- 1Department of Gastroenterology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Medicine, Qingdao University, Qingdao, China
The pathological features of intraductal oncocytic papillary neoplasm (IOPN) of the bile duct include tumor cells that are rich in eosinophilic cytoplasm and arranged in papillary structures. Herein, we report a missed case of IOPN of the bile duct because of concomitant gallstones. A 70-year-old woman was hospitalized with upper abdominal discomfort. The primary diagnosis was choledocholithiasis following imaging examination. However, an unidentified mass was detected after the gallstones were removed. The mass appeared as many papillary protuberances surrounded by fish-egg-like mucosa when viewed by the choledochoscope and was confirmed as IOPN by pathological examination. The patient underwent choledochectomy and no recurrence was observed at the 6-month follow-up examination. In this report, peroral choledochoscopy demonstrated its advantages for the diagnosis of biliary diseases and acquisition of tissue specimens. Therefore, it may solve the challenge related to the lack of preoperative pathological evidence for bile duct tumors.
1 Introduction
Carcinomas of the extrahepatic bile duct are a heterogeneous group of cancers, which are often diagnosed at an advanced stage and exhibit poor patient outcomes. Based on “WHO Classification of Tumours: Digestive System Tumours (5th Edition)”, it can be classified into cholangiocarcinoma, intraductal papillary neoplasm of the bile duct (IPNB), squamous cell carcinoma, adenosquamous carcinoma, and undifferentiated carcinoma. Moreover, IPNB can be further divided into pancreatobiliary, gastric, intestinal, and oncocytic type (1–3). Among these classifications, intraductal oncocytic papillary neoplasm (IOPN) of the bile duct is extremely rare. Only 20 cases with complete information were retrieved from the PubMed database, including only five patients with neoplasm in the extrahepatic bile duct. We encountered a rare case of a patient with IOPN of the extrahepatic bile duct. The novelty of this case is the co-existence of tumor and gallstones, which contributed to the missed diagnosis and is reported here for the first time. This case is also the first report to reveal the choledochoscopic findings of IOPN of the bile duct.
2 Manuscript
2.1 Case report
A 70-year-old woman was hospitalized with upper abdominal discomfort. The patient had a history of carbon monoxide poisoning and was diagnosed with diabetes and hypertension. The physical examination findings were unremarkable. Laboratory tests showed above normal levels of total bilirubin ↑ (81.5 umo1/L; normal value: 3-22 umo1/L), direct bilirubin ↑ (42.9 umo1/L; 0-8 umo1/L), alanine aminotransferase ↑ (330.4 U/L; 7-40 U/L), aspartate aminotransferase ↑ (99.7 U/L; 13-35 U/L), γ-glutamyl transferase ↑ (464.0 U/L; 7-45 U/L), alkaline phosphatase ↑ (138.8 U/L; 50-135 U/L), and C-reactive protein ↑ (5.08 mg/L; 0-5 mg/L). Conversely, the carbohydrate antigen 19-9 (CA19-9) level, carcinoembryonic antigen (CEA) level, and leukocyte count were all normal. Ultrasonography showed a dilated bile duct with a diameter of 1.2 cm. Several hyperechoic masses with a diameter of 0.4-0.8 cm were observed in the bile duct, which exhibited apparent acoustic shadows (Figure 1A). Computed tomography (CT) also revealed multiple slightly high-density masses within the dilated bile duct (Figure 1B). Magnetic resonance cholangiopancreatography (MRCP) demonstrated hypointense masses in the bile duct (Figure 1C).
