- 1Comprehensive Cancer Center, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy
- 2Department of Surgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy
Introduction: Gastric cancer (GC) is the fourth leading cause of cancer-related death worldwide with limited therapeutic options. The aim of this study was to analyze the value of adding surgery to the first-line treatment in patients with oligometastatic GC (OGC).
Methods: This retrospective study included patients with OGC who underwent induction chemotherapy followed by surgery of both primary tumor and synchronous metastasis between April 2012 and April 2022. Endpoints were overall survival (OS) and relapse-free survival (RFS) analyzed by the Kaplan–Meier method. Prognostic factors were assessed with the Cox model.
Results: Data from 39 patients were collected. All cases were referred to our multidisciplinary tumor board (MTB) to evaluate the feasibility of radical surgery. After a median follow-up of 33.6 months (mo.), median OS was 26.6 mo. (95% CI 23.8–29.4) and median RFS was 10.6 mo. (95% CI 6.3–14.8). Pathologic response according to the Mandard criteria (TRG 1–3, not reached versus 20.5 mo. for TRG 4–5; HR 0.23, p=0.019), PS ECOG ≤ 1 (26.7 mo. for PS ≤ 1 versus 11.2 mo. for PS >1; HR 0.3, p=0.022) and a low metastatic burden (26.7 mo. for single site versus 12.9 mo. for ≥2 sites; HR 0.34, p=0.039) were related to good prognosis. No major intraoperative complications nor surgery-related deaths occurred in our series.
Discussion: A sequential strategy of preoperative chemotherapy and radical surgical excision of both primary tumor and metastases was demonstrated to significantly improve OS and RFS. Multidisciplinary evaluation is mandatory to identify patients who could benefit from this strategy.
1 Introduction
Gastric cancer (GC) represents the fifth most diagnosed cancer and the fourth leading cause of cancer-related death (1–3). For the treatment of advanced GC, current guidelines recommend a systemic chemotherapy strategy (4, 5) based on platinum and fluoropyrimidine triplets or doublets combined with anti-HER2 antibodies or immunotherapy based on the molecular assessment (6, 7). Despite these treatments, the median OS for metastatic disease is merely 13.8 months and less than 10% of the patients survive longer than 2 years (8, 9). Thus, new strategies are needed. The role of surgery in metastatic GC is an open debate. In patients identified as having oligometastatic GC (OGC), surgery with curative intention could provide benefit, particularly in those patients with an excellent response to preoperative chemotherapy, achieving the possibility of an R0 intervention (10–14). All available data come from retrospective studies and are controversial. Evidence from randomized controlled prospective trials, such as the REINASSANCE/FLOT-AIO 5 phase 3 study, is anticipated (NCT02578368) (15). The aim of our single-institution experience was to analyze the benefit of adding surgery as another therapeutic option in selected oligometastatic patients after systemic preoperative chemotherapy, retrospectively collecting data from patients treated in a high-volume center for GC treatment.
