- 1Department of Radiation Therapy and Chemotherapy for Cancer Nursing, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
Metastatic choriocarcinoma during viable pregnancy is rare worldwide, and neonate survival following pregnancy termination in the second trimester is uncommon. Here, we report the successful delivery of a pregnancy by a patient with metastatic choriocarcinoma, who received three courses of etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine (EMA-CO) chemotherapy in the second trimester. After multidisciplinary discussions, she was administered paclitaxel and carboplatin (TC) chemotherapy. Regular contractions occurred during her first paclitaxel infusion, and a healthy infant was delivered by cesarean section at 26+4 gestational weeks. Choriocarcinoma was not detected in the placenta. Following delivery of the pregnancy, the patient underwent total treatment comprising one cycle of TC, seven cycles of EMA-CO, and five courses of etoposide, cisplatin, methotrexate, and dactinomycin chemotherapy; her serum level of beta–human chorionic gonadotropin gradually fell after chemotherapy. Uterine and pulmonary metastases shrank, and no distant metastasis or recurrence were found until the eighth course of maintenance treatment with immunotherapy. The patient received periodic chemotherapy for recurrence at the time of publishing this case report. The child was disease-free 15+ months after delivery. Despite serious metastases and complications, metastatic choriocarcinoma diagnosed in the second trimester of pregnancy can be successfully treated with minimal delay by multidisciplinary medical and nursing management.
1 Introduction
Choriocarcinoma is a rare, highly aggressive, malignant trophoblastic neoplasm that is a histologic subtype of gestational trophoblastic neoplasia (GTN) affecting approximately 1 in 40,000 pregnancies in Europe and North America and 9.2 per 40,000 pregnancies in Southeast Asia (1). Coexistence of choriocarcinoma with an intrauterine pregnancy is associated with high mortality rates for both mother (62%) and fetus (65%) (2). In patients with choriocarcinoma, the fetus and accessory tissues are generally abnormal, and choriocarcinoma concurrent with a normal pregnancy is extremely rare, accounting for 1 in 160,000 pregnancies (3). Most patients with choriocarcinoma are diagnosed in the third trimester of pregnancy, and the pregnancy is immediately terminated (4–7). Because of their relatively low molecular weight, chemotherapy drugs can penetrate the placenta and exert adverse effects on fetuses, limiting their use during pregnancy; hence, exposure to chemotherapy during pregnancy is a great concern for mothers and physicians (8). Here, we report a patient with metastatic choriocarcinoma diagnosed in the second trimester of a viable pregnancy, who underwent chemotherapy and had a successful delivery. This case provides a clinical reference for treating and preserving pregnancy in patients with GTN.
2 Case presentation
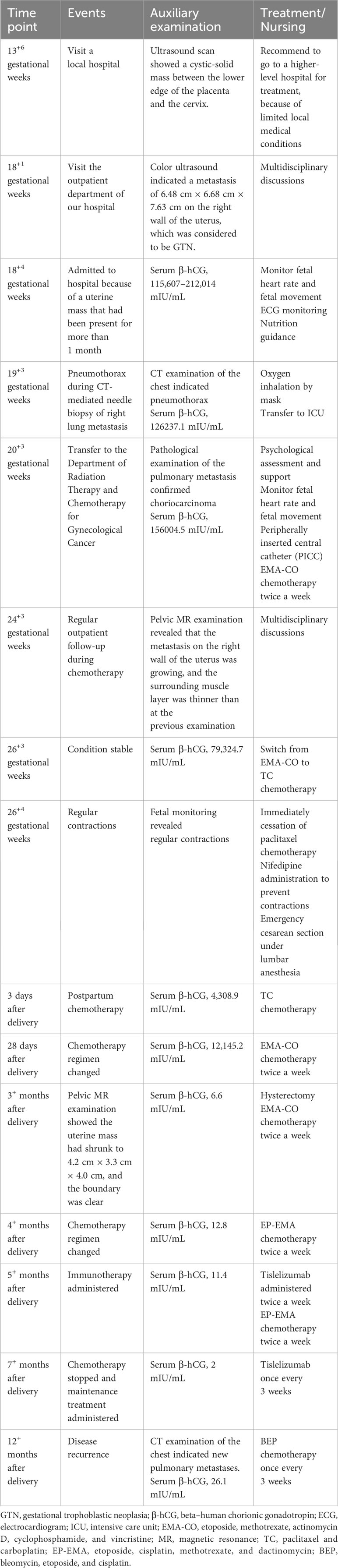
The patient was a 27-year-old pregnant woman (gravida 2, para 0), who had previously undergone six courses of etoposide and cisplatin (EP) chemotherapy due to an “invasive mole” diagnosed at a local hospital in 2018. No vaginal bleeding or discharge were noted during early pregnancy. An ultrasound scan at 13+6 gestational weeks showed a cystic-solid mass between the lower edge of the placenta and the cervix. At 18+1 gestational weeks, color ultrasound examination indicated a metastasis of 6.48 cm × 6.68 cm × 7.63 cm on the right wall of the uterus, which was considered to be GTN. In June 2022, she was admitted at 18+4 gestational weeks, because of the uterine mass that had been present for more than 1 month. Pelvic magnetic resonance (MR) examination showed that the metastatic focus protruded under the serous membrane.