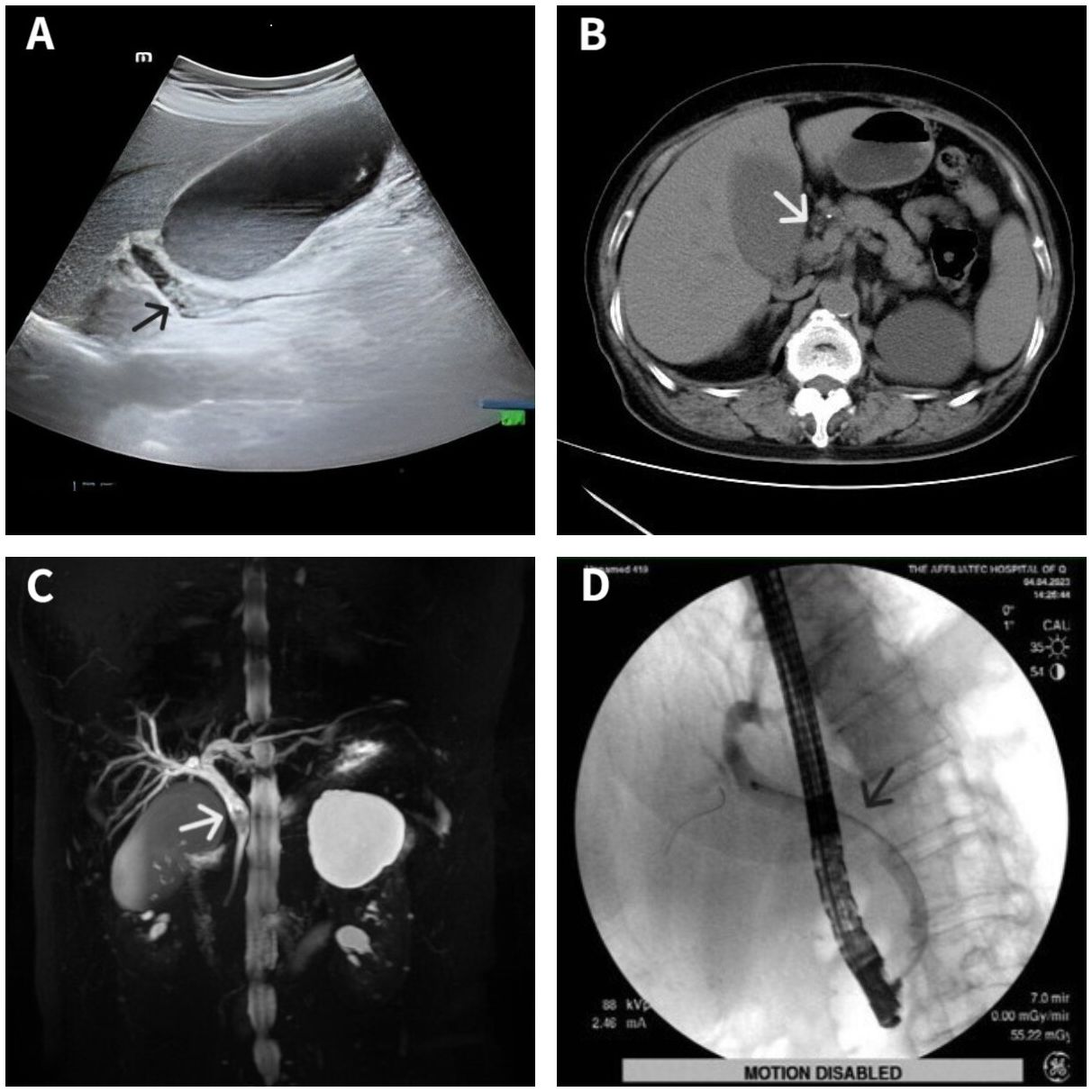
Figure 1 Imaging data of the patient. Ultrasonography showed several hyperechoic masses within the dilated bile duct (A). Computed tomography showed some slightly high-density masses in the bile duct (B). Magnetic resonance cholangiopancreatography showed some hypointense masses within the dilated bile duct (C). Endoscopic retrograde cholangiopancreatography showed a filling defect at the middle bile duct after several stones were removed (D). Lesion locations are marked with arrows.
