG PET/CT in endometrial cancer: systematic review and meta-analysis of the literature. J Nucl Med. (2016) 57:879–85. doi: 10.2967/jnumed.115.170597
73. Zhang X, Song W, Qin C, Song Y, Liu F, Hu F, et al. Uterine uptake of 68Ga-FAPI-04 in uterine pathology and physiology. Clin Nucl Med. (2022) 47:7–13. doi: 10.1097/RLU.0000000000003968
74. Kiran MY, Has Simsek D, Sanli Y, Kuyumcu S. 18F-FDG and 68Ga-FAPI-04 PET/CT in evaluating clear-cell endometrial cancer. Clin Nucl Med. (2023) 48:e87–e8. doi: 10.1097/RLU.0000000000004532
75. van den Heerik A, Horeweg N, de Boer SM, Bosse T, Creutzberg CL. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer. (2021) 31:594–604. doi: 10.1136/ijgc-2020-001822
76. Jamieson A, Huvila J, Thompson EF, Leung S, Chiu D, Lum A, et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol Oncol. (2022) 165:201–14. doi: 10.1016/j.ygyno.2022.02.001
77. Heller SL, Young Lin LL, Melsaether AN, Moy L, Gao Y. Hormonal effects on breast density, fibroglandular tissue, and background parenchymal enhancement. Radiographics. (2018) 38:983–96. doi: 10.1148/rg.2018180035
78. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. (2018) 33:463–79 e10. doi: 10.1016/j.ccell.2018.01.011
79. Gao MQ, Kim BG, Kang S, Choi YP, Park H, Kang KS, et al. Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial-mesenchymal transition-like state in breast cancer cells in vitro. J Cell Sci. (2010) 123:3507–14. doi: 10.1242/jcs.072900
80. Gilardi L, Airo Farulla LS, Demirci E, Clerici I, Omodeo Sale E, Ceci F. Imaging cancer-associated fibroblasts (CAFs) with FAPi PET. Biomedicines. (2022) 10(3):523. doi: 10.3390/biomedicines10030523
81. Syed M, Flechsig P, Liermann J, Windisch P, Staudinger F, Akbaba S, et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging. (2020) 47:2836–45. doi: 10.1007/s00259-020-04859-y
82. Prive BM, Boussihmad MA, Timmermans B, van Gemert WA, Peters SMB, Derks YHW, et al. Fibroblast activation protein-targeted radionuclide therapy: background, opportunities, and challenges of first (pre)clinical studies. Eur J Nucl Med Mol Imaging. (2023) 50:1906–18. doi: 10.1007/s00259-023-06144-0
Keywords: FAPI, breast cancer, gynecologic malignancies, ovarian cancer, cervical cancer, endometrial cancer, PET/CT
Citation: Li T, Zhang J, Yan Y, Tan M and Chen Y (2024) Applications of FAPI PET/CT in the diagnosis and treatment of breast and the most common gynecologic malignancies: a literature review. Front. Oncol. 14:1358070. doi: 10.3389/fonc.2024.1358070
Received: 19 December 2023; Accepted: 21 February 2024;
Published: 05 March 2024.
Edited by:
Abhishek Mahajan, The Clatterbridge Cancer Centre, United KingdomReviewed by:
Xiaotian Xia, Huazhong University of Science and Technology, ChinaCopyright © 2024 Li, Zhang, Yan, Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Chen, Y2hlbnl1ZTU1MjNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.