Five days after admission, chest MR examination indicated multiple pulmonary metastases (maximum size, 1.8 cm × 1.2 cm). Investigation by CT-guided needle biopsy of the right lung metastasis was conducted the following day. She experienced sudden hemoptysis, dyspnea, and thoracalgia during insertion of the CT-guided needle because of pneumothorax. After oxygen inhalation and supportive treatment, pneumothorax did not recur and the patient underwent successful biopsy 2 days later. Pathological examination of the pulmonary metastasis confirmed choriocarcinoma (Figure 1A). Continuing or terminating the pregnancy were both considered high-risk at 20 gestational weeks. The patient and her family decided to continue the pregnancy after being fully informed of the risks.
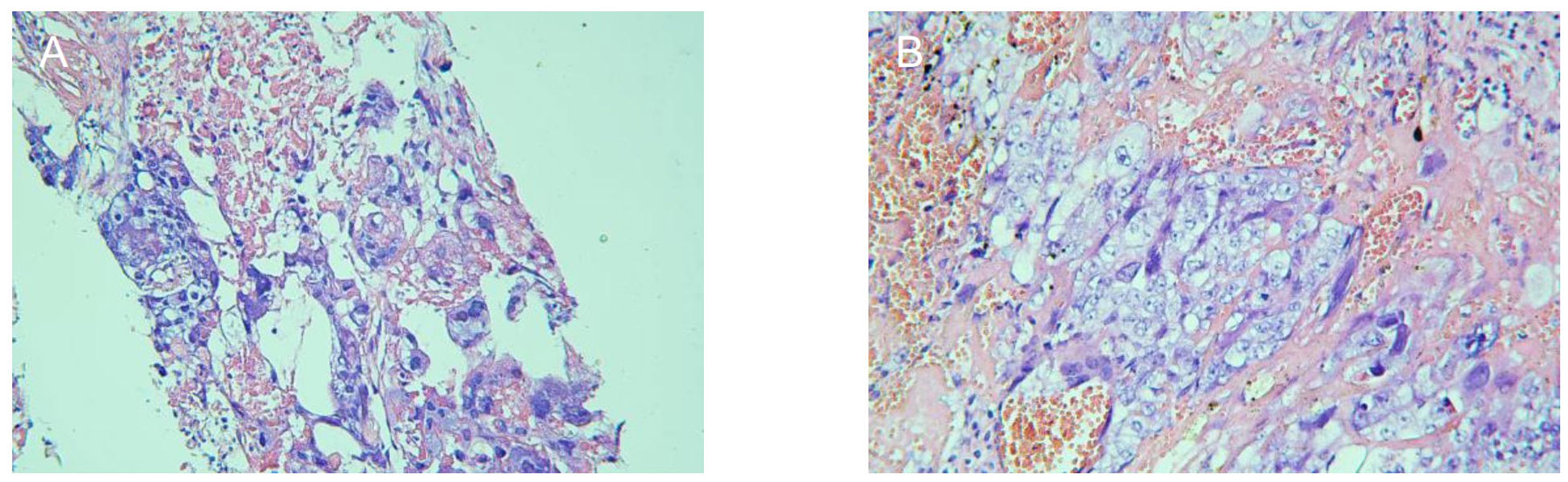
Figure 1 Pathological examination to confirm that the pulmonary metastasis (A) and uterine mass (B) were choriocarcinoma.