The diagnosis was choledocholithiasis based on the above evidence. Therefore, the patient underwent endoscopic retrograde cholangiopancreatography (ERCP). Several stones were removed during the operation. However, a filling defect was still observed at the middle bile duct (Figure 1D). Five days after the stones were removed by ERCP, the patient underwent peroral choledochoscopy. Peroral choledochoscopy (SpyGlass DS II, Boston Scientific Corporation, Delaware, United States) showed that the mass appeared as many papillary protuberances surrounded by fish-egg-like mucosa (Figure 2). The purplish-red lesion extended over half of the circumference of the bile duct. We performed the biopsy under direct vision of the choledochoscope, and the pathological diagnosis was IOPN. The histological appearance was papillary structures with fibrovascular cores, and the tumor cells contained a large amount of eosinophilic cytoplasm and possessed round nuclei. Additionally, cell atypia was light to moderate and local glands were hyperplastic in a crowded state (Figure 3). Finally, no distant metastasis or involvement of regional lymph nodes was apparent. Eleven days after the biopsy under choledochoscopy was performed, the patient underwent choledochectomy. We removed the common bile duct and performed Roux-en-Y choledochojejunostomy. Although, postoperative adjuvant chemotherapy was not performed, no recurrence was detected at 6 months after surgery.

Figure 2 Choledochoscopic findings of intraductal oncocytic papillary neoplasm of the bile duct. Choledochoscopy showed many papillary protuberances (A) surrounded by fish-egg-like mucosa (B).
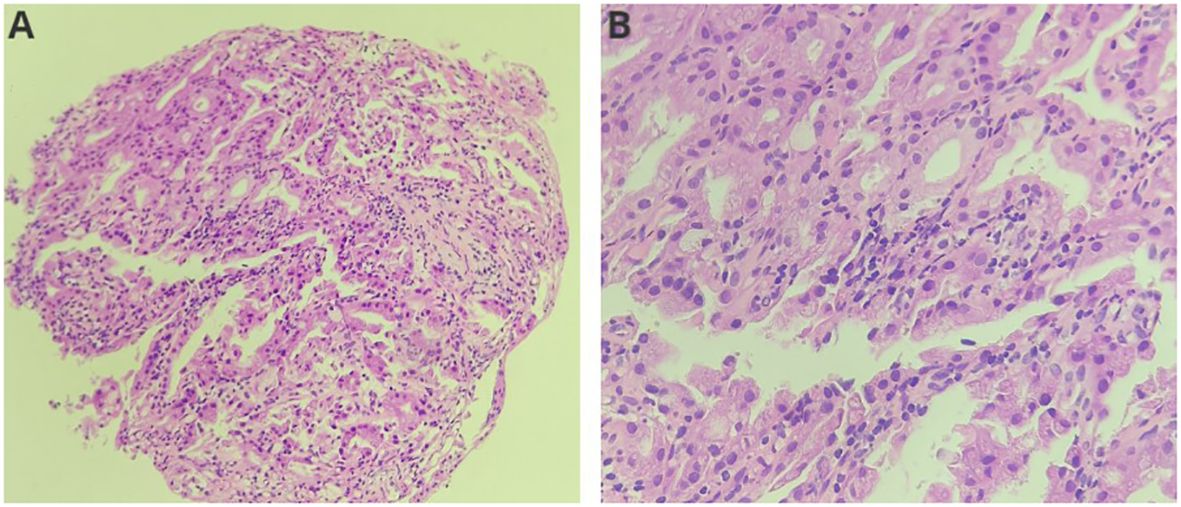
Figure 3 Histological features of the neoplasm ((A): HE, ×200; (B): HE, ×400). The neoplasm showed papillary structures with fibrovascular cores. Neoplasm cells contained a large amount of eosinophilic cytoplasm and round nuclei.
2.2 Literature survey
We found 20 cases with complete data through October 2023 in the PubMed database using “intraductal oncocytic papillary neoplasm of the bile duct” as the search query (4–16). The information related to this search is presented in Table 1. Overall, 12 (60%) patients were from Japan and six (30%) were from the USA. The mean age was 57.2 years (minimum value: 38 years; maximum value: 71 years) and only six (30%) patients were female. Patients had different clinical symptoms, and the most common presentation was abdominal pain. In addition, some patients were found incidentally during health examinations or tests for other diseases.
