- 1Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Department of Clinical Pharmacy and Pharmacy Administration, West China School of Pharmacy, Sichuan University, Chengdu, China
Background: The relationship between serum uric acid (SUA) levels and prostate cancer (PCa) remains controversial. This cross-sectional study investigated the association between SUA levels and PCa incidence.
Methods: A total of 9,776 participants aged ≥40 from the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2020 were included, 503 of whom had PCa. Weighted univariate logistic regression, multivariate logistic regression, and smooth-fitting curve analyses were used to analyze the association between SUA and PCa incidence. Concurrently, the fitted smoothing curves were used to explore the potential non-linear relationships. If non-linearity was observed, a recursive algorithm further calculated the inflection point.
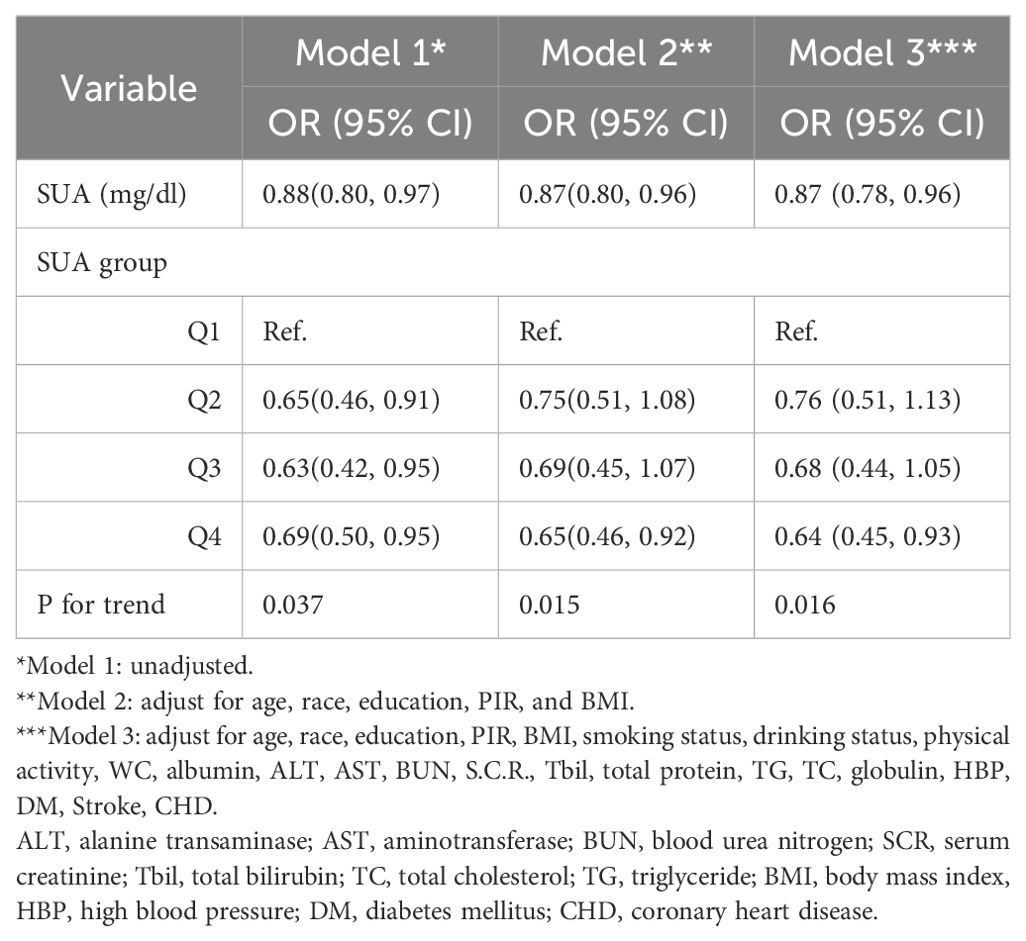
Results: Three models were used to analyze the correlation between SUA levels and PCa incidence. All regression models demonstrated a negative correlation between SUA levels and PCa incidence (model 1: OR = 0.88, 95% CI=0.80–0.97; model 2: OR = 0.87, 95% CI=0.80–0.96; model 3: OR = 0.87, 95% CI=0.78–0.96). According to the trend test, with increasing SUA, the risk of PCa showed a downward trend (three models P for trend = 0.037, 0.015, 0.016). According to the subgroup analysis, a significant negative correlation between SUA and PCa was detected in individuals aged >60 years, non-Hispanic whites, those of other races, and those with hypertension. Moreover, the association between SUA and PCa followed a U-shaped curve among participants without hypertension, and the inflection point of SUA was 5.1 mg/dl.
Conclusions: This cross-sectional study revealed a negative relationship between SUA levels and the risk of PCa, particularly in specific demographic groups. These findings offer a fresh perspective on the role of SUA in PCa patients, potentially paving the way for new approaches for the prevention and treatment of PCa. However, further studies are necessary to validate these findings.
1 Introduction
Prostate cancer (PCa) is one of the most common cancers in men and the fifth leading cause of cancer death globally, with an estimated 1,414,000 new cancer cases and 375,304 deaths in 2020. PCa is the most frequently diagnosed cancer in 112 countries and the leading cause of cancer death in 48 countries (1, 2). Therefore, the disease burden of PCa is expected to increase steadily due to the aging population and economic growth (3). To date, little is known about the causes of PCa. The established risk factors for PCa include age, family history, genetic mutations, and certain diseases (4). Thus, it is necessary to explore whether there are potentially unknown risk factors for PCa to prevent and control its occurrence and development.
Serum uric acid (SUA) is a product of purine metabolism degradation (5). The oxidation of xanthine and hypoxanthine produces SUA by xanthine oxidoreductase (XOR) (6, 7). Earlier studies have linked high SUA levels to several cardiovascular diseases and metabolic syndromes (8). Some recent findings suggest a correlation between SUA and cancer (9). High blood concentrations of SUA can lead to gout. Gout is a form of arthritis arising from SUA accumulation in the bloodstream (10) and is associated with an elevated risk for cancer overall. Gout-associated chronic inflammation, in turn, is associated with increased cancer risk. Another possible explanatory mechanism for the association between gout and carcinogenesis could be genetic instability induced by oxidative stress (11). However, in specific cancer types, the impact of SUA on PCa is still controversial (12, 13). One study showed low SUA levels and increased inflammatory markers; therefore, low SUA levels were risk factors for PCa (14). In contrast, the results of another study suggested that higher SUA levels increase PCa risk (4). Accordingly, we aimed to investigate the association between SUA levels and PCa incidence based on data from the US National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 Data sources and study population
A cross-sectional study method was applied in this study. The study data were collected from the 2007–2020 NHANES datasets (including data from seven 2-year circles). The NHANES is designed to assess health and nutritional status in the U.S. The ethics protocol for the NHANES was approved by The National Center for Health Statistics Research Ethics Review Board, and informed consent was obtained from all participants (15, 16). Weighted statistics were used in this study following National Center for Health Statistics (NCHS) guidelines, and the survey weights were recommended by the NHANES, which combines factors such as sampling probability, non-response adjustment, and post-hoc stratification to ensure the representativeness of the data (relevant instructions and formulas have been added to the manuscript, see https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx; the formula used is Weight=if sddsrvyr in (5,6,7,8,9,10) then MEC12YR = 1/6 * WTMEC2YR), where the weight analysis method helps to reflect the actual situation of the U.S. population more accurately.
