- 1Interdisciplinary Department of Medicine, Section of Diagnostic Imaging, University of Bari Medical School “Aldo Moro”, Bari, Italy
- 2Radiodiagnostic Complex Operating Unit, San Giacomo Hospital, Bari, Italy
- 3Radiology Unit, Ente Ecclesiastico Ospedale Generale Regionale “F. Miulli”, Bari, Italy
- 4Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Naples, Italy
Objective: The prognosis of colorectal cancer has continuously improved in recent years thanks to continuous progress in both the therapeutic and diagnostic fields. The specific objective of this study is to contribute to the diagnostic field through the evaluation of the correlation between superior hemorrhoidal vein (SHV) ectasia detected on computed tomography (CT) and Tumor (T), Node (N), and distant metastasis (M) examination and mesorectal fascia (MRF) invasion in the preoperative staging of rectal cancer.
Methods: Between January 2018 and April 2022, 46 patients with histopathological diagnosis of rectal cancer were retrospectively enrolled, and the diameter of the SHV was evaluated by CT examination. The cutoff value for SHV diameter used is 3.7 mm. The diameter was measured at the level of S2 during portal venous phase after 4× image zoom to reduce the interobserver variability. The parameters evaluated were tumor location, detection of MRF infiltration (defined as the distance < 1 mm between the tumor margins and the fascia), SHV diameter, detection of mesorectal perilesional lymph nodes, and detection of metastasis.
Results: A total of 67.39% (31/46) of patients had SHV ectasia. All patients with MRF infiltration (4/46, 7.14%) presented SHV ectasia (average diameter of 4.4 mm), and SHV was significantly related with the development of liver metastases at the moment of primary staging and during follow-up.
Conclusion: SHV ectasia may be related to metastasis and MRF involvement; therefore, it could become a tool for preoperative staging of rectal cancer.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the fourth most common cause of cancer-related death and, in Western countries, represents about 30% of large bowel cancers (1–5).
The CRC includes a dissimilar group of diseases in terms of mutations and mutagens, representing a challenging field for molecular therapy. Furthermore, the heterogeneity of embryological origins, anatomy, and functions underlines the differences between colon and rectal cancer. More than 30% of patients experience metastasis after primary tumor diagnosis, whereas peritoneal dissemination has long been associated with unfavorable prognosis (6–8).
Rectal cancer has a wide distribution from the seventh decade onward, although diagnoses in patients under 50 are increasing (9).
The median age at diagnosis is 70 years old, with an increase among frailty patients (10). However, many studies demonstrated rapidly increasing incidence rates among adults younger than 50 years (11, 12).
Accurate preoperative staging is mandatory to choose the most precise treatment strategy, taking into account the continuously rising rates of minimally invasive surgery (13–21). It is usually conducted through American Joint Committee on Cancer (AJCC) TNM classification (22, 23). Among the radiological features, the tumor infiltration pattern is strictly related to the patient’s prognosis (24). In particular, the invasion through the rectal wall, expressed by the T stage, is defined by imaging features in the pre-operative evaluation. T stage is related with local recurrence and has a role in the choice between up-front surgery and neo-adjuvant therapy (25–27).
In preoperative staging, rectal ultrasound endoscopy (EUS) is essential for early-stage tumors (T1 and T2). MRI is generally unable to distinguish T1 tumors (growing into the submucosa) from T2 tumors (growing into the muscularis propria) and is considered the standard tool for rectal cancer in more advanced stages (T3–T4) where accuracy in evaluating the infiltration of the mesorectal fascia (MRF) is fundamental.
As highly reported in the literature since the 1990s (28–30), in patients affected by locally advanced T3–T4 and/or N1–N3 low or middle rectal cancers or for tumors with circumferential margin < 1 mm regardless of the site and stage at magnetic resonance imaging (MRI) (31), preoperative radiotherapy followed by surgery represents the curative treatment.
According to the European Society of Medical Oncology guidelines, neoadjuvant chemotherapy is deserved to patients with a grade of infiltration > 5 mm at MRI evaluation (32, 33). MRI has a great sensitivity in the evaluation of T and N stages, approximately of 90%, and is the most accurate tool for the loco-regional staging of rectal cancer (34, 35).
Although radiological and surgical efforts to reduce the side effects of radiotherapy as proctitis, anal incontinence, anastomotic leak or stenosis, the optimal dose of radiotherapy is still debated (36–41).
In this clinical scenario, the most challenging stage to characterize with the standard imaging protocols is the T3, which is related to an overall 5-year survival ranging from 25% to 90%, depending on the T3 subgroup (32, 42–44).
A prognostic role has also been attributed to extramural vascular invasion (EMVI) and involvement of MRF representing poor prognostic factors (45–47). The EMVI is defined as the presence of tumor cells beyond the muscularis propria in endothelium-lined vessels (48, 49), and it is defined by the histological report as lymph-vascular invasion (LVI) (50). EMVI is reported as a risk factor for recurrent disease and metastasis and as a stage independent negative prognostic factor, increasing the risk of developing liver metastases (51, 52).
MRF is considered involved when the distance between the tumor and MRF is ≤1 mm. MRI has the highest accuracy concerning T and N stages and EMVI evaluation; however, the evaluation of EMVI and MRF can be challenging in many cases (53).
The reduced territorial availability of MRI, higher costs, longer execution times, and limited patient characteristics (claustrophobia, marked obesity, metal devices implanted in the body, etc.) reduce the possibility of carrying out an MRI in all patients. On the other hand, the possible presence of marked colorectum stenosis makes the use of the EUS impossible. In these cases, computed tomography (CT) examination and subsequent SHV evaluation become the first choice.
CT can be an alternative diagnostic imaging technique that allows to study of the entire abdomen and pelvis; CT is widely diffuse in clinical practice to assess the preoperative staging of abdominal lymphatic stations and distant metastases. CT is mandatory as 25% of patients affected by CRC have synchronous liver metastases (7, 54–56). Concerning the limited visualization of the mesorectal and the rectal wall, CT cannot be considered the gold standard, as it lacks of contrast resolution, especially for early-stage lesions confined to the rectal wall (32, 57, 58).
On the other hand, CT allows a clear visualization of the vascular anatomy. Concerning venous vascular system of rectum, the superior rectal venous plexus drains into superior hemorrhoidal vein (SHV), which has its origin in the hemorrhoidal plexus and, through this plexus, communicates with the middle and inferior hemorrhoidal veins. The superior rectal vein leaves the lesser pelvis and crosses the left common iliac vessels with the superior rectal artery and is continued upward as the inferior mesenteric vein and finally in the portal vein.
Many diseases are associated with focal or diffuse vascular enlargement of pelvic vessels, among which are pelvic tumors (59). In patients with CRC, it seems to be a variation in the splanchnic circulation. In particular, the SHV ectasia seems to be related to the extramural spreading of the tumor, being a new important negative prognostic factor (60).
