- 1Department of Ultrasound, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 4Department of Ultrasound, Meishan Women and Childrens’ Hospital, Alliance Hospital of West China Second University Hospital, Sichuan University, Meishan, Sichuan, China
Penile schwannoma is an uncommonly seen peripheral nerve tumor, of which penile plexiform schwannomas (PS) is extremely rare that has only been reported in several adults. We present a case of penile PS with a similar lesion in inguinal region in a 9-year-old child, which appeared as painless masses and rapidly growing within one year. Penile ultrasonography suggested well-defined lesions with limited vascularity. Both masses presented with low-to-intermediated signal intensity and no definite enhancement in computed tomography. The lesions were completely resected with minimal intraoperative bleeding, and a diagnose of benign PS was confirmed based on H&E staining and positive S-100 expression in immunohistochemistry. There was no evidence of tumor recurrence or metastasis after 6 months of follow-up. Only 6 cases of penile schwannoma in children were recorded, of which 5 were malignant, and none was PS. The malignancy rate of penile schwannoma in children may be overestimated due to delayed diagnose of benign ones. A rapidly growing penile mass with a suspected metastatic lesion in inguinal region could be easily misdiagnosed as malignant. This case report and literature review is expected to assist clinicians in getting a comprehensive understanding of children penile schwannomas and choosing the best management strategy when faced with this rare condition.
1 Introduction
Schwannoma, the most common peripheral nerve tumor in adults, is an encapsulated tumor composed of Schwann cells, with an incidence of 0.6 per 100,000 people annually (1). They may occur anywhere in the soft tissue but prefer the head, neck and limbs. Primary tumors of the penis, however, are extremely uncommon, with penile schwannomas even rarer, still. Based on histological features and growth patterns, schwannoma can be classified into the following types (2): classic schwannoma, cellular schwannoma, plexiform schwannoma (PS), ancient schwannoma, epithelioid cell schwannoma, and reticular/microcystic schwannoma. PS is a rare subtype, accounting for approximately 4.3% of schwannomas (3). Previously, only six cases of adult penile PS have been reported (4–9). Herein, we present a case of PS occurring in the penis and inguinal region simultaneously in a child. It is extraordinarily rare. Because of this, when a urologist or radiologist encounters such tumors in clinic, it is often difficult to make an accurate diagnosis. The current report presents a detailed description of the clinical course, imaging examination, surgery and pathology. We also compare the characteristics of penile schwannoma between children and adults based on a comprehensive literature review and summarize the features of penile PS previously reported.
2 Case presentation
A previously healthy 9-year-old boy presented with a palpable mass on his penis, which was only the size of a soybean when first discovered one year ago. Subsequently, the mass extended along the dorsal aspect of the entire penis, and another mass was palpable on the left inguinal area six months later. He did not experience any penile pain or urinary obstruction, and no family history of a genetic nerve sheath tumor was reported. Physical examination revealed no café-au-lait spots. A soft subcutaneous mass with moderate mobility, about 5.0 cm in length, was palpable at the base and dorsum of the penis. A similar 2.0 cm nodule appeared in the left inguinal region, without tenderness, but was hardly movable. Blood and urine tests were all within normal range.
Penile ultrasonography suggested a hypoechoic mass extending from the base to the dorsal side of the penis and measured 5.2 x 1.1 x 3.5 cm. The mass showed a clear boundary distinguishing it from the penile corpus cavernosum, with internal linear echogenic septa, slightly enhanced posterior echogenicity, and very limited vascularity inside. A well-defined homogeneous hypoechoic mass without notable septa was found in the left groin area (Figure 1). Both masses presented with low-to-intermediated signal intensity in further evaluation with pelvic computed tomography (CT). No definite enhancement was displayed on postcontrast images (Figure 2).