Among these patients, 15 (75%) had normal tumor markers. However, two (10%) patients had elevated CA19-9, and one (5%) had elevated CEA. Fifteen (75%) patients had intrahepatic bile duct tumors, three (15%) had hilar duct tumors, and two (10%) had common bile duct tumors. CT of the intrahepatic bile duct tumors mainly showed single or multiple cystic masses with or without bile duct dilation, and the cysts were directly connected to the bile ducts. The main CT findings of extrahepatic bile duct tumors were nodular lesions within the dilated bile duct. Similar to these CT findings, magnetic resonance imaging (MRI) of the intrahepatic bile duct tumors typically showed single or multiple cystic lesions of varying sizes. Moreover, extrahepatic bile duct tumors were characterized by papillary protuberances and bile duct dilatation. These lesions appeared hypointense in T1-weighted images and hyperintense in T2-weighted images. Unfortunately, no information about MRCP findings were available, probably because most were intrahepatic bile duct tumors. Among these patients, 17 (85%) lacked pathological diagnosis before surgery. Four patients underwent cytological examination, but three had negative results, while atypical cells were found in one patient. Two (10%) of the pathological specimens were obtained by percutaneous drainage or fine-needle aspiration, and one (5%) of the tissue specimens was obtained by cholangiocarcinoma dochoscopy.
All patients received surgical treatment without adjuvant chemotherapy. The mean follow-up time was 30.7 months (minimum value: 6 months; maximum value: 112 months). Only two (10.5%) patients experienced recurrence while one (5%) lacked a full description.
2.3 Discussion
Few reports exist on IOPN of the bile duct. We only found 20 cases with complete data in PubMed. Importantly, only 19% of the patients had a pathological diagnosis before surgery, which may lead to a misdiagnosis or missed diagnosis. This condition is more likely to happen when patients have concomitant gallstones, as we have reported.
Previous research has suggested that IPNB is more common in older men (17). As a type of IPNB, IOPN may have similar characteristics. The mean age of the patients with IOPN of the bile duct was 57.8 years, and 66.7% of the cases were men. However, the accuracy of these figures may be affected by the small sample size. Additionally, 66.7% of the patients with IOPN of the bile duct were from Asia which may be associated with hepatolithiasis and cholelithiasis (18).
IOPN of the bile duct exhibits no typical clinical manifestations or sensitive tumor markers. Moreover, the tumor can present features similar to those of gallstones when it causes biliary obstruction and infection. CT and MRI of IOPN of the intrahepatic bile duct mainly show single or multiple cystic masses with bile duct dilatation, and the cysts were directly connected to the bile ducts. The typical appearance of other bile duct tumors is the solid mass with irregular margins, which is easier to identify (19). However, biliary cystadenoma/cystadenocarcinoma has a similar presentation to IOPN. The key difference is that the cystic masses of the former are not associated with the bile duct. Moreover, biliary cystadenoma/cystadenocarcinoma only causes the dilatation of the proximal bile duct. In contrast, IOPN can cause diffuse dilatation of intrahepatic and extrahepatic bile ducts when the flow of mucin obstructs the papilla of Vater (18, 20). CT, MRI, and MRCP of IOPN of the extrahepatic bile duct commonly display papillary protuberances within the dilated bile duct. Identifying this condition from other types of the extrahepatic bile duct tumors is difficult, and depends on pathology (21, 22).
Pathology is the gold standard for the diagnosis of IOPN of the bile duct. The main histological manifestations are complex papillary structures with fibrovascular cores (1). Tumor cells typically contain abundant eosinophilic granular cytoplasm and round, large, and uniform nuclei (3, 23, 24). In addition, the nucleolus is obvious (25) and cell atypia is light to moderate. Tumor cells can form an intraepithelial lumen, and some are sieve shaped with a large amount of mucus (17). However, only 19% of the patients with IOPN of the bile duct had pathological diagnosis before surgery because tissue specimen acquisition is extremely difficult.