This study aims to evaluate the correlation between the SHV ectasia, metastasis, and MRF invasion in the preoperative staging of rectal cancer.
Materials and methods
Image acquisition
Between January 2018 and April 2022, all consecutive patients with histopathological diagnosis of rectal cancer were enrolled at the Polyclinic of Bari, Italy, and their data were retrospectively analyzed.
Inclusion criteria:
- diagnosis of rectal cancer;
- informed signed consent to the use of their anonymous data for scientific research; and
- no sign of portal hypertension, cirrhosis, pelvic masses, and splanchnic vein thrombosis (59).
Exclusion criteria:
- any sign of portal hypertension, cirrhosis, pelvic masses, and splanchnic vein thrombosis; and
- lack of consent to participate to the study.
All patients underwent CT examination within 15 days before surgery and histopathological diagnosis as indicated by the standard of care of our institution.
All patients underwent multidisciplinary team discussion before treatment.
CT exams were obtained with a 320-row CT scanner (Multidetector CT Aquillon, Toshiba Medical System, Tokyo, Japan; detector collimation, 0.5 mm; increment, 0.5; 120/87 kVp/mAs).
CT protocol included a non-enhanced scan followed by multiphasic acquisition after the intravenous injection of 1.5 mL/kg of Iopromide (370 mgI/mL) at 2.5 mL/s through the ante-cubital vein using an automatic power injector. The patients were scanned in supine position.
The acquisition was performed from the diaphragm to the pubic symphysis in the non-enhanced and arterial phases; in the portal venous phase, the scan was extended to the thorax. No bowel preparation was performed before CT examination (61).
All CT data were transferred to a workstation equipped with dedicated software for image reconstructions (Vitrea FX 4.1, Vital Images, Minneapolis, Minnesota, USA).
Dataset
Patients underwent surgery following the Italian National Guidelines (Italian Association of Medical Oncology (AIOM)) (62) with curative intent within 3 weeks from CT examination; then, the surgical specimens were submitted to the pathology department for examination. For each patient, we analyzed cancer location (low, middle, and high) and TNM parameters according to the VIII edition of the TNM classification by AJCC (22).
The present retrospective clinical study complied with ethical principles, including the Declaration of Helsinki of the World Medical Association and the additional requirements of Italian law and our Institutional Ethical Committee. In addition, the study was considered free from ethical review as it carries only negligible risk and involves the use of existing data, which contains only non-identifiable human data. The patient signed a written informed consent form approved by the local ethical board.
Preoperative CT scans were examined by two blinded radiologists with 10-year experience in gastrointestinal and oncologic radiology.
According to the literature, the cutoff value for SHV diameter used is 3.7 mm (60); the diameter was measured at the level of S2 vertebral level during portal venous phase after 4× image zoom to reduce the interobserver variability (61). SHV was detected in all patients.
The parameters evaluated were as follows:
- tumor size and location: location of rectal cancer is classified in a cranio-caudal direction basing on the distance of the tumor from the anal verge as low (up to 5 cm), middle (from >5 cm to 10 cm), or high (from >10 cm up to 15 cm);
- detection of MRF infiltration, defined as the distance < 1 mm between the tumor margins and the fascia (26, 48, 63);
- SHV diameter;
- detection of mesorectal perilesional lymph nodes; and
- detection of metastasis.
Statistical analysis
The baseline characteristics of the patients, the SHV ectasia, the presence of synchronous metastasis at CT examination, and the presence of lymph nodes involvement at pathological examination were evaluated by descriptive statistics. The relationship between SHV ectasia and the disease progression was evaluated through the Chi-square test or the Fisher test. P-value was judged statistically significant when less than 0.05.
The interobserver agreement was evaluated by using Cohen’s kappa (K). k > 0.81 assessed an almost complete agreement, and 0.61 < k < 0.8 and 0.41 < k < 0.6 assessed a substantial and a moderate agreement, respectively.
The statistical analysis was performed by using NCSS2007® software.
Results
Forty-six patients were included in our study: 20 men (43.48%) and 26 (56.52%) women with a median age of 62 years. Descriptive statistics of pre-operative staging show that 16/46 (34.78%) patients had low rectal cancer, 18/46 (39.13%) patients had medium rectal cancer, and 12/46 (26.09%) patients had high rectal cancer.
Twelve of the 46 (26.09%) patients had synchronous metastatic involvement at the time of diagnosis of primary tumor.
Neoplastic infiltration of MRF was found in 4/46 (8.69%) patients: None of these patients presented hepatic metastasis. No lung metastases were detected in any patient.
Thirty-one of the 46 (67.39%) patients were SHV positive.
All patients undergoing surgery did not show any MRF infiltration.
At CT examination, 30/46 (65.21%) patients had a suspicion of perirectal lymph nodes.
Postoperative staging of patients undergoing surgery with neoadjuvant radiotherapy with or without chemotherapy after a minimum of 18 months of follow-up shows that 8 of the 31 patients who were SHV positive and M0 developed liver metastasis.
SHV ectasia
The radiological evidence of SHV ectasia was shown in Figures 1A–E. Cohen’s kappa (K) was 0.78, indicating a high grade interrater agreement among the two expert radiologists.