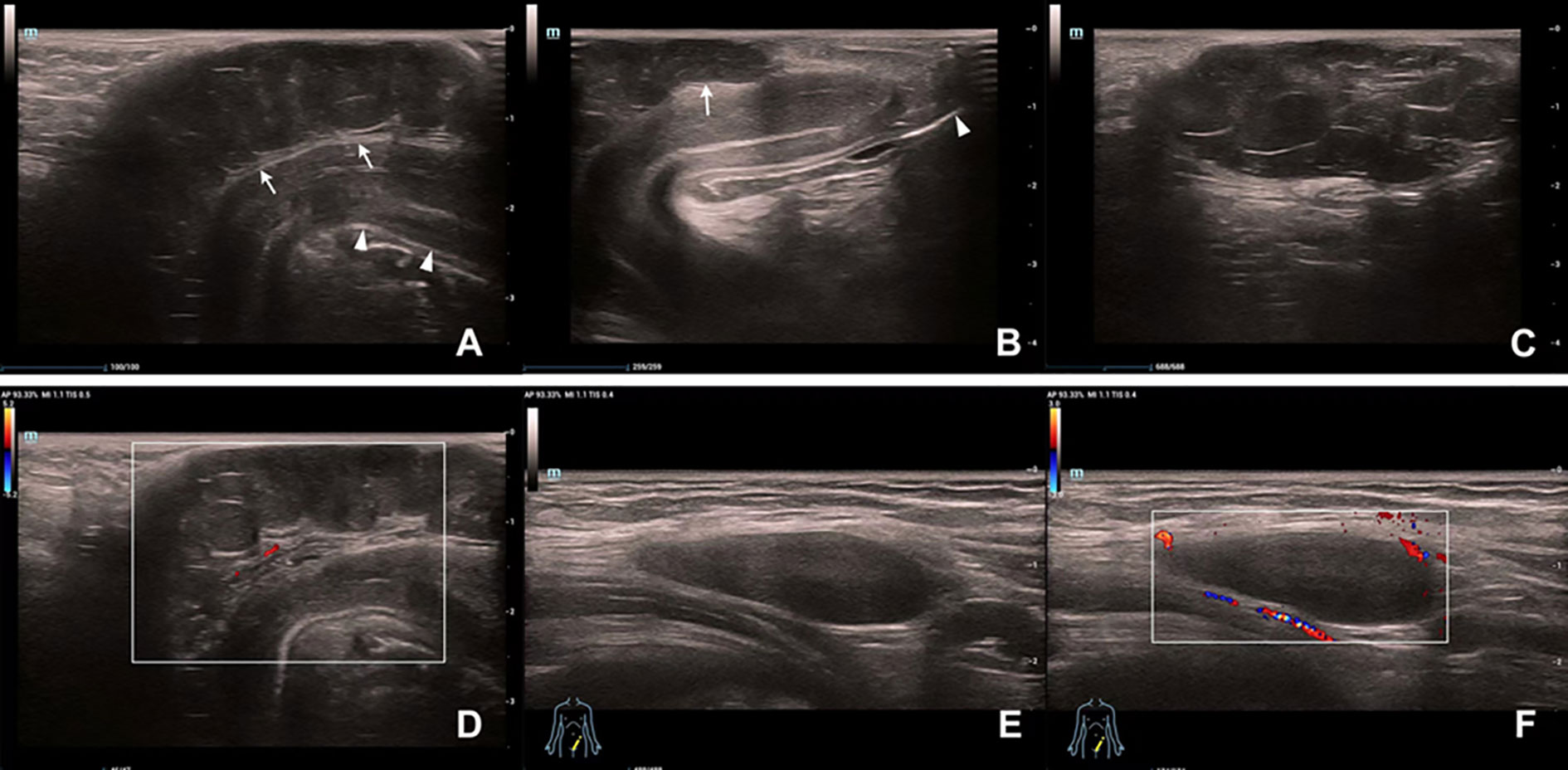
Figure 1 Ultrasound findings of the masses on the penis and in the left groin area. (A) A hypoechoic mass (arrow) with a hyperechoic capsule on the dorsal side of the penile shaft (arrowhead). (B) The distal part of the mass (arrow) does not involve the glans (arrowhead). (C) Posterior acoustic enhancement and multiple linear hyperechoic separations can be seen inside the penile mass. (D) Vascularity of the penile mass was not abundant on color Doppler flow evaluation. (E) A homogeneous hypoechoic mass in the groin area. (F) The inguinal mass showed hypovascularity on Color Doppler ultrasonography.
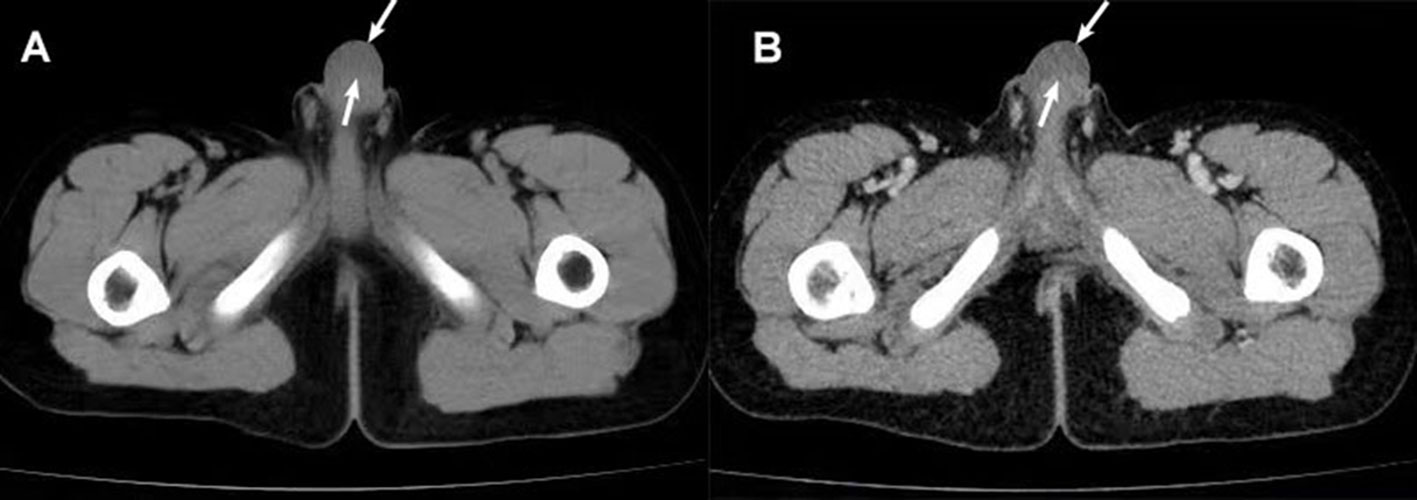
Figure 2 CT scan findings of the penile mass. (A) CT plain scan: a uniform low-density mass (arrow) was seen under the penile skin. (B) Enhanced CT: No definite enhancement of the mass (arrow) after enhancement.