2 Materials and methods
2.1 Study design and population
This study applied the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement (16). We retrospectively collected data from patients who underwent surgery for OGC in Fondazione Policlinico Universitario “Agostino Gemelli”—IRCCS, Rome, between April 2012 and April 2022. All patients were aged 18 years or older, with a new diagnosis of advanced {stage IV according to American Joint Committee on Cancer (AJCC) 8th edition (17)} gastric or gastroesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2, and adequate baseline bone marrow, liver, and renal function. Tumors located proximally in the gastroesophageal junction were classified according to Siewert and Stein (18). Only Siewert type III tumors were included in the analysis. No one had received any previous chemotherapy for advanced disease and was considered eligible to start a doublet or triplet chemotherapy combination as a first-line treatment. All the radiological images and reports were revised at least twice, at diagnosis and preoperatively after induction chemotherapy by the multidisciplinary tumor board (MTB) to assess the TNM stage and disease response to medical treatment. The surgical indication for each OGC patient was discussed by our GC dedicated MTB and defined by the following criteria: (1) confirmed primary GC, (2) resectable hepatic metastasis, (3) minimal peritoneal dissemination [i.e., peritoneal cancer index (PCI) <6, uni- or bilateral Krukenberg tumors], (4) up to three lymph nodes involved in stations categorized as M1 according to AJCC manual 8th edition (17), (5) eligible for radical surgical treatment, and (6) in good general health conditions. Diagnostic laparoscopy and peritoneal cytology were performed to assess the presence of peritoneal dissemination in newly diagnosed advanced GC patients with suspected peritoneal dissemination before starting any kind of treatment as per routine clinical practice and PCI index was used to stratify patients according to living guidelines (4, 19). The decision about the chemotherapy administration was made by medical oncologists according to baseline disease and patient characteristics. The heterogeneity in treatment protocols and number of cycles performed were not considered for statistical analysis. Primitive tumor excision was combined with metastasectomy in one-time surgery. All patients with potentially curable lesions were treated by metastasectomy, gastrectomy, and extended lymphadenectomy (D2) on purpose, in agreement with current guidelines (4, 20). For tumors located in the middle and lower thirds of the stomach, a subtotal gastrectomy was generally preferred, provided that an adequate resection margin was maintained. After total gastrectomy with lymph node dissection, esophagojejunostomy (using a circular stapler, diameter 25 mm) was used routinely for Roux-en-Y reconstruction. In case of subtotal gastrectomy, intestinal continuity was restored by means of Billroth II or Roux-en-Y gastrojejunostomy, at the discretion of the surgeon. Resection was stated as potentially curative (R0 according to the UICC/AJCC staging system (17), if macro- and microscopically no tumor was left following surgery). At the end of the operation, the surgeon resected all lymph nodes from the surgical specimen and identified their distribution according to the Japanese Gastric Cancer Association classification (20). Pathological TNM classification, the status of margins and histological tumor regression grade (TRG) assessed in accordance with the Mandard criteria (21) were described in the pathologists’ report. The patients were monitored for up to 30 days by a surgeon to assess postoperative complications and mortality. All clinical and pathological data were stored in a GC database and retrospectively evaluated for this study. Patient status was investigated by follow-up examination or by telephone contact.
2.2 Endpoints
The primary endpoint was overall survival (OS) defined as the time from diagnosis to death or latest follow-up; the secondary endpoint was recurrence-free survival (RFS) defined as the time from surgery to radiological evidence of relapse. An exploratory analysis was performed to evaluate the survival impact of demographic and clinicopathological factors such as age, sex, ECOG PS, histotype (according to Lauren’s classification) (22, 23), primitive tumor site (esophagogastric junction, fundus/body, and antrum/pylorus) and extension (T), metastatic sites (liver, lymph nodes, and peritoneum) and burden (single metastatic site versus multiple sites), resection margins (R0 versus R1–2), and TRG according to the Mandard criteria (1–3 versus 4–5) (21, 24). Furthermore, we tested a prognostic score based on five relevant prognostic factors: (1) pathological tumor growth (T+) on surgical specimen; (2) presence of liver metastases (L+); (3) peritoneum (P+) or (4) non-locoregional lymph nodes (N+); and (5) presence of macroscopical residual disease on resection margin (R2 resection).
2.3 Statistical analysis
Statistical analyses were performed using SPSS® software, version 29.0 Chicago, IL. The survival curves were generated by the Kaplan–Meier method and compared using the Log-rank test. Demographic and clinicopathological factors were collected and evaluated by univariate analysis among patients who received macroscopically complete resection to evaluate their impact on OS and RFS. Discrete variables were compared using the Chi-square test and continuous variables were compared using independent-samples t-test. p-value <0.05 was considered statistically significant. Only variables that were statistically significant at univariate analysis were tested in multivariate analysis with Cox proportional hazards model to identify independent predictors of specific survival and recurrence.