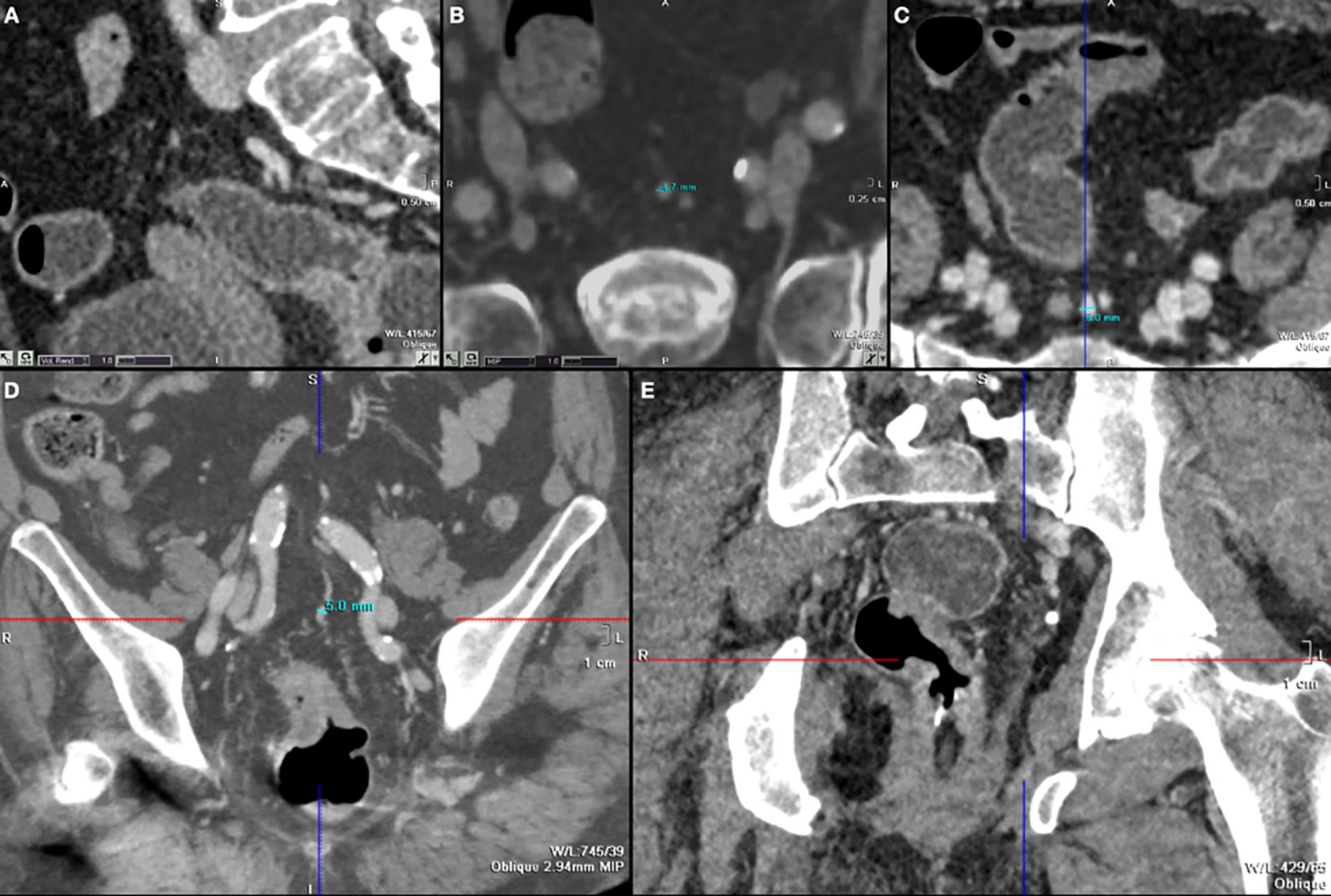
Figure 1 (A) S2 plane to evaluate SHV and S2 (sagittal reconstruction); (B–D) cases of SHV ectasia seen axial plane (B, C) and coronal plane (D); (E) tumor of left lateral wall with MRF invasion and SHV ectasia.
All patients with MRF infiltration (4/46, 7.14%) presented SHV ectasia (average diameter of 4.4 mm).
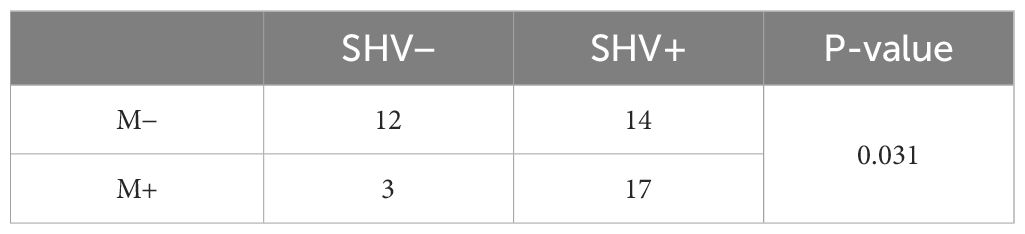
Table 1 shows that 67.39% (31/46) of patients had SHV ectasia. SHV ectasia was significantly related with the development of liver metastases at the moment of primary staging and during follow-up.
Discussion
In our experience, we evaluated the relationship between SHV diameter and T parameter, lymph node involvement, distant metastasis, and MRF infiltration. SHV ectasia may be related to metastasis development.
We found a significant relationship between SHV and advanced disease and disease progression. Hence, in further studies considering our preliminary data, SHV should be considered in the preoperative staging to better stratify the risk classification of disease progression. Our suggestion is to detect the high-risk patient group to perform a more intensive follow-up integrated with liver MRI that can more accurately detect and characterize also small potential liver lesions.
However, it should be underlined that venous vessel enlargement could be due to three principal mechanisms: increasing of venous drainage associated to neoplastic hypervascularization (64), splanchnic vein arterialization due to arterio-venous shunt, and increasing of venous pressure in neoplastic thrombosis (65). Considering this possible bias in patient selection, we preliminarily excluded from this study patients with cirrhosis, portal hypertension, pelvic masses, and splanchnic vein thrombosis, because SHV ectasia is frequent in these patients due to the presence of collateral circulation (59). Patient’s prognosis was affected by tumor invasion of the rectal wall, N stage, and MRF involvement (66, 67).
Following other literature experiences, we chose the cutoff of 3.7 mm to determine SHV ectasia. Some authors established that those patients with SHV diameter equal to or more than 3.7 mm had LVI (26, 60).
The nodal stage is often a challenge for radiologists especially because preoperative staging CT has a limited value in predicting lymph node metastasis in early rectal cancer and it is strongly related to metastatic disease and the treatment (6, 14, 25, 68–74). The study population showed that SHV diameter exceeded the cutoff by 3.7 mm in 79% of patients who had N+ confirmed after surgery and pathological examination. About distant metastasis, 75% of patients with liver metastasis had a SHV enlargement. Thus, patients with SHV diameter equal to or more than 3.7 mm tended to have nodal and distant metastasis.
In addition, we observed that, in the 16 patients who underwent neoadjuvant therapy, 3 did not show SHV ectasia although they had advanced cancer disease. Out of these three patients, two had low rectal cancer, and one had middle rectal cancer. We supposed that a lower rectal cancer, next to the anal verge, could have a different cancer venous vascular drainage, as inferior and/or middle hemorrhoidal vein that could justify no enlargement of SHV.
MRF is considered involved when the distance between the tumor and MRF is ≤1 mm. In our study, all patients with MRF involvement had SHV ectasia; this suggests a possible correlation between these two factors, both predictive of major invasion of the tumor.
Our experience confirms that SHV diameter measurement could be a meaningful tool to analyze LVI, as previously demonstrated in other reports (26, 75). Furthermore, the study suggests that SHV diameter could be a potential marker of MRF involvement.
If this were to be confirmed by further studies, then SHV ectasia may be integrated into the standardized parameters of the structured reporting (SR) for rectal cancer staging. The implementation of SR is important to offer referring physicians and patients an optimal quality of service and to provide researchers with data of the best quality (76, 77).
Obviously, we have to underline that MRF involvement and SHV diameter are useful only if integrated to the standard procedures concerning diagnosis and treatment of rectal cancer.
Nowadays, MRI is the most accurate non-invasive imaging modality to assess local staging at the moment of primary diagnosis (78–80).
MRI, through fast spin echo T2-weighted (FSE T2W), diffusion weighted imaging (DWI), and Apparent diffusion coefficient (APC) sequences, allows to recognize locally advanced diseases suitable of neoadjuvant CRT and to identify poor prognostic factors (81–83).
