
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 27 February 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1357230
Background: Driver oncogene mutations, such as c-ros oncogene 1 (ROS1) and epidermal growth factor receptor (EGFR) were previously believed to be mutually exclusive in non-small cell lung cancer (NSCLC). Only sporadic cases of ROS1 and EGFR co-mutations have been reported. Hence, appropriate treatment options for these patients are still controversial.
Case presentation: A 48-year-old female patient presented at our hospital complaining of a persistent cough that had been ongoing for a month. A chest computed tomography showed a mass in the left lung along with hilar and mediastinal lymphadenopathy. Pathological analysis of bronchoscopic biopsy and lung mass puncture confirmed the presence of lung adenocarcinoma. The patient was diagnosed with stage IIIC left lung adenocarcinoma with a clinical stage of cT2N3M0. Next-generation sequencing analysis conducted at both puncture sites revealed an EFGR 19 deletion mutation combined with ROS1 rearrangement. The lung mass exhibited a higher mutation abundance. Treatment with a combination of third-generation EGFR tyrosine kinase inhibitors (TKIs) and crizotinib yielded satisfactory results. During the follow-up period, the mass significantly reduced and almost disappeared.
Conclusion: The co-mutation of EGFR and ROS1 is a rare phenomenon. Nevertheless, the combination of EGFR-TKI and crizotinib treatment appears to hold promise in providing positive results for patients, with manageable side effects. This therapeutic approach has the potential to enhance patients’ overall prognosis.
Lung cancer is still the leading cause of cancer−related deaths globally, and non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Identifying driver mutations in lung cancer has paved the way for personalized targeted treatments. Therefore, screening patients with lung cancer for oncogenic drivers and administering them with appropriate targeted therapies are of significance (1).
The EGFR gene is located in the 12–14 region of the short arm of chromosome 7. EGFR mutations are common in patients with NSCLC (2), especially among East Asian female patients with lung adenocarcinoma (3). Although various mutations have been detected, almost 90% are located in exons 19 and 21 of the EFGR gene. These include the deletion of amino acids 746–750 in exon 19 [del (746–750)] and a leucine-to-arginine substitution at position 858 in exon 21 (L858R) (4). In 2007, Rikova identified SLC34A2-ROS1 and CD74-ROS1 rearrangements in NSCLC cell lines and patients’ tumors (5), establishing oncogenic c-ros oncogene 1 (ROS1) rearrangements as a therapeutic target in lung cancer. Oncogenic ROS1 gene fusion was found in a distinct subpopulation (about 1 1%–2%) of patients with NSCLC. These patients were typically characterized by adenocarcinoma histology, young age, and a nonsmoking history (6).
Previous studies indicated that ROS1 rearrangements and EGFR mutations were mutually exclusive (5, 6). However, in this study, we presented the case of a patient who exhibited co-mutations in ROS1 and EGFR, and achieved significant remission through the oral administration of two corresponding TKIs. The patient experienced tolerable side effects during the treatment. This case report aimed to provide feasible treatment options for patients with similar co-mutations.
In February 2023, a 48-year-old woman was admitted to our hospital with a 1-month history of cough. She had no previous history of smoking or alcohol consumption and no family history of malignancy. A chest computed (CT) scan tomography revealed a left lower lobe mass measuring approximately 3.6 × 3.5 cm2, along with multiple enlarged mediastinal and hilar lymph nodes.
A pathological examination was conducted to further clarify the nature of the lesion. The biopsy, performed using endoscopic ultrasound-guided transbronchial needle aspiration, confirmed the presence of lung adenocarcinoma. The patient was clinically diagnosed with stage IIIB lung adenocarcinoma, with a clinical stage of cT2bN3M0(he 8th TNM staging system). Additionally, next-generation sequencing (NGS) examination revealed an ROS1 fusion (SLC34A2) (Figures 1A, B) with an abundance of 1.39% and an EGFR exon 19 mutation (Figure 1C) with a mutation abundance of 0.61%. The tumor showed a programmed cell death-ligand1(PD-L1) expression of 60%. We performed a CT-guided puncture biopsy of the lung mass with the patient’s consent to improve accuracy and develop an effective treatment plan. The biopsy confirmed the pathological diagnosis of lung adenocarcinoma. The results of the second NGS test were consistent with the previous test results (Figure 1), except for a higher mutation abundance observed in the EGFR mutation (14.38%) and the ROS1 fusion mutation (5.93%).