Given the size of the tumor and its increasing trend, a surgical removal was planned. Resection of both masses was performed under general anesthesia. The penile mass was between deep and superficial fasciae, with intact capsule and clear margins, which made it easy to separate the mass from adjacent tissue. Beneath the left inguinal ligament, a similar mass was detected on the outer side of the femoral artery. Both lesions were spindle-shaped with yellowish surface and were completely resected with minimal intraoperative bleeding. Intraoperative frozen section pathological examination indicated a benign neural-origin tumor. Thus, no extended excision was performed. The appearance of the penis was not notably impacted postoperatively (Figure 3).
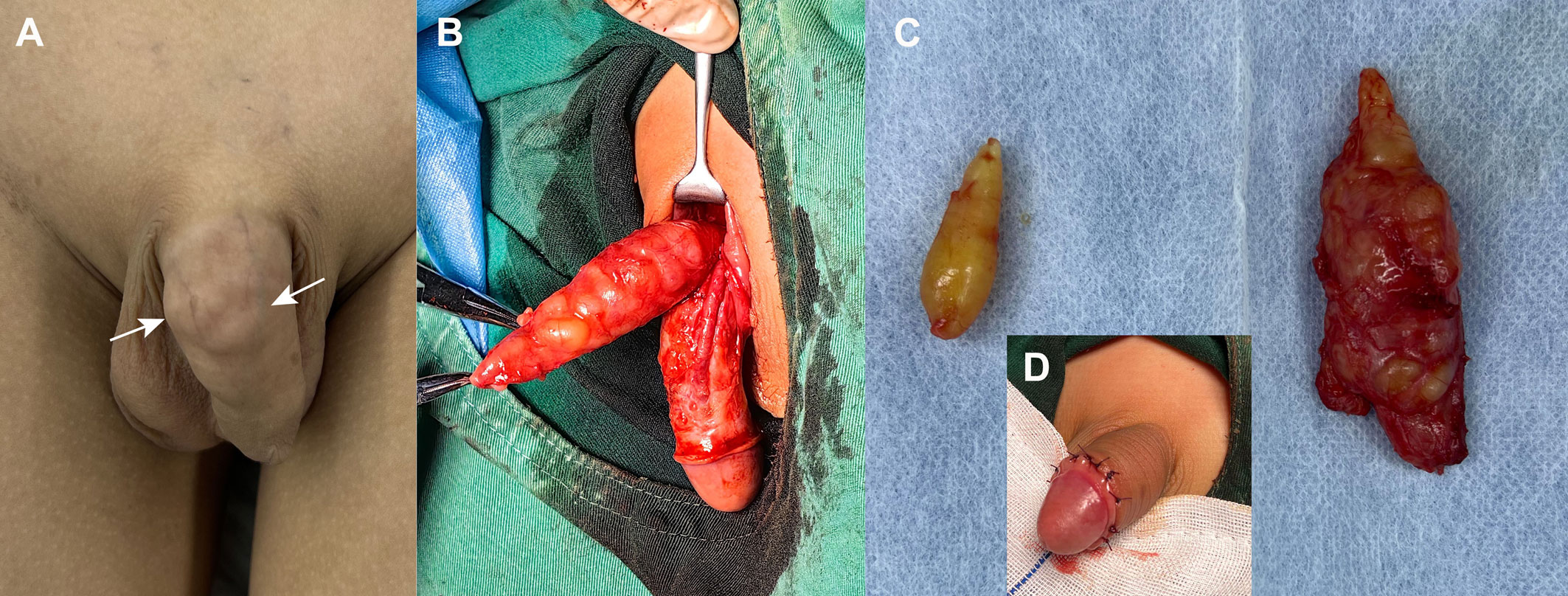
Figure 3 Intraoperative imagings of penile and inguinal schwannomas. (A) Grossly, palpable tissue mass was observed on the dorsum of the penis, near the base of the shaft, with a bumpy, multi-nodular appearance (arrow). (B) Intraoperative findings suggested that the yellow mass between the deep and superficial fascia on the dorsal side of the penis was multi-nodular and demarcated from the corpus cavernosum. (C) Final gross specimens of the penile mass (right) and inguinal mass (left) were all yellow nodular. (D) Postoperatively, the appearance of the penis was less affected.