The identification of a locally advanced disease is mandatory to select the most precise treatment strategy, as the 25% of patients develop local recurrence after surgery and to improve the quality of life after surgery (14, 24, 44, 58, 84, 85).
The differentiation between T2 and T3 needs the MRI and the endorectal US in selected patients (86).
However, MRI has a high risk of over-staging disease due to the modification of muscolaris propria related to penetrating vessels or tissue desmoplastic reaction into the mesenteric fat (49, 87–89).
MRI is also useful for studying the locoregional nodal involvement and the extra-mesorectal lateral nodes, which, if pathological, makes the patients suitable for neoadjuvant chemotherapy (74).
MRI sensitivity is approximately 85% in nodal characterization; however, malignant cells might also be present in nodes < 5 mm of short axis, so our diagnosis power is still lower than our desire (83, 90).
All cases are characterized by a locally advanced disease diagnosed at MRI scan, and, in patients with a middle-low rectal tumor, the neoadjuvant treatment is mandatory before surgery, allowing organ-sparing surgical procedures with lower recurrence rates (87, 91, 92).
Therefore, for the local staging, MRI is the most complete diagnostic modality as it allows to accurately evaluate tumor location, Circumferential resection margin (CRM) involvement, nodal involvement, tumor deposits, or EMVI (93, 94).
Obviously, after any local treatment, it is also considered the gold standard for the restaging to assess the response to therapy (95–97).
At the same time, contrast-enhanced CT of the whole body is mandatory to detect distant metastases and complete the M-staging even in the pre-operative time even after neoadjuvant therapy (98, 99).
The diagnostic performance of CT of liver metastases is high, but it decreases for the lesions < 10 mm (56). In these cases, a diagnostic integration with liver MRI should be performed in selected patients (100, 101).
For this reason, several studies are focusing on the identification of the high risk patients (102–104).
Surely, rectal MRI allows to identify some negative features, such as EMVI, which is related to a higher incidence of developing distant metastases, particularly liver metastases (105–107).
Thus, taking into account what discussed above, the presence of SHV ectasia could also be considered a prognostic feature, suggesting the need of an MRI follow-up (60).
However, this tool should be validated in clinical practice in randomized prospective studies. In addition to radiological imaging, several studies are proposing liquid biopsy to detect circulating DNA that can contribute to the risk stratification of patients affected by CRC (108, 109).
In the era of precision medicine, liquid biopsy associated to imaging features could ensure a personalized follow-up or treatment strategy for different patients (110, 111).
Furthermore, radiomics tools have been proposed to analyze both the primary tumor and the most common site of metastatization.
In particular, many studies focused their attention on liver metastases, not only predicting genetic mutations on liver lesions but also predicting the future development of metachronous liver metastases in apparently healthy liver parenchyma (112, 113).
Currently, both liquid biopsy and radiomics have not already been validated in clinical practice due to the lack of prospective studies on multicentric cohorts; therefore, the analysis of the radiological features can be useful to create the first hybrid tools to create more intensive follow-up for high-risk patients. A more intensive follow-up can identify earlier patients affected by liver metastases and treat them with chemotherapy regimens.
This study has some limitations such as the small number of patients and, overall, the impossibility of comparing CT results with MRI data.
Conclusion
SHV ectasia may be related to metastasis and MRF involvement; a cutoff of 3.7 mm in diameter is considered significant in our experience according to the literature. Therefore, SHV diameter could become an interesting tool to complete the preoperative staging and follow-up of rectal cancer.
Data availability statement
The datasets presented in this article are not readily available because the raw data supporting the conclusions of this article will be made available by the authors, with undue reservation. Requests to access the datasets should be directed toZG90dGNoaWFyYW1vcmVsbGlAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by University of Bari Medical School “Aldo Moro”. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NL: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. NM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. CM: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation. RC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. SB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. PA: Software, Visualization, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. DS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. SG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. AI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EMVI, Extramural vascular invasion; MRF, Mesorectal fascia; MRI, Magnetic Resonance Imaging; CT, Computed Tomography; SHV, Superior hemorrhoidal vein; LVI, Lymph-vascular invasion; SR, structured reporting.
References
1. Losurdo P, Mastronardi M, de Manzini N, Bortul M. Survival and long-term surgical outcomes after colorectal surgery: are there any gender-related differences? Updates Surg. (2022) 74:1337–43. doi: 10.1007/s13304-022-01323-4
2. Avella P, Vaschetti R, Cappuccio M, Gambale F, DEM L, Rafanelli F, et al. The role of liver surgery in simultaneous synchronous colorectal liver metastases and colorectal cancer resections: a literature review of 1730 patients underwent open and minimally invasive surgery. Minerva Surg. (2022) 77:582–90. doi: 10.23736/S2724-5691.22.09716-7
3. Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18010197
4. Zhou F, Ding J. Prognosis and factors affecting colorectal cancer with ovarian metastasis. Updates Surg. (2021) 73:391–8. doi: 10.1007/s13304-021-00978-9
5. Basso C, Gennaro N, Dotto M, Ferroni E, Noale M, Avossa F, et al. Congestive heart failure and comorbidity as determinants of colorectal cancer perioperative outcomes. Updates Surg. (2022) 74:609–17. doi: 10.1007/s13304-021-01086-4