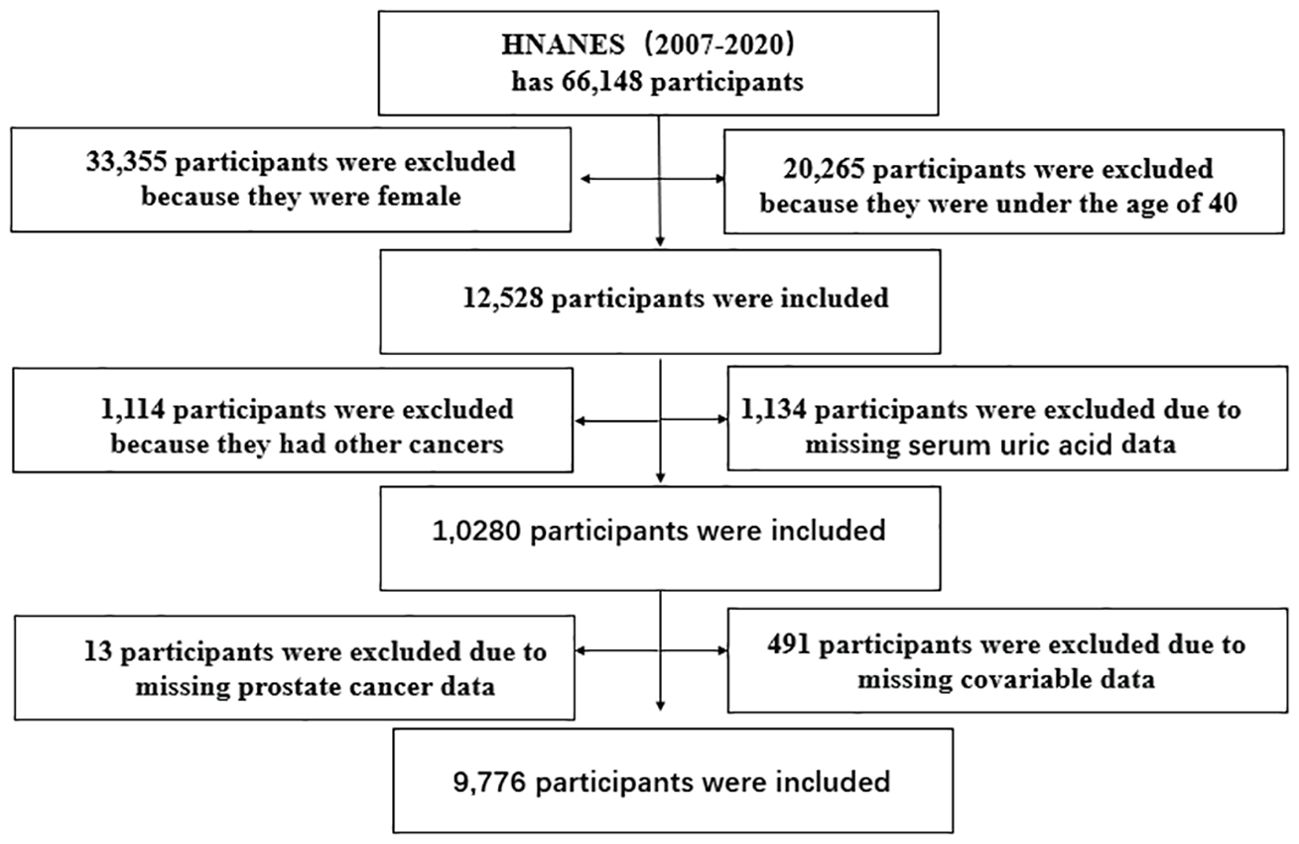
In our study, 66,148 participants were initially selected, and 9,776 men were eventually enrolled in the data analysis (503 patients with PCa); female participants, participants under 40 years old, and those suffering from other cancers were excluded, and those lacking PCa, SUA, and waist circumference (WC) data were excluded. The specific inclusion and exclusion criteria are shown in Figure 1.
2.2 Study variables
All the study variables were collected from well-trained health professionals. All study methods can be downloaded from the NHANES website. The experimental data and laboratory variables were measured following the technical standards issued by the NHANES. The laboratory program manual provided by the NHANES described the exposure variables SUA, mg/dl, and SUA detection methodology in detail.
2.2.1 Outcome variable
PCa incidence was defined according to the participants’ self-reports. When doctors or other health professionals were asked whether they had PCa or prostate malignancy, they responded “Yes”. Complete informed consent was obtained from all participants before the interview and inspection took place.
2.2.2 Covariates
Covariates were determined based on previous studies and our clinical experience and included population statistical data (such as age, race, education, and poverty–income ratio) (16–19) and human measurements (such as body mass index (BMI), WC, and blood pressure). Health-related behaviors (smoking, drinking, physical activity, diabetes mellitus (DM), hypertension, stroke, and coronary heart disease (CHD)). Biochemistry (albumin, ALT, AST, BUN, SCR, total bilirubin (Tbil), total protein, TG, globulin, and total cholesterol (TC)). The diagnostic criteria for diabetes mellitus included the following: (1) self-reported diabetes mellitus, (2) glycosylated hemoglobin ≥6.5%, (3) fasting blood glucose (F.D.G. ≥126 mg/dl), and (4) taking insulin or oral hypoglycemic drugs. The diagnostic criteria for hypertension included the following: (1) systolic blood pressure ≥130 mmHg or diastolic pressure ≥80 mmHg, (2) self-reported hypertension, and (3) blood pressure medication use (20, 21).
2.3 Statistical analyses
The statistical software R (version 4.2.0) and EmpowerStats were used to analyze all the study data. Continuous variables are presented as the means ± SDs (normally distributed data are represented by the median and interquartile range (IQR)). P values were calculated using a weighted linear regression model. The differences between groups for categorical variables were calculated by the chi-square test, with P<0.05. The Epidemiology Strobe guide used weighted single-variable and multivariable logistic regression to analyze the relationship between SUA levels and PCA (22).
Three regression models based on the different confounding factors were created in this study: model 1 (unadjusted model); model 2 (adjusted for age, race, education, poverty, income ratio, BMI adjustment); and model 3 (modified for age, race, education, PIR, BMI, smoking status, drinking status, physical activity, WC, albumin, ALT, AST, BUN, S.C.R., total protein, TG, total protein, globulin, HBP, DM, stroke, CHD).