Hematoxylin-eosin (H&E) staining (Figures 4A, B) showed that fibrous tissue divided the tumor into multiple nodules. A typical pattern of mixed Antoni A and Antoni B areas was demonstrated. The predominant distribution was Antoni A areas, characterized by spindle-shaped cells with palisading nuclei arranged in a fascicular or crisscross pattern. Immunohistochemistry staining demonstrated diffuse expression of S-100 (Figure 4C), mainly in Antoni A areas and primarily concentrated in the nucleus (Figure 4D). Both the penile and inguinal masses were diagnosed as plexiform schwannomas. There was no evidence of tumor recurrence or metastasis after 6 months of follow-up. The patient urinated well and the erectile function of penis was normal.
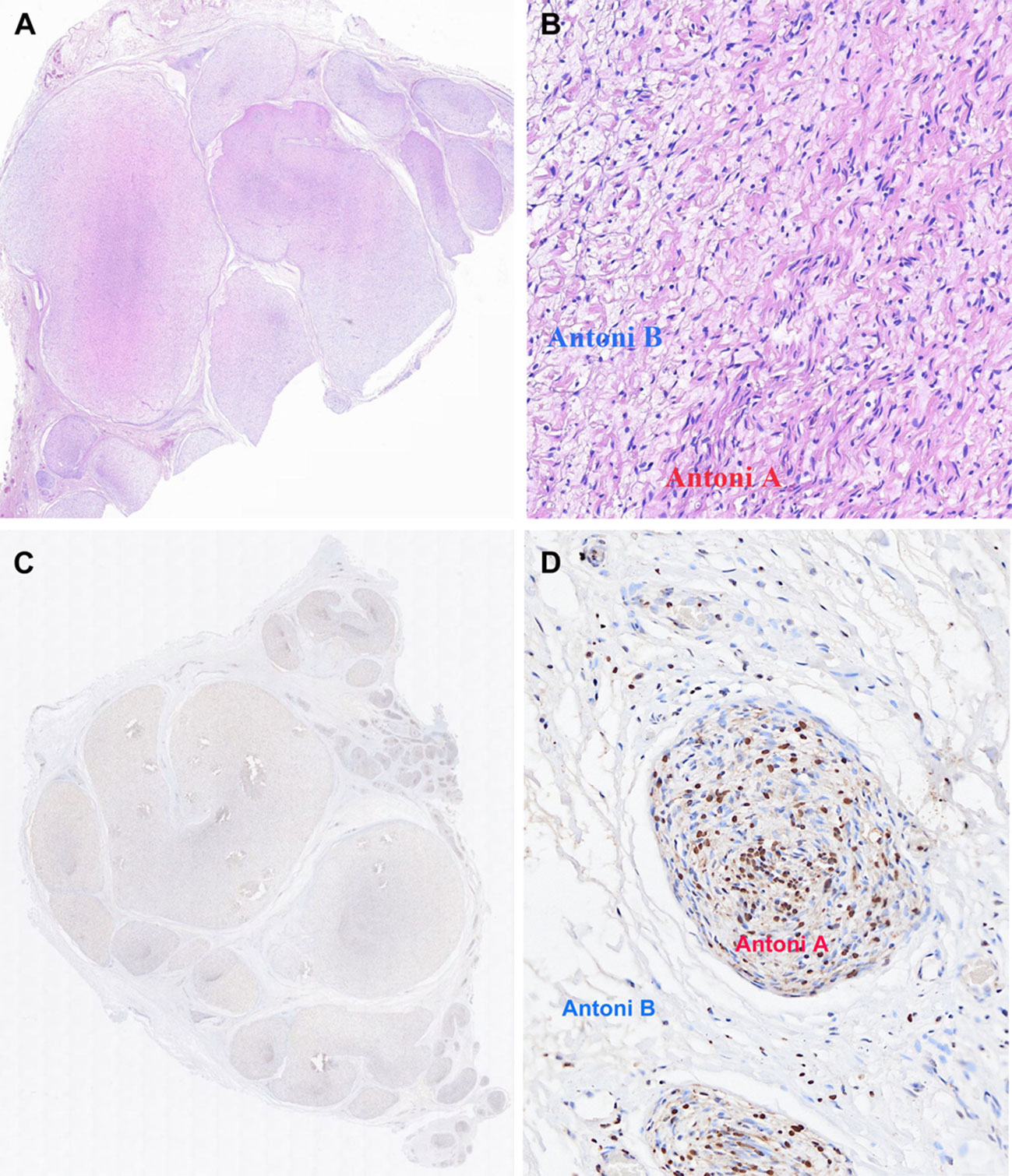
Figure 4 Final pathology of penile mass specimen excised during operation. Hematoxylin-eosin (H&E) stain: (A) The tumor was divided into multiple nodules by fibrous tissue (HE,×10). (B) Hypercellular Antoni A areas: compact spindle cells with no atypia or mitoses arranged in bundles or interlacing fascicles. Hypocellular Antoni B areas: spindle cells arranged haphazardly in a loose stroma. (HE, ×200). Immunohistochemistry stain: (C) Diffuse expression of S100. (D) S100 expression primarily concentrated in the nucleus of Antoni A areas.