6. Ceccarelli G, Rocca A, De Rosa M, Fontani A, Ermili F, Andolfi E, et al. Minimally invasive robotic-assisted combined colorectal and liver excision surgery: feasibility, safety and surgical technique in a pilot series. Updates Surg. (2021) 73:1015–22. doi: 10.1007/s13304-021-01009-3
7. Wang HW, Jin KM, Li J, Wang K, Xing BC. Postoperative complications predict poor outcomes only in patients with a low modified clinical score after resection of colorectal liver metastases: a retrospective cohort study. Updates Surg. (2022) 74:1601–10. doi: 10.1007/s13304-022-01312-7
8. Rotolo S, Di Giorgio A, Santullo F, Attalla El Halabieh M, Lodoli C, Abatini C, et al. Cytoreductive surgery and mitomycin C hyperthermic intraperitoneal chemotherapy with CO(2) recirculation (HIPEC-CO(2)) for colorectal cancer peritoneal metastases: analysis of short-term outcomes. Updates Surg. (2021) 73:1443–8. doi: 10.1007/s13304-021-01034-2
9. Tian Y, Kharazmi E, Brenner H, Xu X, Sundquist K, Sundquist J, et al. Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets. Gastroenterology. (2020) 159:159–68.e3. doi: 10.1053/j.gastro.2020.03.063
10. Abdelfatah E, Ramos-Santillan V, Cherkassky L, Cianchetti K, Mann G. High risk, high reward: frailty in colorectal cancer surgery is associated with worse postoperative outcomes but equivalent long-term oncologic outcomes. Ann Surg Oncol. (2023) 30:2035–45. doi: 10.1245/s10434-022-12970-7
11. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. (2020) 158:341–53. doi: 10.1053/j.gastro.2019.07.055
13. Lohsiriwat V, Lertbannaphong S, Polakla B, Riansuwan W. Implementation of enhanced recovery after surgery and its increasing compliance improved 5-year overall survival in resectable stage III colorectal cancer. Updates Surg. (2021) 73:2169–79. doi: 10.1007/s13304-021-01004-8
14. Peltrini R, Imperatore N, Carannante F, Cuccurullo D, Capolupo GT, Bracale U, et al. Age and comorbidities do not affect short-term outcomes after laparoscopic rectal cancer resection in elderly patients. A multi-institutional cohort study in 287 patients. Updates Surg. (2021) 73:527–37. doi: 10.1007/s13304-021-00990-z
15. Yi X, Liao W, Feng X, Li H, Chen Z, Wang J, et al. An innovative and convenient technique to reduce anastomotic leakage after double stapling anastomosis: laparoscopic demucositized suture the overlapping point of the "dog ear" area. Updates Surg. (2022) 74:1645–56. doi: 10.1007/s13304-022-01282-w
16. Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F, et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. (2019) 144:2992–3000. doi: 10.1002/ijc.32055
17. Bretagnol F, Hatwell C, Farges O, Alves A, Belghiti J, Panis Y. Benefit of laparoscopy for rectal resection in patients operated simultaneously for synchronous liver metastases: preliminary experience. Surgery. (2008) 144:436–41. doi: 10.1016/j.surg.2008.04.014
18. Nasir I, Mureb A, Aliozo CC, Abunada MH, Parvaiz A. State of the art in robotic rectal surgery: marginal gains worth the pain? Updates Surg. (2021) 73:1073–9. doi: 10.1007/s13304-020-00965-6
19. Fiorillo C, Quero G, Menghi R, Cina C, Laterza V, De Sio D, et al. Robotic rectal resection: oncologic outcomes. Updates Surg. (2021) 73:1081–91. doi: 10.1007/s13304-020-00911-6
20. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
21. Aldrighetti L, Boggi U, Falconi M, Giuliante F, Cipriani F, Ratti F, et al. Perspectives from Italy during the COVID-19 pandemic: nationwide survey-based focus on minimally invasive HPB surgery. Updates Surg. (2020) 72:241–7. doi: 10.1007/s13304-020-00815-5
23. Reali C, Bocca G, Lindsey I, Jones O, Cunningham C, Guy R, et al. Influence of incorrect staging of colorectal carcinoma on oncological outcome: are we playing safely? Updates Surg. (2022) 74:591–7. doi: 10.1007/s13304-021-01095-3
24. Gomez Ruiz M, Ballestero Diego R, Tejedor P, Cagigas Fernandez C, Cristobal Poch L, Suarez Pazos N, et al. Robotic surgery for locally advanced T4 rectal cancer: feasibility and oncological quality. Updates Surg. (2023) 75:589–97. doi: 10.1007/s13304-023-01450-6
25. Zhang Q, Li B, Zhang S, Huang Q, Zhang M, Liu G. Prognostic impact of tumor size on patients with metastatic colorectal cancer: a large SEER-based retrospective cohort study. Updates Surg. (2023) 75:1135–47. doi: 10.1007/s13304-023-01533-4
26. Piva C, Panier Suffat L, Petrucci ETF, Manuguerra G, Vittone F, Cante D, et al. Effect of delaying surgery by more than 10 weeks after neoadjuvant therapy in rectal cancer: a single institution experience. Updates Surg. (2022) 74:145–51. doi: 10.1007/s13304-021-01189-y
27. Pechlivanides G, Gourtsoyianni S, Gouvas N, Sougklakos J, Xynos E. Management of the adenocarcinoma of the upper rectum: a reappraisal. Updates Surg. (2021) 73:513–26. doi: 10.1007/s13304-020-00903-6
28. Glynne-Jones R, Hall M. Radiotherapy and locally advanced rectal cancer. Br J Surg. (2015) 102:1443–5. doi: 10.1002/bjs.9930
29. Lin T, Narang A. Advances in radiotherapy for rectal cancer. Surg Oncol Clin N Am. (2023) 32:461–73. doi: 10.1016/j.soc.2023.02.003
30. Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol. (2013) 19:8489–501. doi: 10.3748/wjg.v19.i46.8489
31. Arulampalam T, Sizer B, Lacey N, Motson R. MRI for the assessment of locally advanced rectal cancer - a window of opportunity. Colorectal Dis. (2010) 12:269–70. England. doi: 10.1111/j.1463-1318.2009.02021.x
32. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv22–40. doi: 10.1093/annonc/mdx224
33. Di Candido F, Carvello M, Keller DS, Vanni E, Maroli A, Montroni I, et al. A comparative cost analysis of transanal and laparoscopic total mesorectal excision for rectal cancer. Updates Surg. (2021) 73:85–91. doi: 10.1007/s13304-020-00879-3
34. Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for rectal cancer: staging, mrCRM, EMVI, lymph node staging and post-treatment response. Clin Colorectal Cancer. (2022) 21:10–8. doi: 10.1016/j.clcc.2021.10.007
35. Moreno CC, Sullivan PS, Mittal PK. Rectal MRI for cancer staging and surveillance. Gastroenterol Clin North Am. (2018) 47:537–52. doi: 10.1016/j.gtc.2018.04.005
36. Carlini M, Grieco M, Spoletini D, Menditto R, Napoleone V, Brachini G, et al. Implementation of the gut microbiota prevents anastomotic leaks in laparoscopic colorectal surgery for cancer:the results of the MIRACLe study. Updates Surg. (2022) 74:1253–62. doi: 10.1007/s13304-022-01305-6