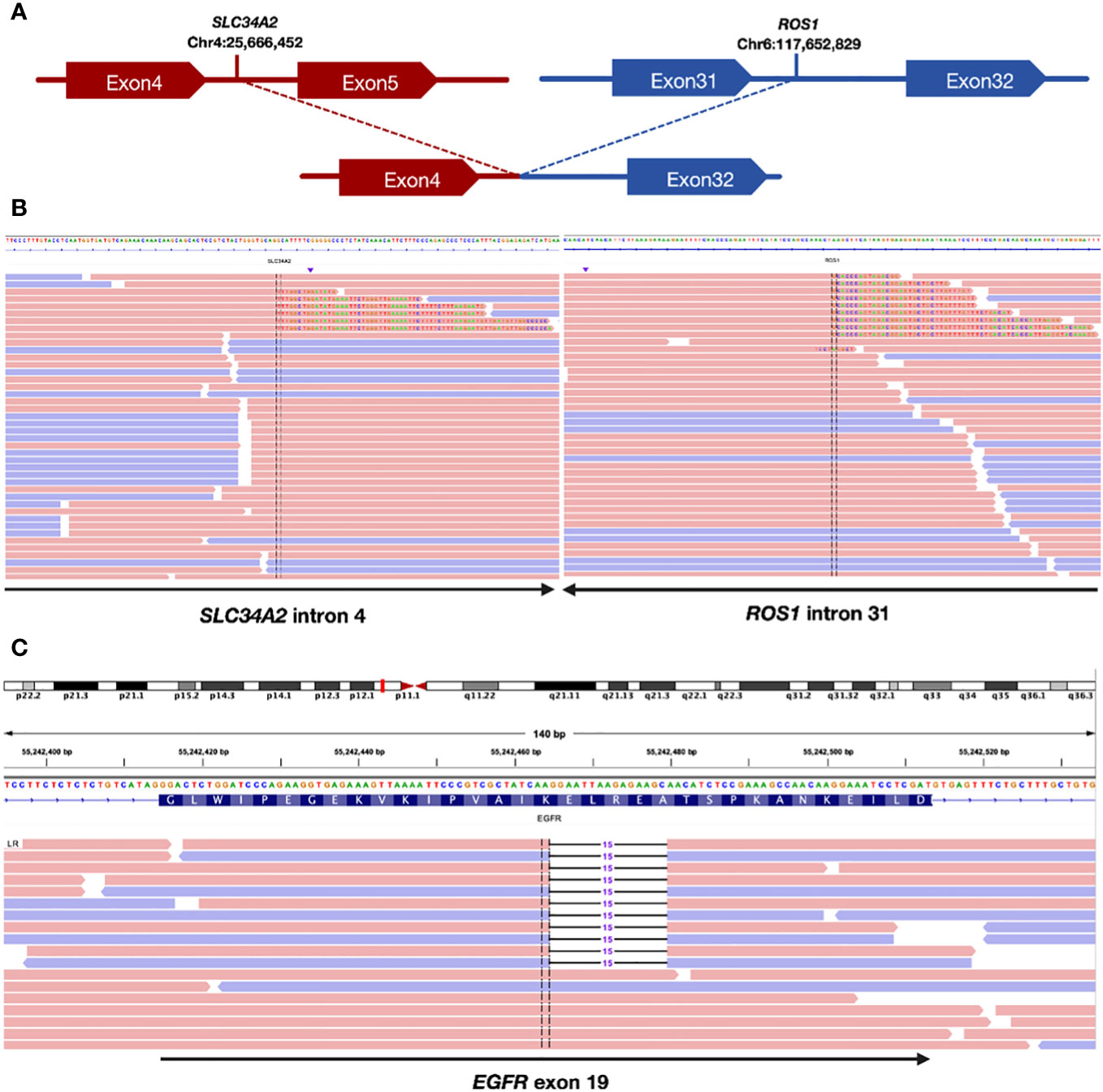
Figure 1 Sequencing assay showed positive ROS1 fusion and EGFR mutation. (A) Schematic indicating the fusion between the SLC34A2 gene exon 4 and ROS1 gene exon 32. (B) Split-read alignment visualizing the breakpoint of the fusion between SLC34A2 and ROS1 sequences. (C) Sequencing read alignment for EGFR exon 19, showing a potential deletion mutation.