The tumor was FIGO stage III (prognostic score, 14); therefore, etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine (EMA-CO) chemotherapy was given at 20+3 gestational weeks. After three cycles of chemotherapy, the chemotherapeutic effect was remarkable, resulting in shrinkage of the pulmonary metastases and a gradual decrease in beta–human chorionic gonadotropin (β-hCG) level; however, pelvic MR examination at 24+3 gestational weeks revealed that the myometrial metastasis was growing. As her condition was stable, the multidisciplinary team decided to switch the EMA-CO regimen to paclitaxel and carboplatin (TC) chemotherapy at 26+3 gestational weeks. Regular contractions occurred during the first paclitaxel infusion at 26+4 gestational weeks. Nifedipine was given to stop the contractions but failed, and the patient and her family refused any other tocolysis. Subsequently, an emergency cesarean section was performed under lumbar anesthesia. A live female infant weighing 640 g was delivered, with Apgar scores of 7, 8, and 8 at 1, 5, and 10 min after delivery. The placenta appeared normal and intact and pathological examination did not reveal any abnormality. The baby was transferred to the neonatal intensive care unit (NICU).
The patient was initially administered one cycle of TC chemotherapy 3 days after delivery and then EMA-CO chemotherapy twice a week. The size of the uterine mass shrank to 4.2 cm × 3.3 cm × 4.0 cm, and its boundary became clear after seven courses of postpartum chemotherapy. The gynecological surgeon considered the uterine mass resistant to chemotherapy and that hysterectomy was feasible. The patient underwent hysterectomy and histological assessment confirmed that the uterine mass was choriocarcinoma (Figure 1B). The EMA-CO regimen was switched to etoposide, cisplatin, methotrexate, and dactinomycin (EP-EMA) chemotherapy after one course of postoperative chemotherapy, because of an unsatisfactory decrease in β-hCG, and tislelizumab was added after one course of EP-EMA for the same reason.
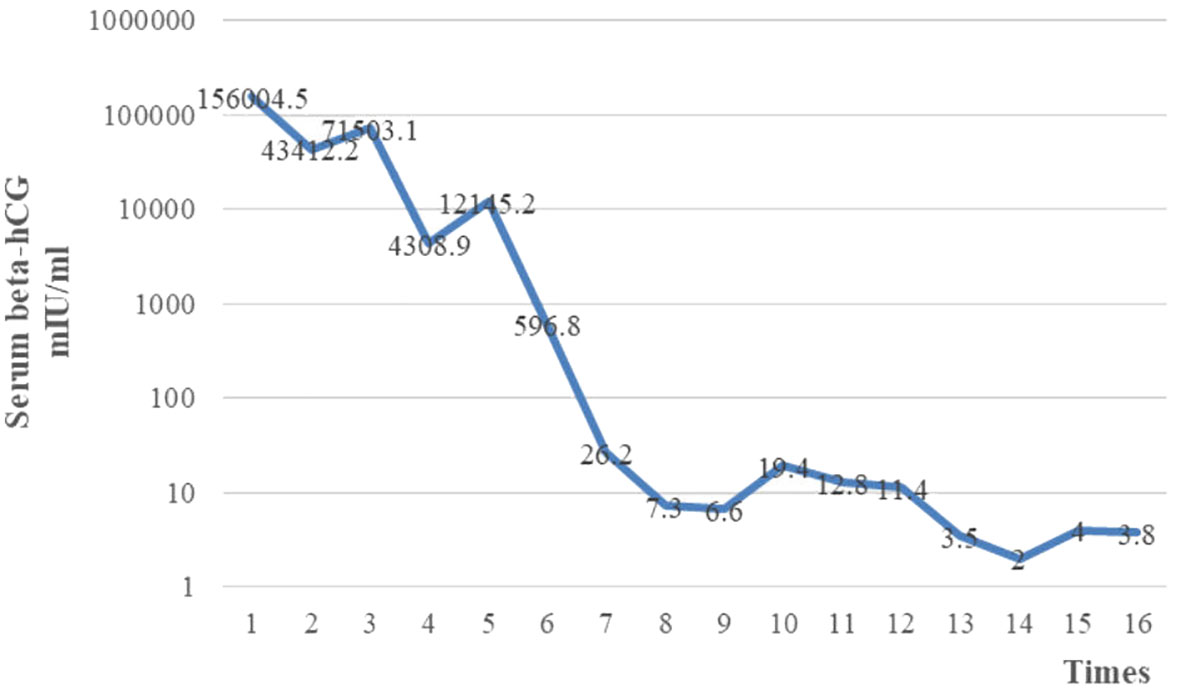