Furthermore, smooth curve fitting and generalized additive models revealed a non-linear relationship between SUA levels and PCa risk. When non-linearity was detected, a recursive algorithm was applied to calculate the inflection point in the correlation between SUA levels and PCa risk (23).
3 Results
3.1 Characteristics of the population
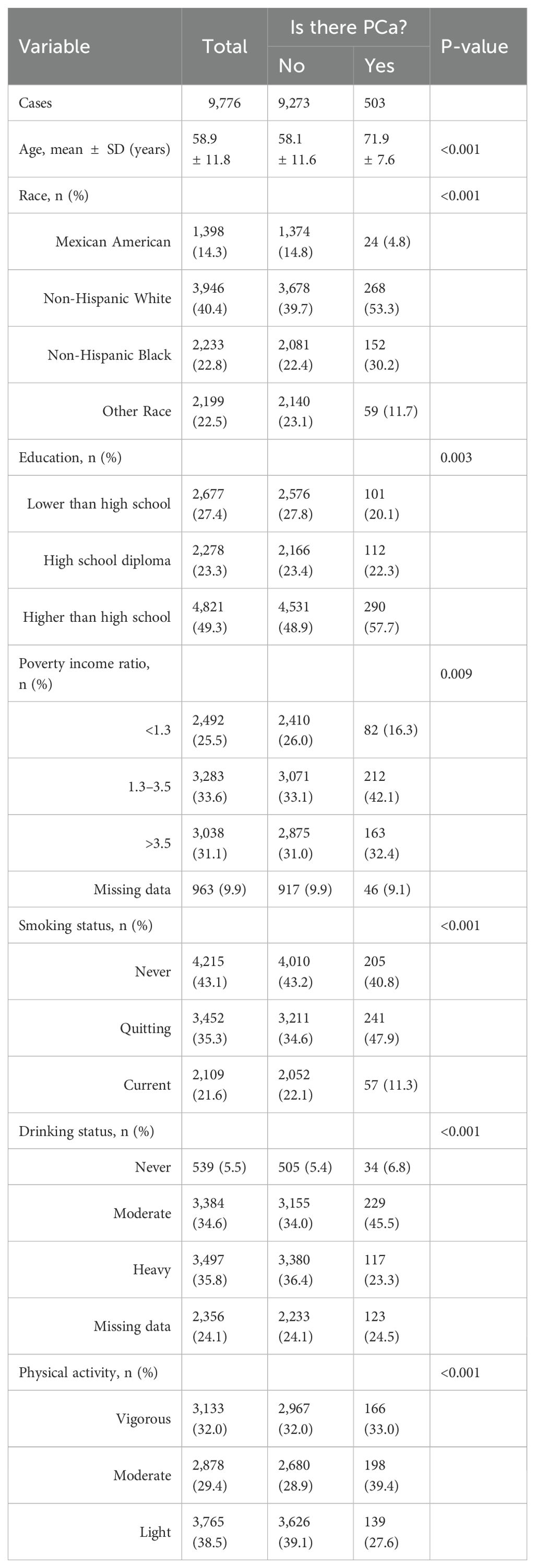
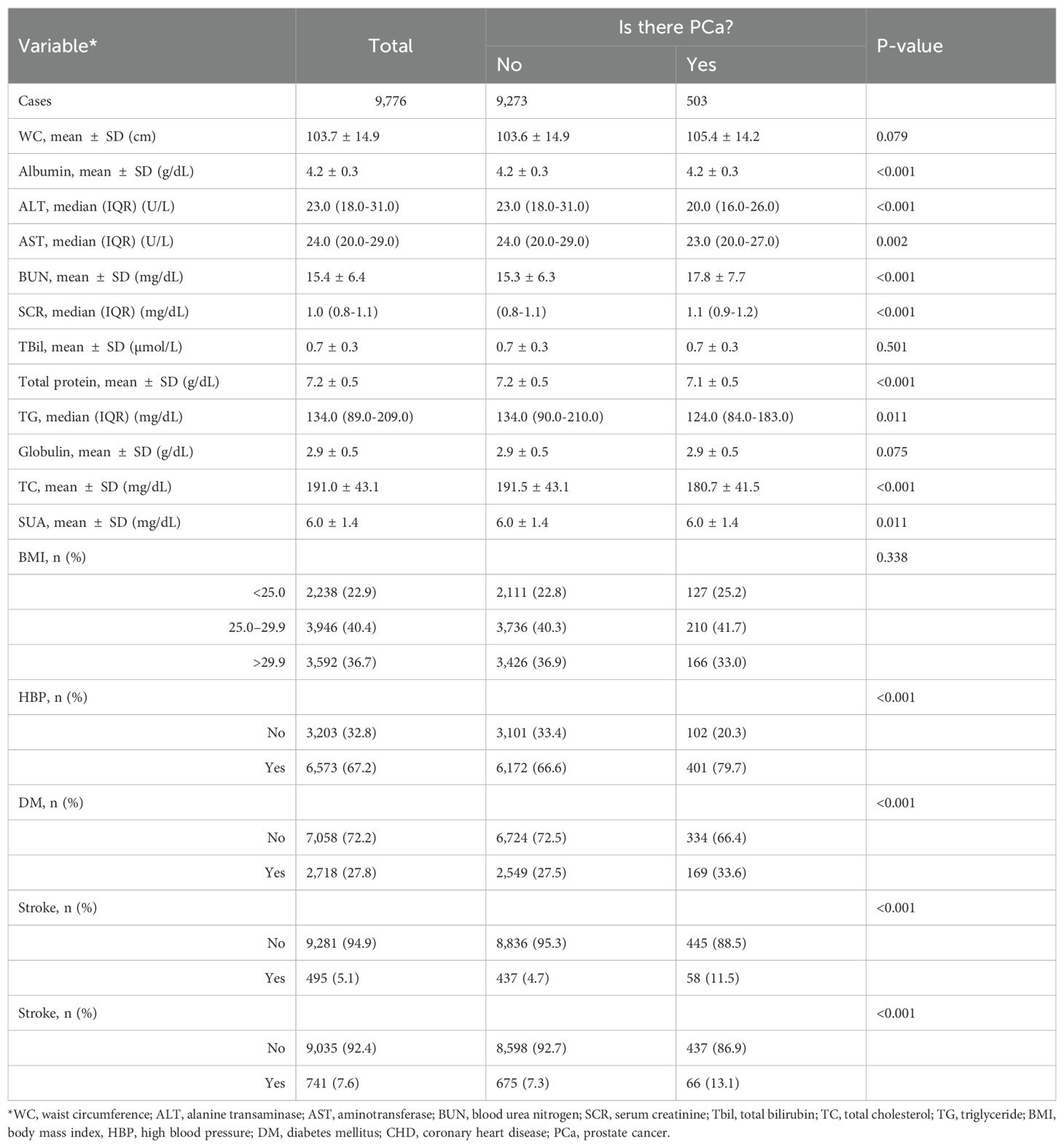
Our study included 9,776 participants; the average age was 58.9 ± 11.8 years. We divided all the participants into a healthy group (9,273 cases) and a PCa group (503 cases). Table 1 shows the basic characteristics of all participants included in this study and Table 2 shows the Healthcare diagnosis data for study participants for all participants. Then, we compared the differences in each variable between the two groups. The results of this study showed that age, race, education, poverty income ratio, smoking status, drinking status, physical activity, albumin, ALT, AST, BUN, SCR, total protein, globulin, SUA, HBP, DM, stroke, and stroke were significantly different between the two groups. WC, TBil, globulin, and BMI were not significantly different between the two groups (Tables 1, 2).