3 Discussion
While the penis and perineum have abundant nerve innervation, schwannoma of penis is a rare condition, with only 47 cases reported. The majority of penile schwannomas are benign, slow-growing neoplasms, and the average patient age was 39.2 years (10). Malignant transformation of these cells is uncommon and only occurs in 9 cases. Penile schwannoma generally presents as a solitary and painless tumor that is most commonly on the dorsal shaft in children. While in adults, these tumors have caused pain, curvature erectile, sexual dysfunction and urinary tract obstruction (1).
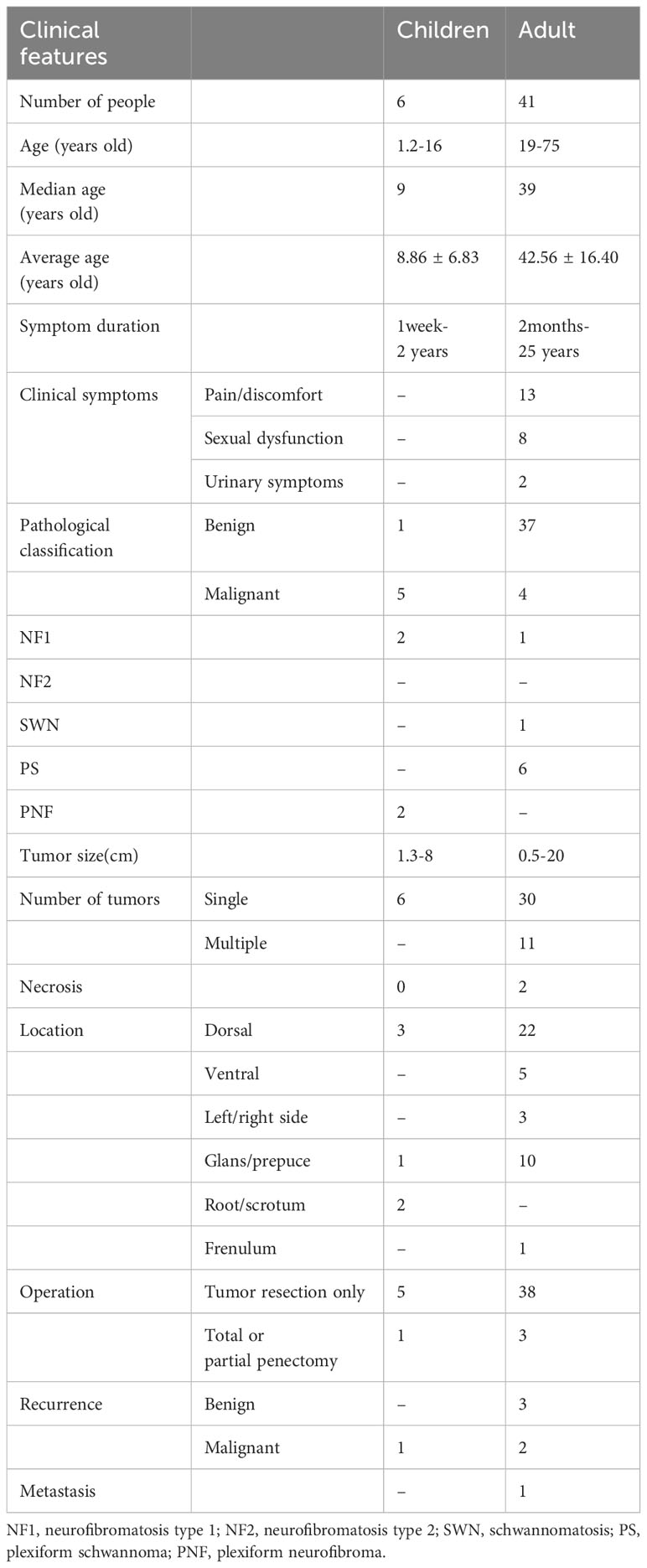
The incidence, growth rate and risk of malignant transformation may differ between pediatric and adult penile schwannomas. Table 1 shows the clinical characteristics of penile schwannomas in adults and children respectively. Penile schwannomas in children were all solitary mass with a shorter duration of illness (1 week-2 years) and a faster growth rate. In 6 children cases, 5 (83%) were diagnosed as malignant, of which 2 were associated with NF1 and 2 underwent malignant transformation from the plexiform neurofibroma (PNF). Only 1 with a malignant lesion developed a recurrence, and no distant metastases were observed. Most of the 41 adult cases had a long course (2 months-25 years) and presented with a single nodule (73.2%). 4 cases (9.8%) were graded as malignant, one of which was associated with NF1. One malignant case presented distant metastasis, and recurrence was observed in 3 benign and 2 malignant cases. Notably, the penile mass may have been present since childhood in some adult cases (4, 5, 11, 12). Delay in seeking medical assistance because of fear or shame of lesion of private parts, tolerable symptoms, lack of medical resources or health education, or tumor excision without pathological proof may result in underestimated incidence and overestimated malignancy rate of penile schwannoma in children.