After four cycles of EP-EMA chemotherapy combined with tislelizumab, her β-hCG level returned to the normal range (Figure 2), and pulmonary metastases were reduced (Figures 3A–C). No distant metastasis or recurrence were found until the eighth course of maintenance treatment with tislelizumab (Figure 3D). The psychological condition of the patient was normal, based on assessment using the Hospital Anxiety and Depression Scale (HADS), which has excellent reliability and validity (9). The patient is undergoing periodic bleomycin, etoposide, and cisplatin (BEP) chemotherapy for recurrence. The baby was discharged 126 days after delivery weighing 2,530 g weight and was disease-free 15+ months after delivery.
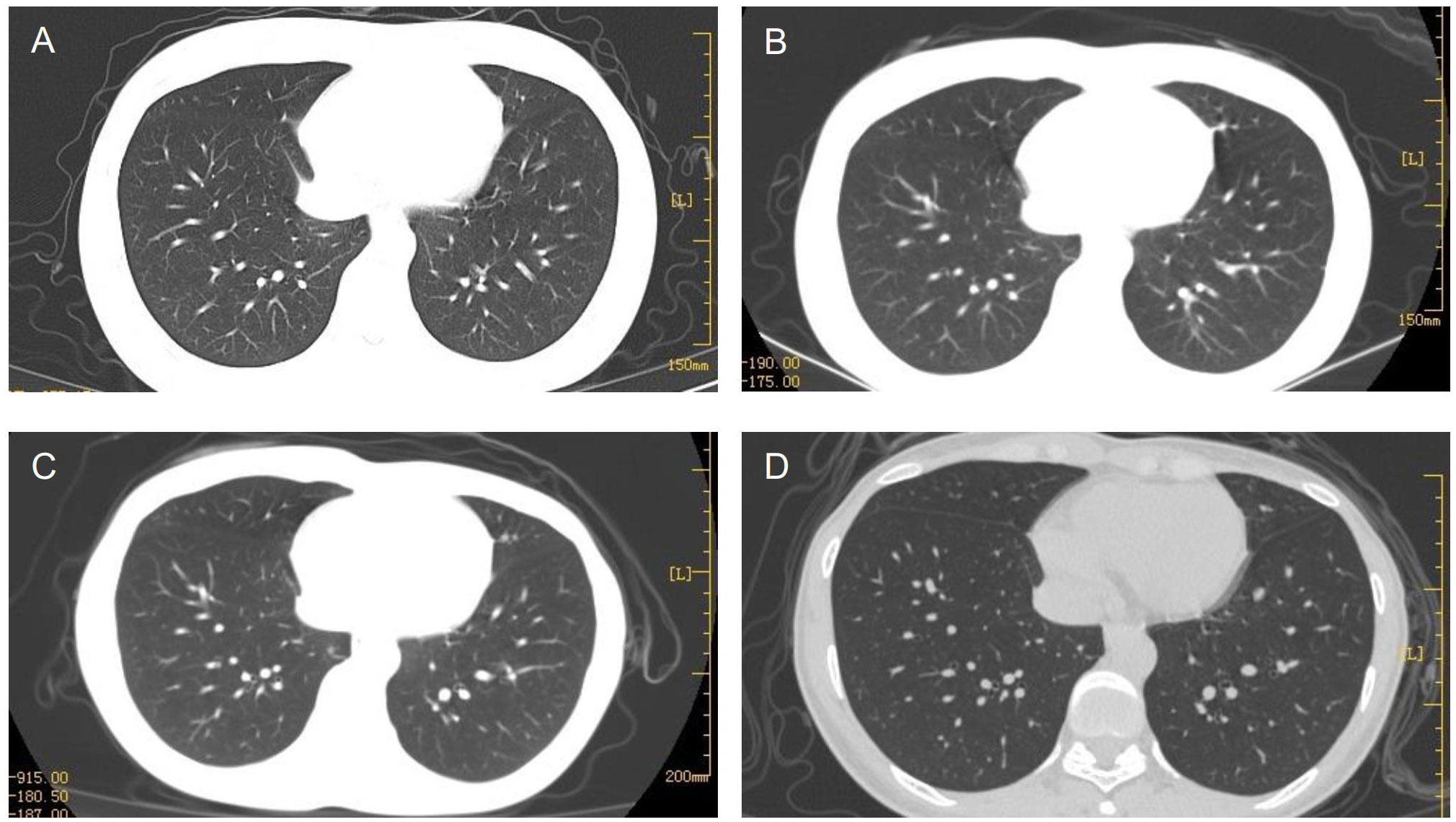
Figure 3 Multiple pulmonary metastases gradually subsided after six cycles (A), 12 cycles (B), and 3 months (C) of chemotherapy. The patient relapsed after eight maintenance treatments with tislelizumab (D).
The clinical course of the patient from initial diagnosis to last follow-up is illustrated in Table 1.
3 Discussion
Choriocarcinomas can originate from either current or previous pregnancies (10), and it is difficult to determine their origin because most women with choriocarcinoma have a previous history of pregnancy. In some metastatic cases, choriocarcinoma has been detected in the placenta by histopathologic examination (11–14); however, it is not routine to conduct pathological assessment of the placenta (15). Short tandem repeat polymorphism (STR) analysis can provide more accurate determination of the origin of choriocarcinomas; for example, according to STR analysis, the choriocarcinoma in a patient described by Ding et al. may have originated from her most recent spontaneous abortion (14). We were unable to confirm the origin of the choriocarcinoma in our case as we did not conduct STR analysis.