37. Grieco M, Marcellinaro R, Spoletini D, Menditto R, Lisi G, Russo G, et al. Laparoscopic right colectomy: changes in surgical technique and perioperative management allow better postoperative results in a comparative series of 361 patients. Updates Surg. (2022) 74:883–90. doi: 10.1007/s13304-022-01287-5
38. Marcellinaro R, Grieco M, Spoletini D, Troiano R, Avella P, Brachini G, et al. How to reduce the colorectal anastomotic leakage? The MIRACLe protocol experience in a cohort in a single high-volume centre. Updates Surg. (2023) 75:1559–67. doi: 10.1007/s13304-023-01588-3
39. Dahiya S, Tisch S, Greenfield J. The effect of GLP-1 receptor agonists in pre-clinical rodent models of Parkinson's disease: A systematic review and meta-analysis. Clin Park Relat Disord. (2022) 6:100133. doi: 10.1016/j.prdoa.2022.100133
40. Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, et al. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. (2016) 59:934–42. doi: 10.1097/DCR.0000000000000665
41. Rondelli F, Pasculli A, De Rosa M, Avenia S, Bugiantella W. Is routine splenic flexure mobilization always necessary in laparotomic or laparoscopic anterior rectal resection? A systematic review and comprehensive meta-analysis. Updates Surg. (2021) 73:1643–61. doi: 10.1007/s13304-021-01135-y
42. Mawdsley S, Glynne-Jones R, Grainger J, Richman P, Makris A, Harrison M, et al. Can histopathologic assessment of circumferential margin after preoperative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for 3-year disease-free survival? Int J Radiat Oncol Biol Phys. (2005) 63:745–52. doi: 10.1016/j.ijrobp.2005.03.003
43. Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, et al. Preoperative treatment of locally advanced rectal cancer. N Engl J Med. (2023) 389:322–34. doi: 10.1056/NEJMoa2303269
44. Perry WRG, Abd El Aziz MA, Duchalais E, Grass F, Behm KT, Mathis KL, et al. Sexual dysfunction following surgery for rectal cancer: a single-institution experience. Updates Surg. (2021) 73:2155–9. doi: 10.1007/s13304-021-01124-1
45. Bae HW, Kim HS, Yang SY, Shin SJ, Chang JS, Koom WS, et al. Upfront chemotherapy and short-course radiotherapy with delayed surgery for locally advanced rectal cancer with synchronous liver metastases. Eur J Surg Oncol. (2021) 47:2814–20. doi: 10.1016/j.ejso.2021.05.018
46. Xie H, Zhou X, Zhuo Z, Che S, Xie L, Fu W. Effectiveness of MRI for the assessment of mesorectal fascia involvement in patients with rectal cancer: a systematic review and meta-analysis. Dig Surg. (2014) 31:123–34. doi: 10.1159/000363075
47. Piozzi GN, Lee TH, Kwak JM, Kim J, Kim SH. Robotic-assisted resection for beyond TME rectal cancer: a novel classification and analysis from a specialized center. Updates Surg. (2021) 73:1103–14. doi: 10.1007/s13304-020-00898-0
48. Taylor A, Slater A, Mapstone N, Taylor S, Halligan S. Staging rectal cancer: MRI compared to MDCT. Abdom Imaging. (2007) 32:323–7. doi: 10.1007/s00261-006-9081-4
49. Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. (2008) 191:1827–35. doi: 10.2214/AJR.08.1004
50. Sohn B, Lim JS, Kim H, Myoung S, Choi J, Kim NK, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. (2015) 25:1347–55. doi: 10.1007/s00330-014-3527-9
51. Kang KA, Jang KM, Kim SH, Kang TW, Cha DI. Risk factor assessment to predict the likelihood of a diagnosis of metastasis for indeterminate hepatic lesions found at computed tomography in patients with rectal cancer. Clin Radiol. (2017) 72:473–81. doi: 10.1016/j.crad.2017.01.011
52. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
53. Tripathi P, Rao SX, Zeng MS. Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis. (2017) 18:2–12. doi: 10.1111/1751-2980.12439
54. Carbone F, Chee Y, Rasheed S, Cunningham D, Bhogal RH, Jiao L, et al. Which surgical strategy for colorectal cancer with synchronous hepatic metastases provides the best outcome? A comparison between primary first, liver first and simultaneous approach. Updates Surg. (2022) 74:451–65. doi: 10.1007/s13304-021-01234-w
55. Avella P, Cappuccio M, Cappuccio T, Rotondo M, Fumarulo D, Guerra G, et al. Artificial intelligence to early predict liver metastases in patients with colorectal cancer. Life (Basel). (2023) 13(10):2027. doi: 10.3390/life13102027
56. Rocca A, Brunese MC, Santone A, Avella P, Bianco P, Scacchi A, et al. Early diagnosis of liver metastases from colorectal cancer through CT radiomics and formal methods: A pilot study. J Clin Med. (2021) 11. doi: 10.3390/jcm11010031
57. Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. (2017) 85:1117–32. doi: 10.1016/j.gie.2017.02.022
58. Sorrentino L, Belli F, Guaglio M, Daveri E, Cosimelli M. Prediction of R0/R+ surgery by different classifications for locally recurrent rectal cancer. Updates Surg. (2021) 73:539–45. doi: 10.1007/s13304-020-00941-0
59. Umeoka S, Koyama T, Togashi K, Kobayashi H, Akuta K. Vascular dilatation in the pelvis: identification with CT and MR imaging. Radiographics. (2004) 24:193–208. doi: 10.1148/rg.241035061
60. Wu CC, Lee RC, Chang CY. Prediction of lymphovascular invasion in rectal cancer by preoperative CT. AJR Am J Roentgenol. (2013) 201:985–92. doi: 10.2214/AJR.12.9657
61. Granata V, Fusco R, Bicchierai G, Cozzi D, Grazzini G, Danti G, et al. Diagnostic protocols in oncology: workup and treatment planning. Part 1: the optimitation of CT protocol. Eur Rev Med Pharmacol Sci. (2021) 25:6972–94. doi: 10.26355/eurrev_202111_27246
63. Dar RA, Chowdri NA, Parray FQ, Shaheen F, Wani SH, Mushtaque M. Pre-operative staging of rectal cancer using multi-detector row computed tomography with multiplanar reformations: single center experience. Indian J Cancer. (2014) 51:170–5. doi: 10.4103/0019-509X.138292
64. Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. (1980) 67:439–42. doi: 10.1002/bjs.1800670619
65. Amato B, Compagna R, Rocca A, Bianco T, Milone M, Sivero L, et al. Fondaparinux vs warfarin for the treatment of unsuspected pulmonary embolism in cancer patients. Drug Des Devel Ther. (2016) 10:2041–6. doi: 10.2147/DDDT.S106153
66. Jin LJ, Chen WB, Zhang XY, Bai J, Zhao HC, Wang ZY. Analysis of factors potentially predicting prognosis of colorectal cancer. World J Gastrointest Oncol. (2019) 11:1206–17. doi: 10.4251/wjgo.v11.i12.1206