3.2 Univariate analysis of factors related to PCa
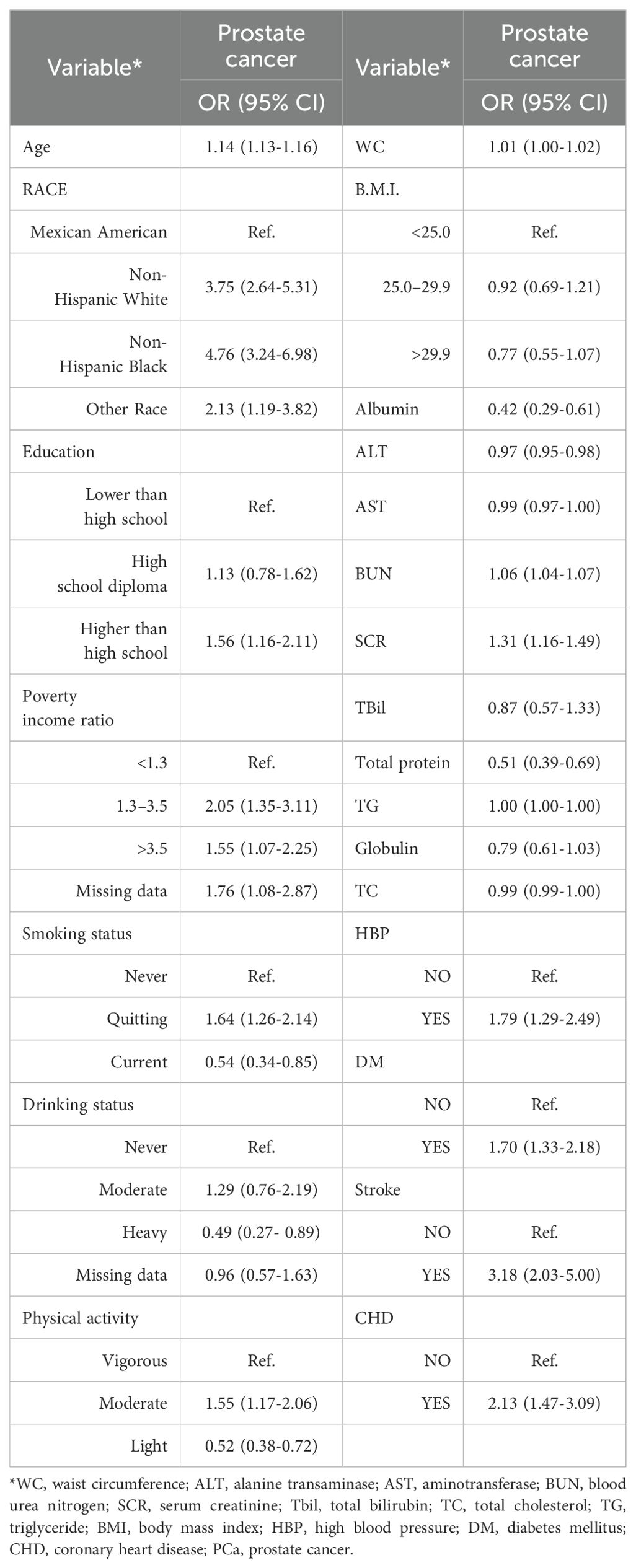
The weighted univariate analysis revealed that age, WC, BUN, SCR, HBP, DM, stroke status, and CHD status were positively associated with PCa incidence (risk factors). Albumin, ALT, and total protein were negatively correlated with PCa incidence and were protective factors. WC, BMI, AST, total bilirubin (Tbil globulin), and total cholesterol (TC) were not significantly different. However, there were different conclusions among the subgroups (Table 3).
3.3 Multivariate analysis of SUA and related factors in PCa patients
Table 4 lists the results of the three regression models. In model 1, SUA was negatively correlated with PCa incidence (OR = 0.88, 95% CI=0.80–0.97), and for each 1-mg/dl increase in SUA, the risk of PCa decreased by 12%. In model 2, SUA was negatively correlated with PCa incidence (OR = 0.87, 95% CI=0.80–0.96), and the risk of PCa decreased by 13% for every 1-mg/dl increase in SUA. In model 2, SUA was inversely associated with PCa incidence (OR = 0.87, 95% CI=0.78–0.96), with each 1-mg/dl increase in SUA associated with a 13% decrease in the risk of PCa.
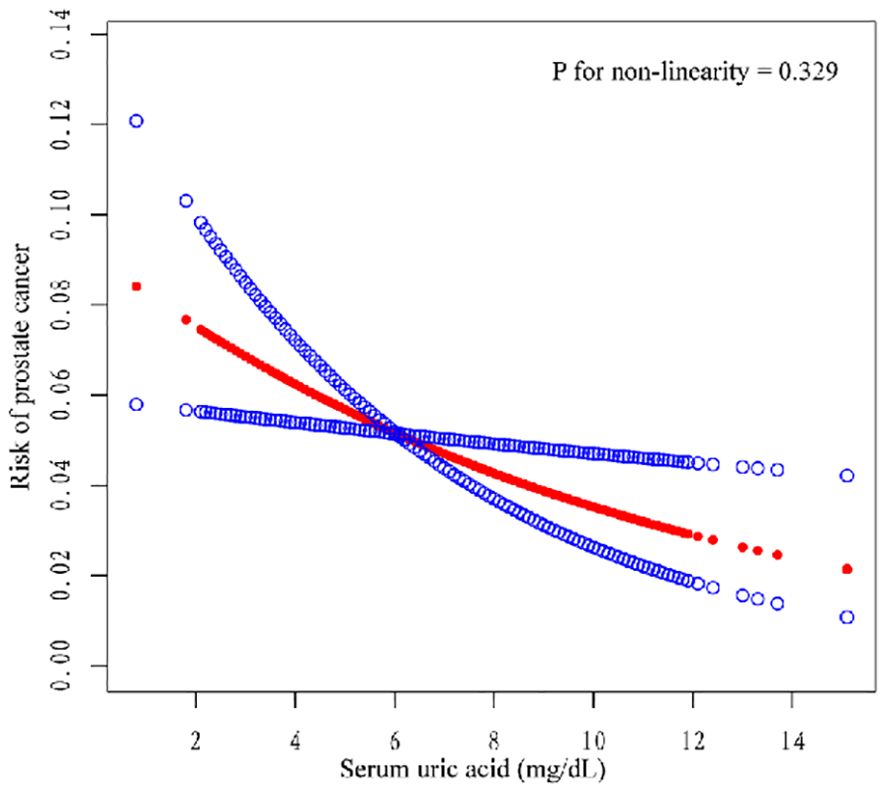
We also conducted subgroup analysis for all the models. In unadjusted model 1, compared with Q1, Q2, Q3, and Q4 were negatively correlated (OR = 0.65, 95% CI=0.46–0.91; OR = 0.63, 95% CI=0.42–0.95; OR = 0.69, 95% CI=0.50–0.95). In model 2, Q4 was negatively correlated with Q1 (OR= 0.65, 95% CI=0.46–0.92). In model 3, Q4 was negatively correlated with Q1 (OR= 0.64, 95% CI=0.45–0.93). The risk of PCA decreased with increasing SUA in all models, and this trend was consistent (three models P for trend = 0.037, 0.015, 0.016). Moreover, we constructed a smooth curve to observe the non-linear relationship between SUA and PCa (P for non-linearity=0.329) (Figure 2).