Vaginal bleeding is the most common clinical manifestation of choriocarcinoma, but symptoms vary widely during pregnancy; for example, metastatic choriocarcinoma was found in a multiparous woman who initial presented with intractable lower back pain (16). The patient described in this study did not experience vaginal bleeding. Further, pulmonary metastases of choriocarcinoma were confirmed without delay after she was admitted. In previous reports, choriocarcinoma metastases have been found in the lung, uterus, brain, and vagina, and other tissues (10, 17), among which choriocarcinoma most often metastasizes to the lung, causing respiratory symptoms, such as cough, hemoptysis, and dyspnea, whereas choriocarcinoma with brain metastasis may cause neurological symptoms. Hence, choriocarcinoma should be considered when a pregnant woman presents with visceral metastases even without vaginal bleeding.
Chemotherapy is the main treatment for choriocarcinoma, with a cure rate of approximately 90%, even for metastatic disease (1); however, there is no consensus on appropriate treatment for choriocarcinoma concurrent with pregnancy, because of its rarity. It is considered better for patients in early pregnancy to terminate the pregnancy and receive standard chemotherapy, whereas antepartum chemotherapy has been suggested for those diagnosed in late pregnancy (10). Among cases described by Ding et al. (14), Bircher et al. (17), and Nabers et al. (18), who received antepartum chemotherapy during the second trimester, live fetuses were delivered after chemotherapy; however, no long-term follow-up of the newborns was conducted. The application of immunotherapy in refractory GTN has also become the subject of increasing attention, since Ghorani et al. (19) reported the first use of pembrolizumab in refractory GTN. The newborn reported in this study was disease-free 15+ months after delivery, even after exposure to chemotherapy in the second trimester. Tislelizumab was added during postpartum chemotherapy. Symptom management was conducted during chemotherapy and immunotherapy. The patient suffered from no side effects, other than mild nausea, vomiting, and myelosuppression. Therefore, we conclude that administration of chemotherapy during the second trimester is relatively safe and that symptom management plays a positive role during chemotherapy and immunotherapy for choriocarcinoma concurrent with pregnancy.