Upon confirming the diagnosis, the patient was administered almonertinib and crizotinib orally. A significant reduction in the primary mass and all lymph nodes was observed after 1 month, and the efficacy was evaluated as a partial response based on the Response Evaluation Criteria In Solid Tumors. However, the patient experienced significant elevations in the levels of creatine kinase (CK) (552.30 U/L; reference range: 40–200 U/L), CK isoenzyme (CK-MB) (58.4 U/L; reference range: 0–25 U/L), and lactate dehydrogenase (LDH) (350.4 U/L; reference range: 120–250 U/L). Fortunately, the patient did not have any symptoms such as muscle soreness. The increase in CK and CK-MB levels aligned with the common adverse reactions of almonertinib (35.5%), indicating that these abnormalities were likely the side effects of almonertinib rather than of crizotinib. Considering the effectiveness of this treatment, we replaced another EGFR-TKI, furmonertinib. As predicted, the levels of CK and CK-MB gradually returned to normal without using any drugs following the discontinuation of almonertinib. The replacement of targeted drugs did not impact the patient’s response to treatment. Continuous monitoring of chest CT scans revealed a gradual reduction in tumor size, almost leading to complete disappearance. The patient has been undergoing treatment for 7 months and currently has stable disease. During subsequent treatment, the patient experienced only a tolerable first-degree rash reaction. The detailed diagnosis and treatment process is shown in Figure 2.
Previous studies have indicated that driver genes are usually mutually exclusive. However, technological advancements have increased the focus on NSCLC cases with dual-positive or even triple-positive driver genes. This is due to the growing feasibility of NGS in clinical practice, which allows for high-sensitivity assays with broad-spectrum genomic targets. The Lung Cancer Mutation Consortium project found that 5% of driver alterations in lung adenocarcinoma were double or multiple mutations (7).
At present, ROS1 rearrangements and EGFR mutations have been found to coexist in a small subgroup of patients with NSCLC (8). In a study by Lin et al., out of 220 ROS1-rearranged patients with NSCLC, only 1 patient was identified with both ROS1 fusion and EGFR-activating mutation (9). Another study reported concurrent genomic alterations with ROS1/EGFR co-occurrence observed in 3.17% of patients with EGFR-mutated NSCLC and 16.67% with ROS1-positive NSCLC (8). Additionally, Yanjiao Mao et al. found concomitant ALK/ROS1 fusion genes in 3.1% of patients with EGFR-mutated lung adenocarcinoma (10). The variation in concurrent mutation rates and associated partners could be attributed to the differences in detection methods, sample types, ethnic backgrounds, and tumor heterogeneity. However, the specific incidence still needs to be confirmed in future studies. This case report presented a case of EGFR and ROS1 common mutations combined with high PD-L1 expression and outlined our proposed treatment plan.
Significant progress has been made in the medical therapy of lung cancer in the last decade, largely due to the introduction of targeted therapy. Currently, tyrosine kinase inhibitors (TKIs) are considered a standard treatment for patients with advanced non-small cell lung cancer (NSCLC) who have specific gene mutations. These TKIs have significantly improved the prognosis for these patients (11). For patients with “classic” EGFR mutations (Ex19Dels and L858R), third-generation EGFR-TKIs have been shown to significantly increase progression-free survival and overall survival. Crizotinib, which has been extensively studied in both prospective and retrospective clinical trials (12), has demonstrated remarkable effectiveness in patients with advanced NSCLC carrying ROS1 alterations (13). It was approved by the US Food and Drug Administration and the European Medicines Agency in 2016.
Targeted therapy remains the preferred treatment for patients with co-mutations; however, determining the appropriate TKI for use still poses a challenge (10). Only sporadic reports exist on the selection of targeted drugs due to the rarity of EGFR and ROS1 co-mutations (14). Among these rare reports, there were cases of obvious response to EGFR TKI but ineffective to crizotinib (10, 15), and cases of response to crizotinib but ineffective to EGFR TKI (10, 16). Among patients with EGFR-activating mutations, approximately 70%–80% respond to EGFR-TKIs, whereas the remaining 20%–30% show a poor response, of which 7%–12% progress despite treatment with EGFR-TKIs, suggesting a risk for primary or intrinsic resistance (17).