3.4 Subgroup analysis of the correlation between SUA and PCa incidence status
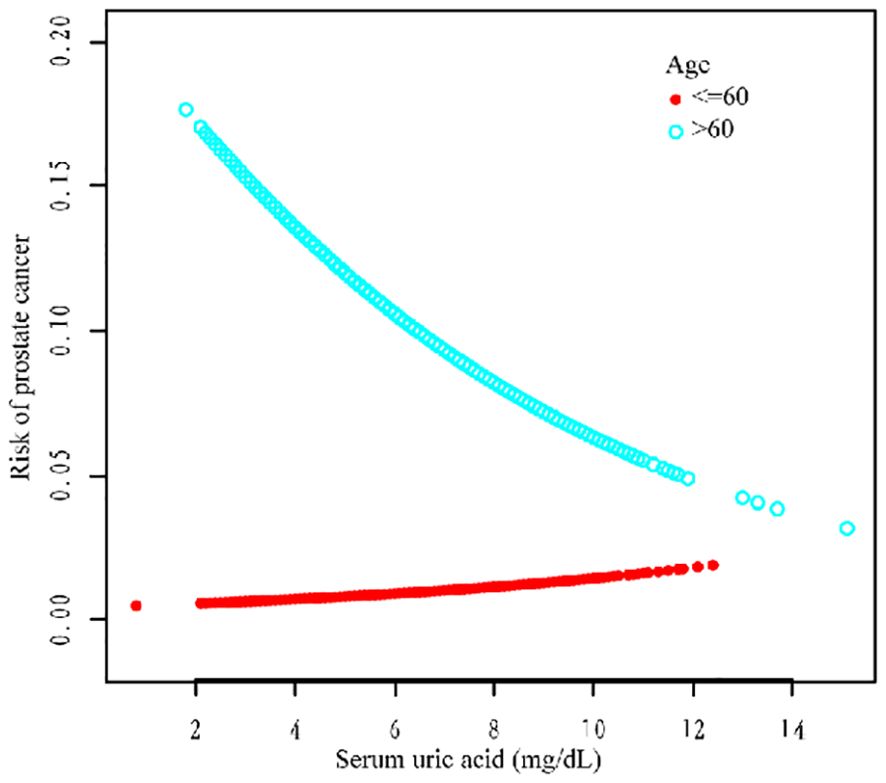
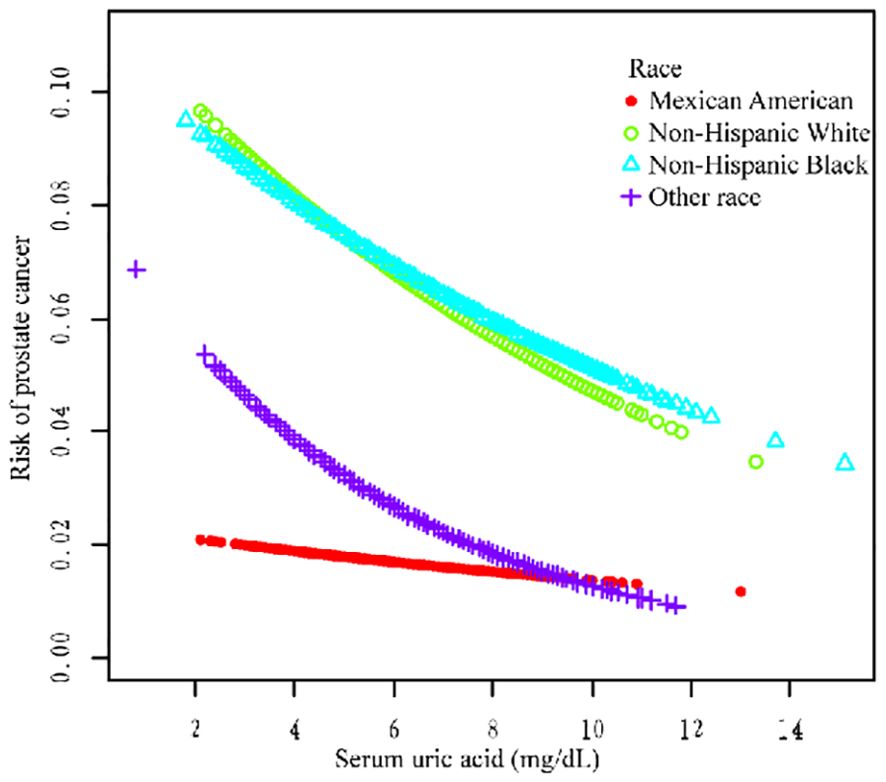
We performed a stratified subgroup analysis of age, race, and HBP to further analyze whether the relationship between SUA and PCa existed stably in the different subgroups (Table 5). In the >60-year age group, there was a negative correlation (OR = 0.87, 95% CI=0.80–0.95; OR = 0.87, 95% CI=0.79–0.95; OR = 0.85, 95% CI=0.76–0.94) in all three models. In the race group, non-Hispanic White ethnicity and other races were negatively correlated according to the three models (OR = 0.85, 95% CI=0.75–0.96; OR = 0.87, 95% CI=0.78–0.98; OR = 0.85, 95% CI=0.75–0.97; and OR = 0.76, 95% CI=0.59, 0.99; OR = 0.71, 95% Cl=0.54, 0.93; OR = 0.69, 95% Cl=0.54, 0.90). In the HBP group, hypertension was negatively correlated with hypertension according to all the models (OR = 0.84, 95% CI=0.76–0.93; OR = 0.86, 95% CI=0.78–0.95; OR = 0.87, 95% CI=0.77–0.98) (Table 5 for details).