Most reports on choriocarcinoma concurrent with viable pregnancy documented that the patient terminated their pregnancy immediately after diagnosis, common reasons for which have included life-threatening symptoms of the mother and fetus and concerns about chemotherapy-induced fetal abnormalities (4–7, 11). Adjustment to the psychological transition from having a healthy pregnancy to a malignant choriocarcinoma is extremely challenging for patients and their families, who may experience negative emotions, such as sadness, anxiety, and shock, as well as isolation, fertility concerns, and worries about recurrence (12). A cross-sectional study in Australia indicated that depression and sexual dysfunction were the most important adverse effects of GTN (20). Our patient was encouraged to express her feelings, and, with the support of her family, no psychological issues were detected, based on her HADS scores each time she was admitted to our hospital.
4 Conclusion
Metastatic choriocarcinoma is extremely rare in women who are carrying a viable pregnancy, but our case emphasizes that it must be considered when a visceral metastasis is identified in a pregnant woman, even in the absence of vaginal bleeding. Pathological confirmation is essential for proper management of such cases. Chemotherapy, together with symptom management, is relatively safe during the second trimester and can improve survival of both the mother and fetus. Regular contractions and other related symptoms should be closely observed during chemotherapy, and the medical team should be ready for emergency cesarean section. Moreover, psychological support is important to help the patient and her family cope with the transition from healthy pregnancy to also having malignant choriocarcinoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT: Writing – original draft. JY: Writing – review & editing. XD: Writing – review & editing. TC: Writing – review & editing. YH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the patient and her husband for providing background information and allowing us to publish this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. (2010) 203:531–9. doi: 10.1016/j.ajog.2010.06.073
2. Steigrad SJ, Cheung AP, Osborn RA. Choriocarcinoma co-existent with an intact pregnancy: case report and review of the literature. J Obstet Gynaecol Re. (1999) 25:197–203. doi: 10.1111/j.1447-0756.1999.tb01147.x
3. Ganapathi KA, Paczos T, George MD, Goodloe S, Balos LL, Chen F. Incidental finding of placental choriocarcinoma after an uncomplicated term pregnancy. Int J Gynecol Pathol. (2010) 29:476–8. doi: 10.1097/PGP.0b013e3181d81cc2
4. Shen C, Ai L, Li K, Cao Y, Wu H, Sun D. Choriocarcinoma brain metastasis in a patient in the third trimester: a case report. J Med Case Rep. (2021) 15:417. doi: 10.1186/s13256-021-02808-3
5. Mamelak AN, Withers GJ, Wang X. Choriocarcinoma brain metastasis in a patient with viable intrauterine pregnancy. J Neurosurg. (2002) 97:477–81. doi: 10.3171/jns.2002.97.2.0477
6. Hookins B, Vatsayan A. Intraplacental choriocarcinoma and fetomaternal haemorrhage and maternal disseminated intravascular coagulopathy in a term pregnancy: a case report. Case Rep Women’s Health. (2020) 27:e216. doi: 10.1016/j.crwh.2020.e00216
7. Thagard AS, Dubil EA, Lee A, Allard J, Zelig CM. The use of middle cerebral artery Doppler ultrasonography to guide delivery of a viable pregnancy complicated by metastatic gestational choriocarcinoma. Australas J Ultrasound Med. (2013) 16:93–6. doi: 10.1002/j.2205-0140.2013.tb00171.x