Concomitant genetic alterations were found to be associated with primary resistance in patients with EGFR-mutated NSCLC (17, 18). These co-existing mutations included T790M and KRAS and the activation of the downstream PI3K-Akt due to factors such as PTEN loss or PIK3CA mutation (17). The mechanism of co-mutation is not clear. Research suggests that two driver alterations can develop within the same clone of tumor cells and may potentially work together during cancer development (19). Two hypotheses have been proposed to explain the coexistence of multiple oncogenic drivers in NSCLCs. Increasing evidence has shown that genetic instabilities in cancer cells lead to genetic and phenotypic heterogeneities within tumors, indicating that different genetic alterations may occur in different tumor cells rather than in a single clone (16). The interactions between EFGR and ROS1 in patients with co-mutations are not well understood. Previous case reports have tried to use EGFR-TKI or crizotinib alone, and no reports exist exploring the combination of EGFR-TKI and crizotinib.
Current genetic testing methods cannot determine which oncogene aberration is more dominant in the same sample. The results of genetic testing may provide us with some clues. The relative levels of phospho-EGFR can be used to predict the efficacy of targeted treatment in EGFR mutants (20). In addition, gene abundance can also be used as a predictor of targeted therapy (21). The rate of ROS1 rearrangement in lung cancer is relatively low, and limited research exists exploring the relationship between ROS1 mutation abundance and lung cancer prognosis. In NSCLCs, CD74–ROS1 is the most common ROS1 fusion (~44%), followed by EZR–ROS1 (16%), SDC4–ROS1 (14%), and SLC34A2–ROS1 (10%) (12). Some scholars have found that SDC4 and SLC34A2 are more likely to coexist with EGFR mutations and ALK translocations (8). In our case, NGS analysis of mediastinal lymph nodes and the primary lung mass indicated the presence of SLC34A2-ROS1 rearrangements along with an EGFR 19 Del mutation, with variations in gene mutation abundance. This phenomenon indicated tumor heterogeneity, which might lead to different dominant targets. Therefore, for this patient, we chose third-generation EGFR-TKI combined with crizotinib, which yielded positive results.
Previous studies have indicated that PD-L1 expression decreases in EGFR-mutated NSCLC compared with EGFR wild-type NSCLC (22). This is because activating the EGFR pathway can induce PD-L1 expression in NSCLC cell lines (23). Currently, data on the expression of PD-L1 in patients with common mutations are lacking. In our case, both tests revealed high expression of PD-L1. The impact of PD-L1 expression on the efficacy of targeted therapy remains controversial. Only patients without actionable driver mutations can benefit from immunotherapy-based treatment, whereas the efficacy of ICIs in patients with actionable driver mutations, regardless of PD-L1 expression, has been limited (24, 25). However, immunotherapy combined with chemotherapy may provide new hope for patients resistant to the third−generation EGFR−TKIs (26). Further research is needed to determine whether ICIs can benefit patients with co-mutations in the future.
In summary, it is necessary to perform full gene detection in NSCLC patients. Dealing with overlapping mutations in driver genes poses significant challenges when tailoring individualized therapy for patients with NSCLC. The role of EGFR and ROS1 inhibitors in patients with dual-positive mutations is unclear in current and previous studies. Further investigation is needed to understand the molecular mechanisms that determine responsiveness to EGFR-TKIs and ROS1-TKIs, as well as potential combination or sequential treatment strategies for this specific subgroup with co-alterations. The case reported in this study provides valuable insights for future research in this area.
The clinical data supporting the conclusions of this article will be made available by the authors.
This study was approved by the Ethics and Scientific Committeeof Hubei University of Medicine with approval number XYY2021002. Written informed consent was obtained from the individual for the publication of any potentially identifiable images included in this article.