67. Maeda K, Shibutani M, Otani H, Nagahara H, Ikeya T, Iseki Y, et al. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. (2015) 7:111–7. doi: 10.4251/wjgo.v7.i8.111
68. Zhang Y, Ge L, Weng J, Tuo WY, Liu B, Ma SX, et al. Neoadjuvant chemotherapy for patients with resectable colorectal cancer liver metastases: A systematic review and meta-analysis. World J Clin Cases. (2021) 9:6357–79. doi: 10.12998/wjcc.v9.i22.6357
69. Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. (2018) 55:330–79. doi: 10.1067/j.cpsurg.2018.08.004
70. Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. (2018) 105:1210–20. doi: 10.1002/bjs.10838
71. Rocca A, Scacchi A, Cappuccio M, Avella P, Bugiantella W, De Rosa M, et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int J Med Robot. (2021) 17:e2330. doi: 10.1002/rcs.2330
72. Morelli L, Di Franco G, Guadagni S, Rossi L, Palmeri M, Furbetta N, et al. Robot-assisted total mesorectal excision for rectal cancer: case-matched comparison of short-term surgical and functional outcomes between the da Vinci Xi and Si. Surg Endosc. (2018) 32:589–600. doi: 10.1007/s00464-017-5708-5
73. Khan SM, Emile SH, Barsom SH, Ahsan SO. Development of the 'PREDICT' score through a systematic review and meta-analysis of the predictive parameters for locoregional recurrence after total mesorectal excision. Updates Surg. (2021) 73:35–46. doi: 10.1007/s13304-020-00853-z
74. Takeyama H, Danno K, Nishigaki T, Yamashita M, Oka Y. Surgical technique for mesorectal division during robot-assisted laparoscopic tumor-specific mesorectal excision (TSME) for rectal cancer using da Vinci Si surgical system: the simple switching technique (SST). Updates Surg. (2021) 73:1093–102. doi: 10.1007/s13304-020-00901-8
75. Bayar S, Saxena R, Emir B, Salem RR. Venous invasion may predict lymph node metastasis in early rectal cancer. Eur J Surg Oncol. (2002) 28:413–7. doi: 10.1053/ejso.2002.1254
76. Granata V, Caruso D, Grassi R, Cappabianca S, Reginelli A, Rizzati R, et al. Structured reporting of rectal cancer staging and restaging: A consensus proposal. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13092135
77. Granata V, Faggioni L, Grassi R, Fusco R, Reginelli A, Rega D, et al. Structured reporting of computed tomography in the staging of colon cancer: a Delphi consensus proposal. Radiol Med. (2022) 127:21–9. doi: 10.1007/s11547-021-01418-9
78. Cianci R, Cristel G, Agostini A, Ambrosini R, Calistri L, Petralia G, et al. MRI for rectal cancer primary staging and restaging after neoadjuvant chemoradiation therapy: how to do it during daily clinical practice. Eur J Radiol. (2020) 131:109238. doi: 10.1016/j.ejrad.2020.109238
79. Reginelli A, Clemente A, Sangiovanni A, Nardone V, Selvaggi F, Sciaudone G, et al. Endorectal ultrasound and magnetic resonance imaging for rectal cancer staging: A modern multimodality approach. J Clin Med. (2021) 10. doi: 10.3390/jcm10040641
80. Karbhari A, Baheti AD, Ankathi SK, Haria PD, Choudhari A, Katdare A, et al. MRI in rectal cancer patients on 'watch and wait': patterns of response and their evolution. Abdom Radiol (NY). (2023) 48:3287–96. doi: 10.1007/s00261-023-04003-y
81. Zhong G, Xiao Y, Zhou W, Pan W, Zhu Q, Zhang J, et al. Value of endorectal ultrasonography in measuring the extent of mesorectal invasion and substaging of T3 stage rectal cancer. Oncol Lett. (2017) 14:5657–63. doi: 10.3892/ol
82. Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, et al. MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology. (2018) 287:833–43. doi: 10.1148/radiol.2018172300
83. Gollub MJ, Arya S, Beets-Tan RG, dePrisco G, Gonen M, Jhaveri K, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY). (2018) 43:2893–902. doi: 10.1007/s00261-018-1642-9
84. Horesh N, Freund MR, Garoufalia Z, Gefen R, Nagarajan A, Suarez E, et al. Total neoadjuvant therapy is a predictor for complete pathological response in patients undergoing surgery for rectal cancer. J Gastrointest Surg. (2022) 26:2579–84. doi: 10.1007/s11605-022-05463-1
85. Ghezzi TL, Tarta C, Contu PC, Lazzaron AR, Contin BM, Kliemann LM, et al. Distal resection margins in rectal cancer specimens: differences in assessment between surgeons and pathologists and the influence of neoadjuvant chemoradiation. Updates Surg. (2021) 73:1787–93. doi: 10.1007/s13304-021-01102-7
86. Ghoneem E, Shabana ASA, El Sherbini M, Zuhdy M, Eldamshety O, Gouda M, et al. Endoluminal ultrasound versus magnetic resonance imaging in assessment of rectal cancer after neoadjuvant therapy. BMC Gastroenterol. (2022) 22:542. doi: 10.1186/s12876-022-02628-9
87. Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. (2018) 28:1465–75. doi: 10.1007/s00330-017-5026-2
88. Lee S, Kassam Z, Baheti AD, Hope TA, Chang KJ, Korngold EK, et al. Rectal cancer lexicon 2023 revised and updated consensus statement from the Society of Abdominal Radiology Colorectal and Anal Cancer Disease-Focused Panel. Abdom Radiol (NY). (2023) 48:2792–806. doi: 10.1007/s00261-023-03893-2
89. Chen HLR, Seow-En I, Chok AY, Ngo NT, Cheng TL, Tan KE. The role of magnetic resonance tumour regression grade in the prediction of regression and survival of rectal adenocarcinoma after long-course chemoradiotherapy: a cohort study. Ann Med Surg (Lond). (2023) 85:842–8. doi: 10.1097/MS9.0000000000000441
90. Liu H, Zhao Y, Yang F, Lou X, Wu F, Li H, et al. Preoperative prediction of lymph node metastasis in colorectal cancer with deep learning. BME Front. (2022) 2022:9860179. doi: 10.34133/2022/9860179
91. Bianco F, Incollingo P, Falato A, De Franciscis S, Belli A, Carbone F, et al. Short stump and high anastomosis pull-through (SHiP) procedure for delayed coloanal anastomosis with no protective stoma for low rectal cancer. Updates Surg. (2021) 73:495–502. doi: 10.1007/s13304-021-01022-6
92. Aliyev V, Piozzi GN, Bulut A, Guven K, Bakir B, Saglam S, et al. Robotic vs. laparoscopic intersphincteric resection for low rectal cancer: a case matched study reporting a median of 7-year long-term oncological and functional outcomes. Updates Surg. (2022) 74:1851–60. doi: 10.1007/s13304-022-01396-1
93. Crimì F, Angelone R, Corso A, Bao QR, Cabrelle G, Vernuccio F, et al. Diagnostic accuracy of state-of-the-art rectal MRI sequences for the diagnosis of extramural vascular invasion in locally advanced rectal cancer after preoperative chemoradiotherapy: dos or maybes? Eur Radiol. (2023) 33:6852–60. doi: 10.1007/s00330-023-09655-4