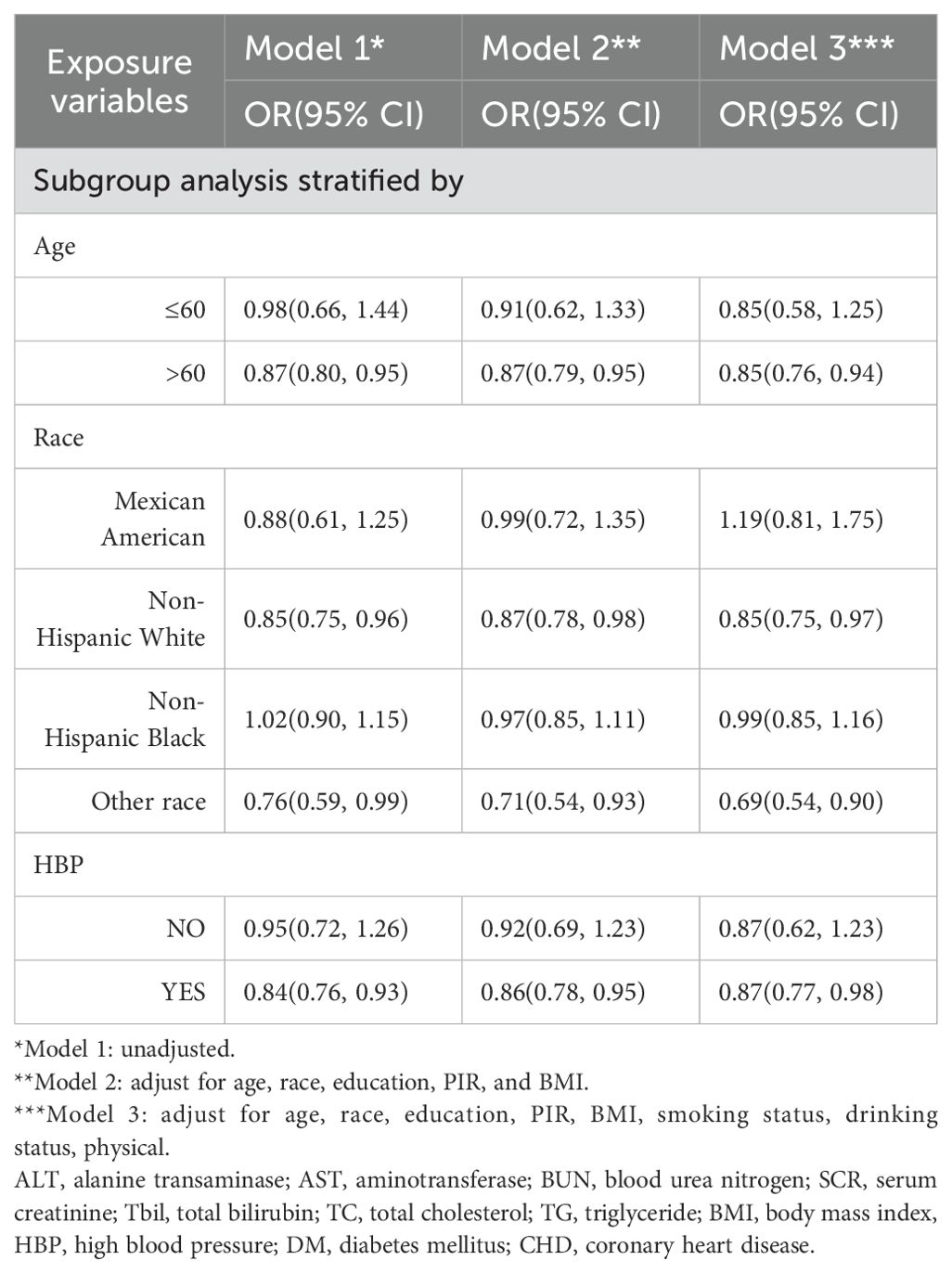
Table 5. The relationship between SUA and PCa, stratified by age, race, and high blood pressure, was weighted.
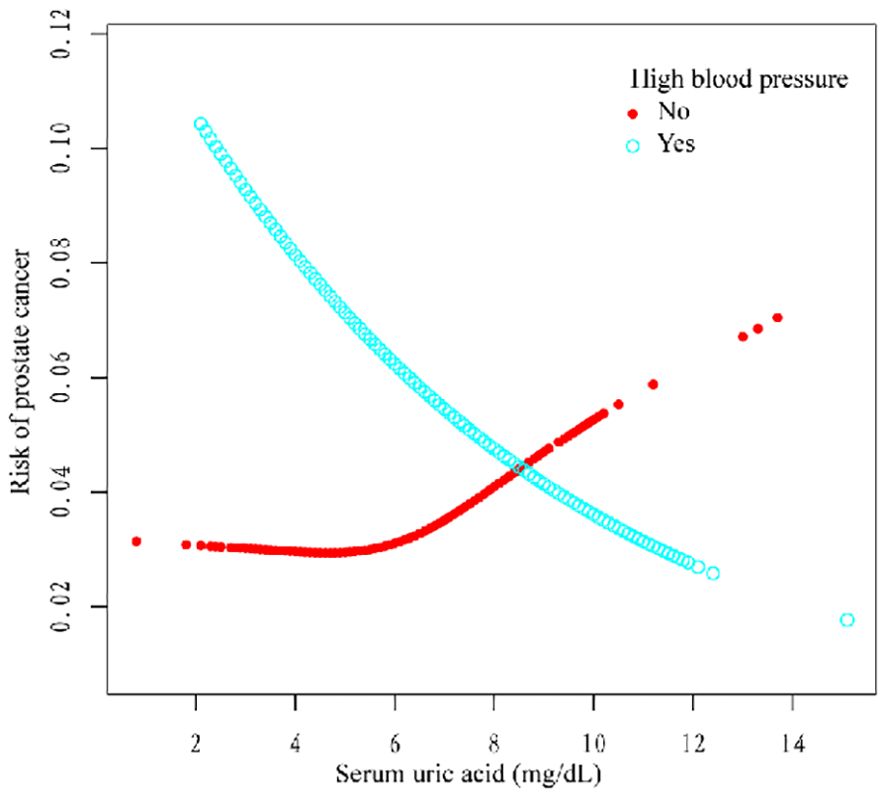
Additionally, we used a smoothing curve to explore the potential non-linear relationships among age, race, and HBP (Figures 3–5). The results showed that the association between SUA and PCa followed a U-shaped curve among participants without hypertension, and the inflection point of SUA was 5.1 mg/dl (Table 6).