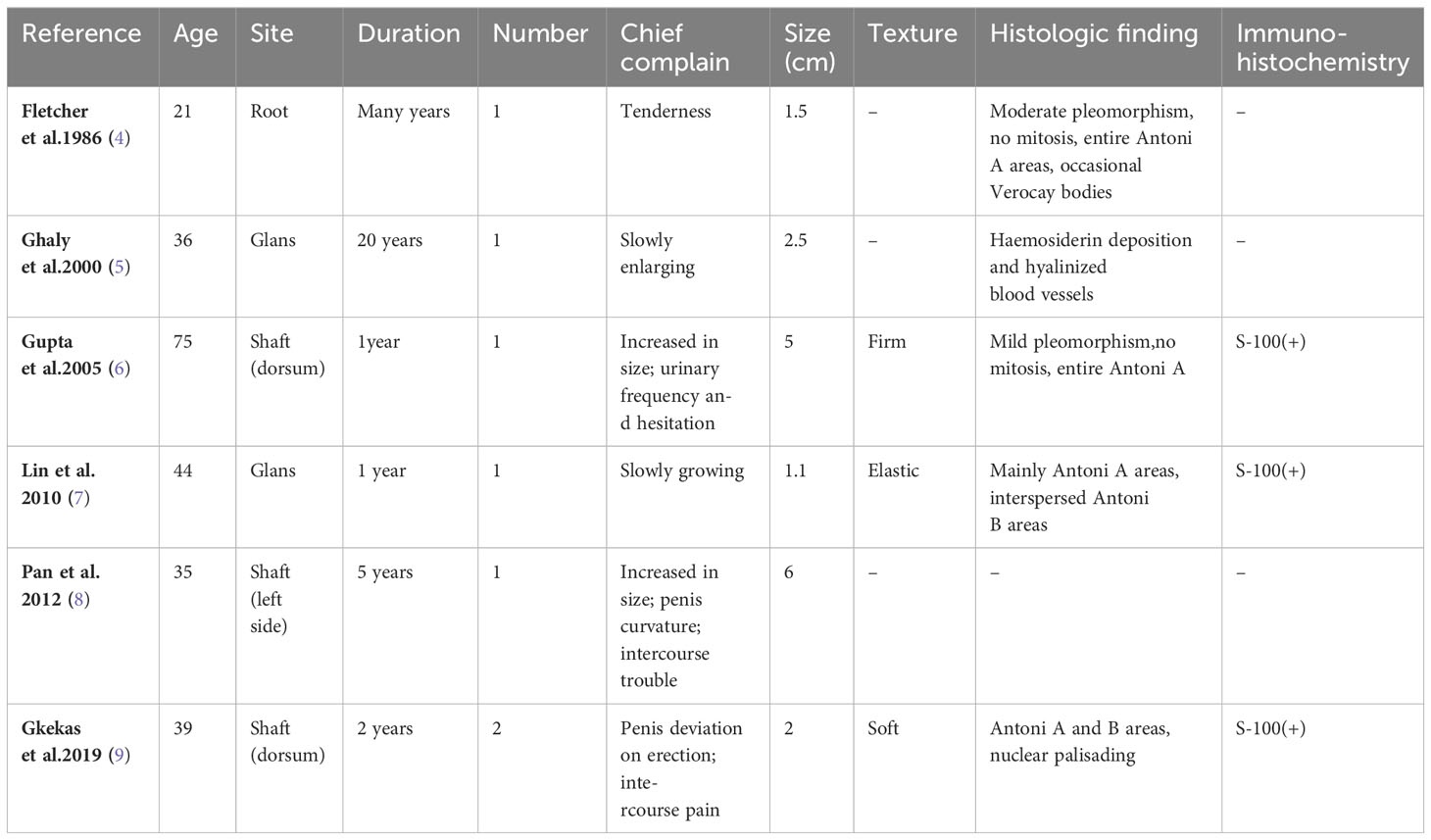
PS represents a rare variant of schwannoma and is characterized by a plexiform or multinodular growth pattern (6). Only 6 cases of penile PS, all adults, were recorded. (Table 2). The median age was 30 years (21-75 years), and the median mass size was 1.75 cm (1.1-6.0 cm). The most prevalent site is the penile shaft, which was found in 4 cases (66.7%) and all experienced varying degrees of pain or discomfort. In the remaining 2 cases, tumors appeared on the glans without apparent symptoms. Except for one case of glans penis, the course of other diseases is relatively short (1-5 years). PS is previously unreported in the penis in the pediatric age group. Our case delineates the first of which to occur in a patient this young with a tumor size greater than 5.0 cm in the penis. This lump rapidly grew to over 5cm within one year, accompanied by a similar lesion in the inguinal region, which could be easily misdiagnosed as a malignant tumor with inguinal metastasis.
Imaging studies can assist in diagnosing penile schwannomas, although their specificity is limited. Ultrasonography usually shows a well-demarcated and uniform hypoechoic mass with posterior acoustic enhancement (13). Cystic or necrotic degeneration is rarely seen (14). Hypervascularity may occur in 38% of penile schwannomas (15). Although without increased vascularity, the lesions did appear multiple internal hyperechoic lines in our case, which have never been reported. CT scans often reveal homogeneous low-density nodules with clear, smooth borders and a slightly higher-density peripheral capsule. MRI features include elliptical tumors with eccentric growth along nerve trunks, appearing as low to moderate signals on T1-weighted images and high signals on T2-weighted images (13). Preoperative fine-needle aspiration biopsy can preliminarily determine whether the mass is malignant, and sharp radiating pain during the biopsy can serve as a crucial clue to a nerve-derived tumor (6).