ZW: Writing – review & editing. ZZ: Writing – review & editing. DZ: Writing – original draft. ZL: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (Grants number: 82200214), Key Research and Development Project of Hubei (Grants number: 2022BCE028), Young and middle-aged talent program of Hubei Education Bureau (Grants number: Q20222101), Platform Special Fund for Scientific Research of Xiangyang No.1 People’s Hospital (Grants number: XYY2022P05) and Instructional projects of Hubei Provincial Health and Health Commission (WJ2023F074).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA (2014) 311(19):1998–2006. doi: 10.1001/jama.2014.3741
2. Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol (2022) 85:253–75. doi: 10.1016/j.semcancer.2022.04.002
3. Shi Y, Au JS-K, Thongprasert S, Srinivasan S, Tsai C-M, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol (2014) 9(2):154–62. doi: 10.1097/JTO.0000000000000033
4. Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer – search and destroy. Eur J Cancer. (2006) 42(1):17–23. doi: 10.1016/j.ejca.2005.07.031
5. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell (2007) 131(6):1190–203. doi: 10.1016/j.cell.2007.11.025
6. Azelby CM, Sakamoto MR, Bowles DW. ROS1 targeted therapies: current status. Curr Oncol Rep (2021) 23(8):94. doi: 10.1007/s11912-021-01078-y
7. Kris MG, Johnson BE, Kwiatkowski DJ. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC). J Clin Oncol (2011) 29(18_suppl):CRA7506–CRA. doi: 10.1200/jco.2011.29.18_suppl.cra7506
8. Li D, Jiang H, Jin F, Pan L, Xie Y, Zhang L, et al. Concurrent classic driver oncogenes mutation with ROS1 rearrangement predicts superior clinical outcome in NSCLC patients. Genes Genomics (2023) 45(1):93–102. doi: 10.1007/s13258-022-01326-w
9. Lin JJ, Ritterhouse LL, Ali SM, Bailey M, Schrock AB, Gainor JF, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non-small cell lung cancer. J Thorac Oncol (2017) 12(5):872–7. doi: 10.1016/j.jtho.2017.01.004
10. Mao Y, Wu S. ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther (2017) 10:3399–404. doi: 10.2147/OTT.S133349
11. Melichar B. Biomarkers in the management of lung cancer: changing the practice of thoracic oncology. Clin Chem Lab Med (2023) 61(5):906–20. doi: 10.1515/cclm-2022-1108
12. Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat Rev Clin Oncol (2021) 18(1):35–55. doi: 10.1038/s41571-020-0408-9
13. Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med (2014) 371(21):1963–71. doi: 10.1056/NEJMoa1406766
14. Masago K, Kuroda H, Takahashi Y, Oya Y, Sasaki E, Sakakura N, et al. Synchronous driver gene alterations (EGFR L858R, T790M, and ROS1) rearrangements in a patient with early-stage lung adenocarcinoma. Cancer Genet (2022) 268-269:124–7. doi: 10.1016/j.cancergen.2022.09.010
15. Ju L, Han M, Zhao C, Li X. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer. (2016) 95:94–7. doi: 10.1016/j.lungcan.2016.03.005
16. Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol (2015) 26(2):348–54. doi: 10.1093/annonc/mdu530
17. Kim GW, Song JS, Choi C-M, Rho JK, Kim SY, Jang SJ, et al. Multiple resistant factors in lung cancer with primary resistance to EGFR-TK inhibitors confer poor survival. Lung Cancer. (2015) 88(2):139–46. doi: 10.1016/j.lungcan.2015.01.023
18. Lee JK, Shin JY, Kim S, Lee S, Park C, Kim JY, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol (2013) 24(8):2080–7. doi: 10.1093/annonc/mdt127
19. Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res (2008) 14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168
20. Yang J-J, Zhang X-C, Su J, Xu C-R, Zhou Q, Tian H-X, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res (2014) 20(5):1383–92. doi: 10.1158/1078-0432.CCR-13-0699
21. Zhou Q, Zhang X-C, Chen Z-H, Yin X-L, Yang J-J, Xu C-R, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol (2011) 29(24):3316–21. doi: 10.1200/JCO.2010.33.3757
22. Dong Z-Y, Zhang J-T, Liu S-Y, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology (2017) 6(11):e1356145. doi: 10.1080/2162402X.2017.1356145
23. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discovery (2013) 3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310
24. Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li X, et al. Immune checkpoint inhibitors in EGFR-mutated NSCLC: dusk or dawn? J Thorac Oncol (2021) 16(8):1267–88. doi: 10.1016/j.jtho.2021.04.003
25. Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann Oncol (2020) 31(5):599–608. doi: 10.1016/j.annonc.2020.01.065
Keywords: non-small cell lung cancer, EGFR, ROS1, tyrosine kinase inhibitors, co-mutation
Citation: Wu Z, Zhang Z, Zhang D and Li Z (2024) Remarkable response to third-generation EGFR-TKI plus crizotinib in a patient with pulmonary adenocarcinoma harboring EGFR and ROS1 co-mutation: a case report. Front. Oncol. 14:1357230. doi: 10.3389/fonc.2024.1357230
Received: 17 December 2023; Accepted: 30 January 2024;
Published: 27 February 2024.
Edited by:
Anthonie J. Van Der Wekken, University Medical Center Groningen, NetherlandsReviewed by:
Marthe Paats, Erasmus Medical Center, NetherlandsCopyright © 2024 Wu, Zhang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengyan Li, bHp5MTk5MTAzMTAxMDBAMTYzLmNvbQ==; Dongdong Zhang, emhhbmdkb25nZG9uZ0B3aHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.