94. Wang KX, Yu J, Xu Q. Histogram analysis of dynamic contrast-enhanced magnetic resonance imaging to predict extramural venous invasion in rectal cancer. BMC Med Imaging. (2023) 23:77. doi: 10.1186/s12880-023-01027-0
95. Popiţa AR, Rusu A, Muntean V, Cadariu PA, Irimie A, Lisencu C, et al. Preoperative MRI accuracy after neoadjuvant chemoradiation for locally advanced rectal cancer. Med Pharm Rep. (2023) 96:258–68. doi: 10.1016/S0140-6736(13)61649-9
96. Thompson HM, Bates DDB, Pernicka JG, Park SJ, Nourbakhsh M, Fuqua JL 3rd, et al. MRI assessment of extramural venous invasion before and after total neoadjuvant therapy for locally advanced rectal cancer and its association with disease-free and overall survival. Ann Surg Oncol. (2023) 30:3957–65. doi: 10.1245/s10434-023-13225-9
97. Zhang W, Zhou H, Jiang J, Zhu Y, Zou S, Jiang L, et al. Neoadjuvant chemotherapy with modified FOLFOXIRI for locally advanced rectal cancer to transform effectively EMVI and MRF from positive to negative: results of a long-term single center phase 2 clinical trial. BMC Cancer. (2023) 23:592. doi: 10.1186/s12885-023-11103-x
98. Shahzadi I, Zwanenburg A, Lattermann A, Linge A, Baldus C, Peeken JC, et al. Analysis of MRI and CT-based radiomics features for personalized treatment in locally advanced rectal cancer and external validation of published radiomics models. Sci Rep. (2022) 12:10192. doi: 10.1038/s41598-022-13967-8
99. Jensen CT, Wong VK, Wagner-Bartak NA, Liu X, Padmanabhan Nair Sobha R, Sun J, et al. Accuracy of liver metastasis detection and characterization: Dual-energy CT versus single-energy CT with deep learning reconstruction. Eur J Radiol. (2023) 168:111121. doi: 10.1016/j.ejrad.2023.111121
100. Görgec B, Hansen IS, Kemmerich G, Syversveen T, Abu Hilal M, Belt EJT, et al. MRI in addition to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): an international, multicentre, prospective, diagnostic accuracy trial. Lancet Oncol. (2023). doi: 10.1016/S1470-2045(23)00572-7
101. Joskowicz L, Szeskin A, Rochman S, Dodi A, Lederman R, Fruchtman-Brot H, et al. Follow-up of liver metastases: a comparison of deep learning and RECIST 1. 1. Eur Radiol. (2023) 33:9320–7. doi: 10.1007/s00330-023-09926-0
102. Patra A, Lakhani A, Augustine A, Mohapatra P, Eapen A, Singh A, et al. Predicting positive radial margin on restaging MRI of patients with low rectal cancer: can we do better? Indian J Radiol Imaging. (2024) 34:85–94. doi: 10.1055/s-0043-1774300
103. Tran CG, Goffredo P, Mott SL, Suraju MO, Kohn JF, Mishra A, et al. Conditional overall survival after diagnosis of non-metastatic colon cancer: impact of laterality, MSI, and KRAS status. Ann Surg Oncol. (2024) 31:142–51. doi: 10.1245/s10434-023-14443-x
104. Zheng P, Ye C, Liu H, Gao X, Huang H. Adjuvant chemotherapy decision-making in stage II colon adenocarcinoma associated with patients' age and high-risk factors. Int J Colorectal Dis. (2023) 39:3. doi: 10.1007/s00384-023-04581-9
105. Zhong X, Feng N, Ouyang B, Zhao D, Lei L, Peng J, et al. Modifiable risk factors in high-risk groups of colorectal cancer screening: A cross-sectional study with propensity score method. Risk Manag Healthc Policy. (2023) 16:2673–83. doi: 10.2147/RMHP.S435727
106. Chen M, Ma Y, Song YW, Huang J, Gao YH, Zheng J, et al. Survival outcomes of different neoadjuvant treatment regimens in patients with locally advanced rectal cancer and MRI-detected extramural venous invasion. Cancer Med. (2023) 12:20523–37. doi: 10.1002/cam4.6625
107. Alshuhri MS, Alduhyyim A, Al-Mubarak H, Alhulail AA, Alomair OI, Madkhali Y, et al. Investigating the feasibility of predicting KRAS status, tumor staging, and extramural venous invasion in colorectal cancer using inter-platform magnetic resonance imaging radiomic features. Diagnostics (Basel). (2023) 13. doi: 10.3390/diagnostics13233541
108. Chang L, Zhang X, He L, Ma Q, Fang T, Jiang C, et al. Prognostic value of ctDNA detection in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy: A systematic review and meta-analysis. Oncologist. (2023) 28:e1198–e208. doi: 10.1093/oncolo/oyad151
109. Yang Y, Zhang J, Zhang W, Wang Y, Zhai Y, Li Y, et al. A liquid biopsy signature of circulating extracellular vesicles-derived RNAs predicts response to first line chemotherapy in patients with metastatic colorectal cancer. Mol Cancer. (2023) 22:199. doi: 10.1186/s12943-023-01875-y
110. Xue K, Liu L, Liu Y, Guo Y, Zhu Y, Zhang M, et al. Radiomics model based on multi-sequence MR images for predicting preoperative immunoscore in rectal cancer. Radiol Med. (2022) 127(7):702–713. doi: 10.1007/s11547-022-01507-3
111. Jafri H, Mushtaq S, Baig S, Bhatty A, Siraj S. Comparison of KRAS gene in circulating tumor DNA levels vs histological grading of colorectal cancer patients through liquid biopsy. Saudi J Gastroenterol. (2023) 29:371–5. doi: 10.4103/sjg.sjg_85_23
112. Chiloiro G, Cusumano D, de Franco P, Lenkowicz J, Boldrini L, Carano D, et al. Does restaging MRI radiomics analysis improve pathological complete response prediction in rectal cancer patients? A prognostic model development. Radiol Med. (2022) 127(1):11–20. doi: 10.1007/s11547-021-01421-0
Keywords: computed tomography, CT, rectal cancer, superior hemorrhoidal vein, tumor diagnosis, prediction
Citation: Lucarelli NM, Mirabile A, Maggialetti N, Morelli C, Calbi R, Bartoli S, Avella P, Saccente D, Greco S and Ianora Stabile AA (2024) The role of superior hemorrhoidal vein ectasia in the preoperative staging of rectal cancer. Front. Oncol. 14:1356022. doi: 10.3389/fonc.2024.1356022
Received: 14 December 2023; Accepted: 09 July 2024;
Published: 05 August 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Fabrizio Urraro, University of Campania Luigi Vanvitelli, ItalyFederica Rubbino, Humanitas Research Hospital, Italy
Copyright © 2024 Lucarelli, Mirabile, Maggialetti, Morelli, Calbi, Bartoli, Avella, Saccente, Greco and Ianora Stabile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Morelli, ZG90dGNoaWFyYW1vcmVsbGlAZ21haWwuY29t
†These authors have contributed equally to this work
 Nicola Maria Lucarelli
Nicola Maria Lucarelli Alessandra Mirabile2†
Alessandra Mirabile2† Chiara Morelli
Chiara Morelli Simona Bartoli
Simona Bartoli Pasquale Avella
Pasquale Avella Sara Greco
Sara Greco