8. Beksac K, Orgul G, Ozyuncu O, Yurdakok M, Altundag K, Beksac MS. Chemotherapy during pregnancy: Cases of Hodgkin’s and non-Hodgkin’s lymphoma, chronic myeloid leukemia, breast cancer, nasopharyngeal cancer, and choriocarcinoma. Oncol Res Treat. (2017) 40:441–5. doi: 10.1159/000473880
9. Vodermaier A, Millman RD. Accuracy of the Hospital Anxiety and Depression Scale as a screening tool in cancer patients: a systematic review and meta-analysis. Support Care Cancer. (2011) 19:1899–908. doi: 10.1007/s00520-011-1251-4
10. Yu P, Diao W, Jiang X. A successfully treated metastatic choriocarcinoma coexistent with pregnancy: a case report of a 4-year follow-up. Med (Baltimore). (2016) 95:e3505. doi: 10.1097/MD.0000000000003505
11. Lee E, Cho H. A Case of intraplacental choriocarcinoma with pulmonary metastasis. Case Rep Oncol. (2020) 12:802–6. doi: 10.1159/000503816
12. Hensley JG, Shviraga BA. Metastastic choriocarcinoma in a term pregnancy: a case study. MCN Am J Matern Child Nurs. (2014) 39:8–15, 16-17. doi: 10.1097/NMC.0b013e3182a8de5b
13. Monteiro S, Burling M, Doyle H. Late diagnosis of intraplacental choriocarcinoma co-existing with fetomaternal haemorrhage causing fetal demise: A case report. Case Rep Women’s Health. (2021) 31:e341. doi: 10.1016/j.crwh.2021
14. Ding W, Zhang N, Rao Y, Xu X, Nie T, Qu P. A successfully treated multiple metastatic choriocarcinoma coexistent with live fetus: a case report and literature review. Front Oncol. (2022) 11:777707. doi: 10.3389/fonc.2021.777707
15. Wu Y, Ren P, Chen J, Ai L. A case of pregnancy with choriocarcinoma complicated by a cerebral hemorrhage and lung metastasis. Case Rep Oncol. (2021) 14:1182–8. doi: 10.1159/000516802
16. Huang L, Huang S, Lee A, Hung T. Choriocarcinoma in a viable pregnancy with the rare presentation of intractable lower back pain. Taiwan J Obstet Gyne. (2021) 60:1098–102. doi: 10.1016/j.tjog.2021.09.024
17. Bircher C, Smith RP, Seckl MJ, Brown D, Short D, Rees H, et al. Metastatic choriocarcinoma presenting and treated during viable pregnancy: a case report. BJOG: Int J Obstet Gynecol. (2011) 118:1672–5. doi: 10.1111/j.1471-0528.2011.03062.x
18. Nabers J, Splinter TA, Wallenburg HC, Ten Kate FJ, Oosterom R, Hilvering C. Choriocarcinoma with lung metastases during pregnancy with successful delivery and outcome after chemotherapy. Thorax. (1990) 45:416–8. doi: 10.1136/thx.45.5.416
19. Ghorani E, Kaur B, Fisher RA, Short D, Joneborg U, Carlson JW, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. (2017) 390:2343–5. doi: 10.1016/S0140-6736(17)32894-5
Keywords: gestational choriocarcinoma, metastases, viable pregnancy, chemotherapy, second trimester, case report
Citation: Tian Y, Yu J, Dan X, Chen T and He Y (2024) Case report: Metastatic choriocarcinoma in the second trimester of a viable pregnancy with successful delivery and outcome after chemotherapy. Front. Oncol. 14:1345011. doi: 10.3389/fonc.2024.1345011
Received: 27 November 2023; Accepted: 12 February 2024;
Published: 08 March 2024.
Edited by:
Mihaela Carmen Cristea, Regeneron Pharmaceuticals, Inc., United StatesReviewed by:
Kefeng Shen, Huazhong University of Science and Technology, ChinaMark Wakabayashi, Regeneron Pharmaceuticals, Inc., United States
Copyright © 2024 Tian, Yu, Dan, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yalin He, aGV5YWxpbjA4MjVAc2luYS5jb20=
 Yalin Tian
Yalin Tian Jiayi Yu1,2
Jiayi Yu1,2