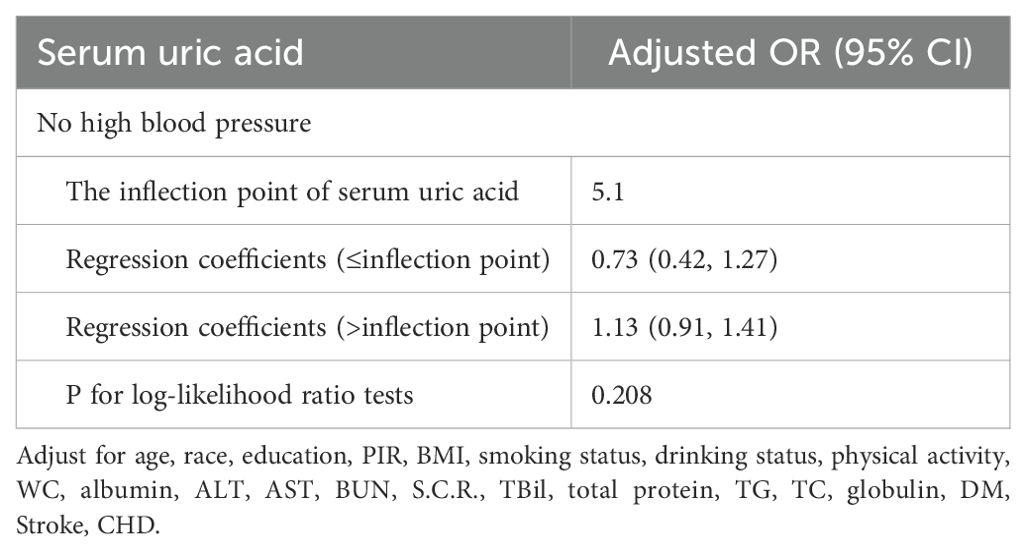
Table 6. The results of a two-piecewise linear regression model between serum uric acid and prostate cancer.
4 Discussion
A negative relationship between SUA level and the risk of PCa was found in this cross-sectional study, particularly in the age >60 years, non-Hispanic white, other race, and hypertension subgroups. In addition, the correlation between SUA and PCa followed a U-shaped curve among the groups without hypertension, and the inflection point of SUA was 5.1 mg/dl. This study was the first to investigate the relationship between SUA and PCa based on data from the NHANES. SUA is a protective factor in PCa patients, and with increasing SUA levels, the risk of PCa decreases.
At present, the relationship between SUA and PCa is still controversial. Several studies have reported that SUA protects against cancer by increasing antioxidant capacity (9). Nevertheless, other study results contradict the proposed antioxidant and protective effects of SUA against cancer and suggest that high SUA concentrations are associated with outcomes, possibly reflecting more serious prognostic indications (24). Another population-based cohort study revealed a J-shaped relationship between the baseline UA level and PCa-related mortality risk (25). There are two possible explanations for these different conclusions. First, some preliminary research has missed potential variables that may result in different outcomes. However, this study included mostly common risk factors for PCa as covariates to increase the accuracy of the results. Second, some studies included only a few PCa patients, which may have led to the lack of relevance. This study uses a weighted analysis method to represent the data of American men aged more than 40 years from 2007 to 2020. Therefore, a larger sample size was included to support the conclusions of this study. Therefore, the conclusions of this study have additional credibility.
For further analysis, we also conducted a subgroup analysis. The results of the subgroup analysis showed that SUA and PCa had a significant negative association in the age >60, non-Hispanic white, other race, and hypertension subgroups. Moreover, the association between SUA and PCa followed a U-shaped curve among participants without hypertension, and the inflection point of SUA was 5.1 mg/dl. Several studies have shown a correlation between SUA and high blood pressure (26). Hyperuricemia (an SUA level greater than 6.8 mg/dl) was associated with an increased risk of uncontrolled hypertension and resistance to antihypertensive therapy (27–29). However, the relationship between hypertension and PCa still needs further exploration.
Something that needs special clarification: The SUA level is not as high as possible; hyperuricemia can also damage health. Therefore, this study’s conclusions may apply to protecting against PCa at a higher uric acid concentration in the normal range, especially among specific people, such as those aged >60 years, non-Hispanic whites, other races, and those with hypertension. However, additional studies need to be conducted to verify these conclusions.
4.1 Potential mechanism
SUA may act as an antioxidant by scavenging reactive oxygen species (ROS) (29). ROS scavenging can reduce the oxidative stress-induced apoptosis of cancer cells, thereby promoting their growth and survival (30). One study reported that SUA plays a major role in stimulating immune cells (31). This effect of SUA may be due to its protective effect against malignancy. In brief, a reduction in SUA levels may be related to cancer, on the one hand, through reduced antioxidant capacity and, on the other hand, through suppression of the immune system. These studies all support the conclusion that SUA is a protective factor against cancer, which is also consistent with the conclusion of this study.
4.2 Strength of this study
Compared with previous studies, this study has the following advantages. First, this study actively explored the potential cause of PCA and investigated the relationship between SUA levels and PCA. Second, the data used in this study were obtained from the NHANES database. The data come from the real world and have the characteristics of standardization and large sample sizes. In addition, this study adjusted for different mixed factors, and a multi-regression analysis model was established for the data analysis to increase the accuracy of the results.
4.3 Limitations of this study
There are several limitations to this study. First, this study used a cross-sectional survey design. Therefore, causality cannot be inferred, and further prospective research should be conducted based on the findings of this study. Second, this study data came from the U.S. database, and whether the conclusions can be applied to other countries and regions requires additional research. Moreover, PCa data in this study were collected via self-reports rather than laboratory examinations; thus, there was some recall bias. Finally, although we adjusted for several potential influencing factors, some factors that were not considered may still impact the results.
5 Conclusion
PCa is one of the most common cancers worldwide and accounts for many cancer-related deaths. This study investigated the possible protective factors of PCa. The results of this study reveal one possibility: SUA can serve as an oxidant and has a certain potential protective effect on PCa, especially in some populations. However, additional basic research and prospective studies are needed to verify this conclusion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the NCHS Research Ethics Review Board((https://www.cdc.gov/nchs/nhanes/irba98.htm). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. HL: Data curation, Methodology, Resources, Software, Writing – original draft. ZH: Methodology, Supervision, Writing – original draft. LW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The National Key Clinical Specialties Construction Program supported this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AST, aminotransferase; BUN, blood urea nitrogen; SCR, serum creatinine; TBil, total bilirubin; TC, total cholesterol; TG, triglyceride; BMI, body mass index; HBP, high blood pressure; DM, diabetes mellitus.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Wang L, Lu B, He M, Wang Y, Wang Z, Du L, et al. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health. (2022) 10:811044. doi: 10.3389/fpubh.2022.811044
3. Wang L, Lu B, He M, Wang Y, Wang Z, Du L, et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. (2020) 77:38–52. doi: 10.1016/j.eururo.2019.08.005
4. Deng Y, Huang J, Sang Wong MC. Association between serum uric acid and prostate cancer risk in East Asian populations: a Mendelian randomization study. Eur J Nutr. (2023) 62:1323–9. doi: 10.1007/s00394-022-03076-7
5. Jiang M, Ren L, Chen S, Li G. Serum uric acid levels and risk of eight site-specific cancers: a Mendelian randomization study. Front Genet. (2021) 12:608311. doi: 10.3389/fgene.2021.608311
6. Hille R. Molybdenum-containing hydroxylases. Arch Biochem Biophys. (2005) 433:107–16. doi: 10.1016/j.abb.2004.08.012
7. Wu L, Wu JT. Serum uric acid is a marker of inflammation and a marker predicting the risk of developing CVD, stroke, renal failure and cancer. J BioMed Lab Sci. (2007) 20:1–2.
8. Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney Injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. (2023) 15:175–81. doi: 10.1007/s11906-013-0344-5
9. Taghizadeh N, Vonk JM, Boezen HM. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control. (2014) 25:1075–80. doi: 10.1007/s10552-014-0408-0
10. Ganz M, Alessandro C, Jacobs M, Miller D, Gejerman Y, Okoye F, et al. Investigating the anti-inflammatory effect of allopurinol on the prevention of prostate cancer. Cureus. (2023) 15:e40058. doi: 10.7759/cureus.40058
11. Kukko V, Kaipia A, Talala K, Taari K, Tammela TLJ, Auvinen A, et al. Allopurinol and prostate cancer survival in a Finnish population-based cohort. Prostate Cancer Prostatic Dis. (2024) 27:73–80. doi: 10.1038/s41391-022-00597-4
12. Sangkop F, Singh G, Rodrigues E, Gold E, Bahn A. Uric acid: A modulator of prostate cells and activin sensitivity. Mol Cell Biochem. (2016) 414:187–99. doi: 10.1007/s11010-016-2671-8
13. Siroosbakht S, Rezakhaniha S, Namdari F, Rezakhaniha B. Is there a relationship between serum uric acid levels and lower urinary tract symptoms, prostate volume, and PSA in men without cancer? A prospective population-based study. Andrologia. (2021) 53:e14200. doi: 10.1111/and.v53.10
14. Benli E, Cirakoglu A, Ayyıldız SN, Yüce A. Comparison of serum uric acid levels between prostate cancer patients and a control group. Cent Eur J Urol. (2018) 71:242–7. doi: 10.5173/ceju.2018.1619
15. Gong R, Pu X, Cheng Z, Ding J, Chen Z, Wang Y, et al. The association between serum cadmium and diabetes in the general population: A cross-sectional study from NHANES(1999–2020). Front.Nutr. (2020) 9:966500. doi: 10.3389/fnut.2022.966500
16. Xu K, Yan Y, Cheng C, Li S, Liao Y, Zeng J, et al. The relationship between serum albumin and prostate-specific antigen: A analysis of the National Health and Nutrition Examination Survey, 2003-2010. Front Public Health. (2023) 11:1078280. doi: 10.3389/fpubh.2023.1078280
17. Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National health and nutrition examination survey, 2015-2018: sample design and estimation procedures. Vital Health Stat. (2020) 184):1–35.
18. Wang J, Liu F, Kong R, Han X. Association between globulin and diabetic nephropathy in type2 diabetes mellitus patients: A cross-sectional study. Front Endocrinol (Lausanne). (2022) 13:890273. doi: 10.3389/fendo.2022.890273
19. Tan M-Y, Mo C-Y, Li F. The association between serum uric acid and hypertriglyceridemia: evidence from the national health and nutrition examination survey (2007-2018). Front Endocrinol (Lausanne). (2023) 14:1215521. doi: 10.3389/fendo.2023.1215521
20. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Himmelfarb CD, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. (2018) 71:1269–324. doi: 10.1161/HYP.0000000000000066
21. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15. doi: 10.2337/dc21-S002
22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
23. Hou X-z, Liu E-q, Liu S-q, Lv H, Cui H-f, Han J, et al. The negative association between serum albumin levels and coronary heart disease risk in adults over 45 years old: a cross−sectional survey. Sci Rep. (2023) 13:672. doi: 10.1038/s41598-023-27974-w
24. Strasak AM, Rapp K, Hilbe W, Oberaigner W, Ruttmann E, Concin H, et al. VHM&PP Study Group The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. (2007) 18:1893–7. doi: 10.1093/annonc/mdm338
25. Lee YHA, Chan JSK, Leung CH, Hui JMH, Dee EC, Ng K, et al. Association between serum uric acid and prostate cancer mortality in androgen deprivation therapy: A population-based cohort study. Cancer Med. (2023) 12:17056–60. doi: 10.1002/cam4.v12.16
26. Wang S, Shu Z, Tao Q, Yu C, Zhan S, Li L, et al. Uric acid and incident chronic kidney disease in a large health check-up population in Taiwan. Nephrol (Carlton). (2011) 16:767–76. doi: 10.1111/j.1440-1797.2011.01513.x
27. Cho J, Kim C, Kang DR, Park JB. Hyperuricemia and uncontrolled hypertension in treated hypertensive patients: k-MetS Study. Med (Baltimore). (2016) 95:e4177. doi: 10.1097/MD.0000000000004177
28. Cicero AFG, Rosticci M, Fogacci F, Grandi E, D'Addato S, Borghi C, et al. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med. (2017) 37:38–42. doi: 10.1016/j.ejim.2016.07.026
29. Borghi C, Tubach F, De Backer G, Dallongeville J, Guallar E, Medina J, et al. Lack of control of hypertension in primary cardiovascular disease prevention in Europe: results from the EURIKA study. Int J Cardiol. (2016) 218:83–8. doi: 10.1016/j.ijcard.2016.05.044
30. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. (2017) 18:567–79. doi: 10.1016/j.jnutbio.2006.10.007
Keywords: prostate cancer, serum uric acid, curve fitting, cross-sectional study, logistic regression, NHANES
Citation: Yan Y, Lin H, He Z and Wang L (2024) Serum uric acid and prostate cancer: findings from the NHANES (2007–2020). Front. Oncol. 14:1354235. doi: 10.3389/fonc.2024.1354235
Received: 20 January 2024; Accepted: 06 September 2024;
Published: 25 October 2024.
Edited by:
Ali Ata Moazzami, Swedish University of Agricultural Sciences, SwedenReviewed by:
Hong Weng, Wuhan University, ChinaMauricio Rodriguez-Dorantes, National Institute of Genomic Medicine (INMEGEN), Mexico
Paulina Nastaly, Medical University of Gdansk, Poland
Hong-Yue Lai, China Medical University, Taiwan
Mansoor-Ali Vaali-Mohammed, King Saud University, Saudi Arabia
Ruijie Xie, University of South China, China
Copyright © 2024 Yan, Lin, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Wang, d2xpbl9zY3VAc2N1LmVkdS5jbg==
 Yu Yan
Yu Yan Hong Lin
Hong Lin Zhiyao He
Zhiyao He Ling Wang
Ling Wang