Some methods are used to obtain specimens of extrahepatic bile duct tumors, such as cytology brushing and fluoroscopic biopsy by ERCP, endoscopic ultrasonography-guided biopsy, and bile cytology (22). However, the sensitivity of cytological examination in the diagnosis of biliary stricture was only 45% (26). Although four patients with IOPN of the bile duct underwent cytological examination, three had negative results while atypical cells were found in one patient. Reports indicate that the sensitivity of fluoroscopic biopsy is 50% (27). However, the use of this method in patients with IOPN of the bile duct has not been reported. Tissue specimens from intrahepatic bile duct tumors can be obtained by percutaneous biopsy. However, this method is not recommended for cystic lesions because of the risk of bile leakage and needle tract seeding (28). Although obtaining a preoperative pathological diagnosis is suggested, the acquisition of specimens is challenging.
Peroral choledochoscopy may be the solution to this problem. The third-generation SpyGlass peroral choledochoscope was launched in 2018 with the advantages of visual examination and targeted biopsies. We can directly identify the tumors and stones visually and detect precancerous lesions of the biliary mucosa. A meta-analysis showed that the specificity and sensitivity of choledochoscopic visual diagnosis were 86% and 93%, respectively (29). In our case, choledochoscopy of IOPN showed many papillary protuberances surrounded by fish-egg-like mucosa. The reason may be that the tumor cells arrange in papillary shape and the surrounding mucosa presents intraepithelial micropapillary or flat neoplastic lesion (30). Their grades and subtypes are similar or identical to the main tumor (17). Therefore, the surrounding mucosal cells also contain a large amount of eosinophilic cytoplasm and mucin, which makes the cells swollen and shaped like fish eggs. The choledochoscopic features of this disease have not been previously reported. Additionally, we can perform the biopsy under the direct vision of choledochoscope and it has a higher sensitivity than that of fluoroscopic biopsy for the diagnosis of biliary stricture (31, 32). Choledochoscopy can also be used to treat difficult bile duct stones (33) and perform radiofrequency ablation of bile duct tumors (34).
Complete resection is currently considered the most effective treatment for IOPN of the bile duct. Tumor metastasis to other organs or systems was not detected in any of the 21 patients and they all underwent surgical treatment without adjuvant chemotherapy. Only 10% of the patients had recurrence (the mean follow-up time was 29.5 months). Therefore, the prognosis of the disease appears excellent, but this assertion needs to be confirmed by additional studies.
In conclusion, IOPN of the bile duct is extremely rare. The main clinical features are summarized based on only a few cases. Therefore, many aspects need to be further studied and confirmed. Imaging examination may lead to misdiagnosis or missed diagnosis, especially when the tumor is accompanied by gallstones. However, use of peroral choledochoscopy may solve this clinical problem.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HZ: Data curation, Investigation, Resources, Visualization, Writing – original draft. YM: Formal analysis, Resources, Validation, Writing – review & editing. BC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Science and Technology Benefiting Project of Qingdao West Coast Area (Grant Number: 2020-58).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. 5th ed. Lyon: International Agency for Research on Cancer (2019).
2. Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. (2011) 35:512–21. doi: 10.1097/PAS.0b013e3182103f36
3. Nakanuma Y, Kakuda Y, Uesaka K. Characterization of intraductal papillary neoplasm of the bile duct with respect to the histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Gut Liver. (2019) 13:617–27. doi: 10.5009/gnl18476
4. Martin RC, Klimstra DS, Schwartz L, Yilmaz A, Blumgart LH, Jarnagin W. Hepatic intraductal oncocytic papillary carcinoma. Cancer. (2002) 95:2180–7. doi: 10.1002/cncr.10934
5. Terada T, Taniguchi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. (2004) 54:116–23. doi: 10.1111/j.1440-1827.2004.01594.x
6. Nakanishi Y, Zen Y, Hirano S, Tanaka E, Takahashi O, Yonemori A, et al. Intraductal oncocytic papillary neoplasm of the bile duct: the first case of peribiliary gland origin. Hepato-Biliary-Pancreatic. (2009) 16:869–73. doi: 10.1007/s00534-009-0070-1
7. Tanaka M, Fukushima N, Noda N, Shibahara J, Kokudo N, Fukayama M. Intraductal oncocytic papillary neoplasm of the bile duct: clinicopathologic and immunohistochemical characteristics of 6 cases. Hum Pathol. (2009) 40:1543–52. doi: 10.1016/j.humpath.2009.03.014
8. Liszka L, Pajak J, Zielińska-Pajak E, Krzych L, Gołka D, Mrowiec S, et al. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: a description of five new cases and review based on a systematic survey of the literature. Hepato-Biliary-Pancreatic. (2010) 17:246–61. doi: 10.1007/s00534-010-0268-2
9. Cocieru A, Kesha K, El-Fanek H, Saldinger PF. Hepatic intraductal oncocytic papillary carcinoma. Am J Surg. (2010) 199:e57–8. doi: 10.1016/j.amjsurg.2009.06.019
10. Kato Y, Konishi M, Kinoshita T, Takahashi S, Gotohda N, Kinoshita T. Intraductal oncocytic papillary neoplasm of the extrahepatic bile duct: report of a case. Surg Today. (2012) 42:1240–3. doi: 10.1007/s00595-012-0233-6
11. Kakisaka T, Kamiyama T, Yokoo H, Nakanishi K, Wakayama K, Tsuruga Y, et al. An intraductal papillary neoplasm of the bile duct mimicking a hemorrhagic hepatic cyst: a case report. World J Surg Oncol. (2013) 11:111. doi: 10.1186/1477-7819-11-111
12. Watanabe A, Suzuki H, Kubo N, Araki K, Kobayashi T, Sasaki S, et al. An oncocytic variant of intraductal papillary neoplasm of the bile duct that formed a giant hepatic cyst. Rare Tumors. (2013) 5:e30. doi: 10.4081/rt.2013.e30
13. Jurczyk MF, Zhu B, Villa C, DeFrias D, Lin X. Cytomorphology of intraductal oncocytic papillary neoplasm of the liver. Diagn Cytopathology. (2014) 42:895–8. doi: 10.1002/dc.23073
14. Tong A, Veillette G, Budhai A, Gilet A. Intraductal oncocytic papillary neoplasm: a benign hepatic cystic neoplasm. BMJ Case Rep. (2017) 2017:bcr2016218139. doi: 10.1136/bcr-2016-218139
15. Tsujimae M, Sakai A, Masuda A, Inomata N, Masuda S, Gonda M, et al. A Case in which an Intraductal Papillary Neoplasm of the Bile Duct Was Surgically Resected 12 Years after the Initial Diagnosis. Internal Med (Tokyo Japan). (2020) 59:2879–83. doi: 10.2169/internalmedicine.4891-20
16. Liu Q, Wang Z, Yu C, Zhu J, Liu C, Li X, et al. Intraductal oncocytic papillary neoplasm arising in Peutz-Jeghers Syndrome bile duct: a unique case report. Diagn Pathol. (2022) 17:96. doi: 10.1186/s13000-022-01275-8
17. Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, et al. Intraductal papillary neoplasm of bile duct: Updated clinicopathological characteristics and molecular and genetic alterations. J Clin Med. (2020) 9:3991. doi: 10.3390/jcm9123991
18. Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, et al. Intraductal papillary neoplasm of the bile duct: Clinical, Imaging, and pathologic features. AJR Am J Roentgenology. (2018) 211:67–75. doi: 10.2214/ajr.17.19261
19. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. (2014) 60:1268–89. doi: 10.1016/j.jhep.2014.01.021
20. Mocchegiani F, Vincenzi P, Conte G, Nicolini D, Rossi R, Cacciaguerra AB, et al. Intraductal papillary neoplasm of the bile duct: The new frontier of biliary pathology. World J Gastroenterol. (2023) 29:5361–73. doi: 10.3748/wjg.v29.i38.5361
21. Zhou JX, Liang BL, Xu LY, Huang SQ. [MR cholangiopancreatography and MR imaging in the diagnosis of extrahepatic cholangiocarcinoma]. Zhonghua zhong liu za zhi [Chinese J oncology]. (2004) 26:421–3.