Typical schwannoma is characterized by a single nodule enveloped by a fibrous capsule. The nodule is mainly composed of spindle-shaped cells, often palisading, with densely cellular zone of Antoni A areas, and hypocellular Antoni B areas consisting of sparse spindle cells in myxoid stroma. PS presents as multiple nodules primarily composed of Antoni A areas. Immunohistochemically, they show similar diffusely strong expression of the neural marker protein S100. Penile PS in adults often demonstrates mild to moderate pleomorphism. In other locations, PS has abundant cell numbers, necrosis, increased nuclear pleomorphism, high mitotic activity and the possibility of local recurrence in children, but without metastatic potential (3). The higher mitotic activity and increased cellularity may be responsible for the rapid increase in mass size. Notably, the penile PS in the current report showed predominant nuclear distribution that differs from the diffuse and uniform expression of S100 in the cytoplasm and nucleus observed in some adult cases (1, 16). This disparity may be attributed to the variance between penile PS in children and adults, however, additional case studies are required to validate this conjecture.
Schwannomas are primarily isolated and sporadic tumors, while multifocality may be associated with schwannomatosis. Inactivating mutations in tumor suppressor genes, such as SMARCB1, LZTR1 and NF2, often give rise to schwannomatosis. A loss of function in the SMARCB1 and LZTR1 gene is also a causative factor in certain childhood tumors and syndromes that may affect intelligence, such as atypical teratoid/rhabdoid tumors, Coffin-Siris syndrome and Noonan syndrome (17). Out of 87 cases of head and neck PS reported by Berg JC, 9 were associated with a syndrome, of which 4 were children (3). Therefore, genetic syndromes or certain specific tumors should be considered promptly in children with multiple schwannomas or PS. There have been no reports of childhood penile PS. It is currently unclear whether penile PS behaves biologically like the head and neck PS.
PS should be identified with PNF. PNF is closely related to NF1 and has a 2%-5% risk of malignant transformation, which increases to 10%-30% when symptoms are present (17). The tumors consist of Schwann cells, fibroblasts, and perineural cells, without capsules or typical Antoni A/B regions. Immunohistochemistry shows weak positivity for S100. Furthermore, unlike penile PS, the aggressive growth of PNF can lead to arterial steal from the corpus cavernosum, directly causing erectile dysfunction (18).
Penile schwannomas are well-encapsulated lesions that generally attach eccentrically to nerves. Thus, they are easily removable without any appreciable nerve insult (16). PS have a higher local recurrence rate, possibly due to irregular and multifocal growth patterns, making it challenging to define clear tumor margins during excision, leading to inadequate removal (6). Close follow-up after resection is necessary to monitor penile functional recovery and tumor recurrence. Only 4 of 47 cases underwent total or partial penectomy. One case involved a 14-month-old child with a preoperative diagnosis of malignant schwannoma. The patient underwent complete penectomy, partial ureterectomy, perineal urethrostomy, partial scrotectomy, and testicular relocation, followed by radiation. Though the child survived, complex urogenital reconstruction and penile transplantation were required (19). When dealing with penile tumors in children, the decision to perform complete or partial penile excision should be made judiciously to avoid overtreatment. In cases of uncertainty, intraoperative frozen section biopsies can help determine the appropriate surgical extent.
This case report summarizes the features of penile PS that are difficult to detect in clinical practice and compares the characteristics of penile schwannoma between children and adults. However, it is based on a single case, which may not be representative, thereby limiting its clinical applicability. Collecting and summarizing more case series would provide a more comprehensive and systematic understanding of penile PS in children, benefiting a larger number of patients. Besides, the follow-up period was relatively short. We will continue to track and follow up, and if necessary, we will provide follow-up reports.