22. Cadamuro M, Al-Taee A, Gonda TA. Advanced endoscopy meets molecular diagnosis of cholangiocarcinoma. J Hepatol. (2023) 78:1063–72. doi: 10.1016/j.jhep.2023.01.027
23. Wang T, Askan G, Adsay V, Allen P, Jarnagin WR, Memis B, et al. Intraductal oncocytic papillary neoplasms: Clinical-pathologic characterization of 24 cases, with an emphasis on associated invasive carcinomas. Am J Surg Pathol. (2019) 43:656–61. doi: 10.1097/pas.0000000000001226
24. Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. (2012) 57:787–93. doi: 10.1016/j.jhep.2012.05.008
25. Stendahl K, Gilani SM, Basturk O, Hui P, Sigel C, Cai G. Intraductal papillary neoplasm of the bile duct: Cytomorphologic and molecular features. Cancer Cytopathology. (2023) 131:37–49. doi: 10.1002/cncy.22637
26. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of Malignant biliary strictures: a systematic review and meta-analysis. Gastrointestinal Endoscopy. (2015) 81:168–76. doi: 10.1016/j.gie.2014.09.017
27. Nishikawa T, Tsuyuguchi T, Sakai Y, Sugiyama H, Tawada K, Mikata R, et al. Factors affecting the accuracy of endoscopic transpapillary sampling methods for bile duct cancer. Digestive Endoscopy. (2014) 26:276–81. doi: 10.1111/den.12140
28. Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. (2020) 69:1382–403. doi: 10.1136/gutjnl-2020-321299
29. Kulpatcharapong S, Pittayanon R, Kerr SJ, Rerknimitr R. Diagnostic performance of digital and video cholangioscopes in patients with suspected Malignant biliary strictures: a systematic review and meta-analysis. Surg Endoscopy. (2022) 36:2827–41. doi: 10.1007/s00464-021-08571-2
30. Nakanuma Y, Sudo Y. Biliary tumors with pancreatic counterparts. Semin Diagn Pathol. (2017) 34:167–75. doi: 10.1053/j.semdp.2016.12.013
31. Walter D, Peveling-Oberhag J, Schulze F, Bon D, Zeuzem S, Friedrich-Rust M, et al. Intraductal biopsies in indeterminate biliary stricture: Evaluation of histopathological criteria in fluoroscopy- vs cholangioscopy guided technique. Digestive liver Dis. (2016) 48:765–70. doi: 10.1016/j.dld.2016.03.013
32. Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointestinal Endoscopy. (2012) 75:347–53. doi: 10.1016/j.gie.2011.09.020
33. Leung JW, Chung SS. Electrohydraulic lithotripsy with peroral choledochoscopy. BMJ (Clinical Res ed). (1989) 299:595–8. doi: 10.1136/bmj.299.6699.595
Keywords: carcinoma of the bile duct, intraductal oncocytic papillary neoplasm of the bile duct, gallstones, missed diagnosis, choledochoscopy
Citation: Xie C, Zhang H, Meng Y and Cao B (2024) A missed case of intraductal oncocytic papillary neoplasm associated with missed stones in extrahepatic bile duct: a case report. Front. Oncol. 14:1349914. doi: 10.3389/fonc.2024.1349914
Received: 18 January 2024; Accepted: 17 April 2024;
Published: 21 May 2024.
Edited by:
Pasquale Cianci, Azienda Sanitaria Localedella Provincia di Barletta Andri Trani (ASL BT), ItalyReviewed by:
Mostafa Kotb, Alexandria University, EgyptCarlos Bilreiro, Champalimaud Foundation, Portugal
Asit Kumar Manna, The University of Utah, United States
Copyright © 2024 Xie, Zhang, Meng and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Cao, cXljYW9iaW5AMTI2LmNvbQ==
 Cong Xie
Cong Xie Hang Zhang1,2
Hang Zhang1,2 Bin Cao
Bin Cao