4 Conclusion
Penile schwannomas in children are rare and preoperative diagnosis can be challenging. We present the first case of pediatric penile PS accompanied by a similar lesion in the inguinal region, which was completely removed without causing nerve damage. Although the rate of malignancy may be overestimated, it should be noted that penile schwannomas in children may differ from those in adults, as they may grow faster and be bound up with genetic syndromes or certain specific tumors that affect intelligence. Care also should be taken to differentiate them from PNF, as the former is benign while the latter carries a high risk of malignant transformation. Comprehensive understanding of penile schwannomas in children is essential to make the correct diagnosis and determine the optimal management approach.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XQ: Writing – original draft, Writing – review & editing. YT: Writing – original draft. YF: Writing – original draft. DM: Writing – original draft, Formal analysis. LW: Writing – original draft, Data curation. HP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim SH, Ahn H, Kim KH, Kim DS, Yang HJ. Penile schwannoma mistaken for hemangioma: a rare case report and literature review. Transl Androl Urol. (2021) 10:2512–20. doi: 10.21037/tau-21-239
2. Magro G, Broggi G, Angelico G, Puzzo L, Vecchio GM, Virzì V, et al. Practical approach to histological diagnosis of peripheral nerve sheath tumors: an update. Diagnostics. (2022) 12:(6):1463. doi: 10.3390/diagnostics12061463
3. Berg JC, Scheithauer BW, Spinner RJ, Allen CM, Koutlas IG. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol. (2008) 39:633–40. doi: 10.1016/j.humpath.2007.10.029
4. Fletcher CDM, Davies SE. Benign plexiform (multinodular) schwannoma: a rare tumour unassociated with neurofibromatosis. Histopathology. (1986) 10:971–80. doi: 10.1111/j.1365-2559.1986.tb02595.x
5. Ghaly AF, Orange GV. Not every penile lump is a wart! Schwannoma of the penis. Int J STD AIDS. (2000) 11:199–200. doi: 10.1258/0956462001915543
6. Gupta SK, Singh S, Kumar CP, Marwah N, Gupta A. Fine needle aspiration cytology of a non-ulcerated penile lump. Cytopathology. (2005) 16:312–4. doi: 10.1111/j.1365-2303.2005.00231.x
7. Lin TC, Wu PY, Lin TY, Lee TL. An infrequent plexiform variant of schwannoma of the glans penis: a rare finding. Asian J Androl. (2010) 12:455–7. doi: 10.1038/aja.2009.96
8. Pan F, Li B, Kunwar KJ, Zhang Q, Xiao Y, Zeng F. Neuroimage: giant plexiform schwannoma of the penis. Eur Neurol. (2013) 69:118–8. doi: 10.1159/000342238
9. Gkekas C, Kalyvas V, Symeonidis EN, Malioris A, Papathanasiou M, Kalinderis N, et al. Plexiform schwannoma of the penis: A rare subtype of genital schwannoma. Case Rep Urol. (2019) 2019:1–4. doi: 10.1155/2019/1752314
10. Nguyen AH, Smith ML, Maranda EL, Punnen S. Clinical features and treatment of penile schwannoma: a systematic review. Clin Genitourin. Cancer. (2016) 14:198–202. doi: 10.1016/j.clgc.2015.12.018
11. Chan WP, Chiang SS, Huang AH, Lin CN. Penile frenulum neurilemoma: A rare and unusual genitourinary tract tumor. J Urol. (1990) 144:136–7. doi: 10.1016/S0022-5347(17)39394-1
12. Kumar GP, Sukumar S, Bhat SH, Nambiar A. Schwannoma of the penis: A common tumour at a rare site. Scand. J Urol Nephrol. (2006) 40:166–7. doi: 10.1080/00365590500499776
13. Song Z, Zhang Z, Xu S, Qiu Y, Ouyang J. Multiple penile schwannomas. Urol Case Rep. (2022) 43:102107. doi: 10.1016/j.eucr.2022.102107
14. Lee CH, Wu CJ, Chen YL, Huang GS, Tang SH. Multiple penile schwannomas and their magnetic resonance imaging characteristics. J Androl. (2012) 33:167–9. doi: 10.2164/jandrol.111.012997
15. Huang LC, Wang HZ, Chu YC, Ng KF, Chuang CK. Clinicopathological presentation and management of penile schwannoma. Sexual Med Rev. (2020) 8:615–21. doi: 10.1016/j.sxmr.2019.12.001
16. Mejri R, Dali KM, KaysChaker, Mokhtar B, Ben Rhouma S, Nouira Y. Isolated penile schwannoma: A rare case report. Urol Case Rep. (2022) 40:101866. doi: 10.1016/j.eucr.2021.101866
17. Schraepen C, Donkersloot P, Duyvendak W, Plazier M, Put E, Roosen G, et al. What to know about schwannomatosis: a literature review. Br J Neurosurg. (2022) 36:171–4. doi: 10.1080/02688697.2020.1836323
18. Issa B, Mansour E, Jabbour G, Chikhani C, Mansour H, Jabbour M. Plexiform penile neurofibroma: A case report of a rare entity in a pre-pubertal child. Urology. (2021) 156:124–6. doi: 10.1016/j.urology.2021.06.001
Keywords: schwannoma, penis, inguinal region, plexiform, children
Citation: Qi X, Tan Y, Feng Y, Ma D, Wang L and Pang H (2024) Case report and literature review: plexiform schwannoma in the penile and inguinal region in a child. Front. Oncol. 14:1356000. doi: 10.3389/fonc.2024.1356000
Received: 14 December 2023; Accepted: 30 January 2024;
Published: 01 March 2024.
Edited by:
Francesca Sanguedolce, University of Foggia, ItalyReviewed by:
Santosh Kumar Mondal, Kalyani (AIIMS Kalyani), IndiaHui Lu, Zhejiang University, China
Indranil Chakrabarti, Kalyani (AIIMS Kalyani), India
Copyright © 2024 Qi, Tan, Feng, Ma, Wang and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Houqing Pang, cGFuZ2hvdXFpbmc3NUAxNjMuY29t
 Xiaoying Qi
Xiaoying Qi Yifei Tan1,2
Yifei Tan1,2 Houqing Pang
Houqing Pang