3 Results
3.1 Demographic and clinicopathological characteristics of patients
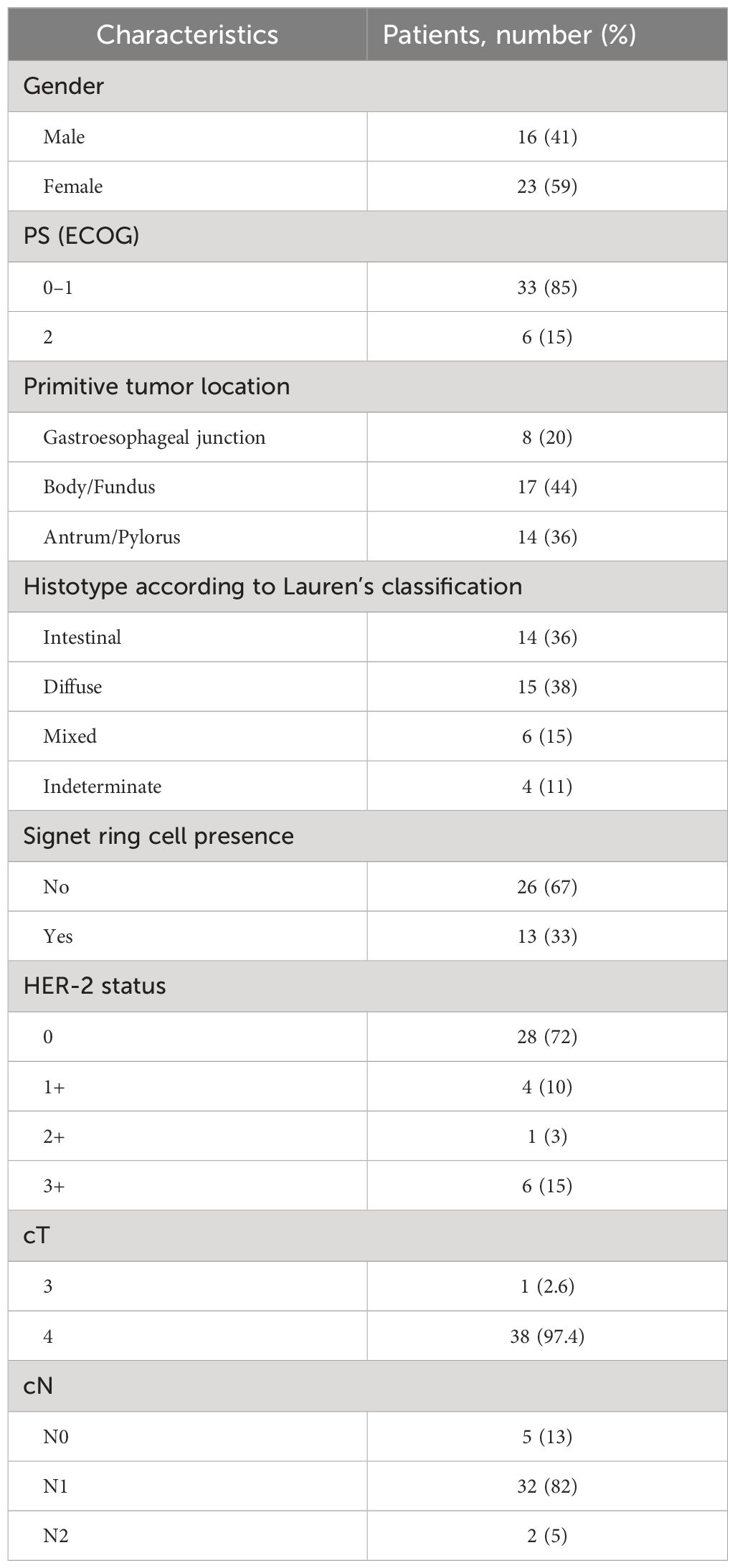
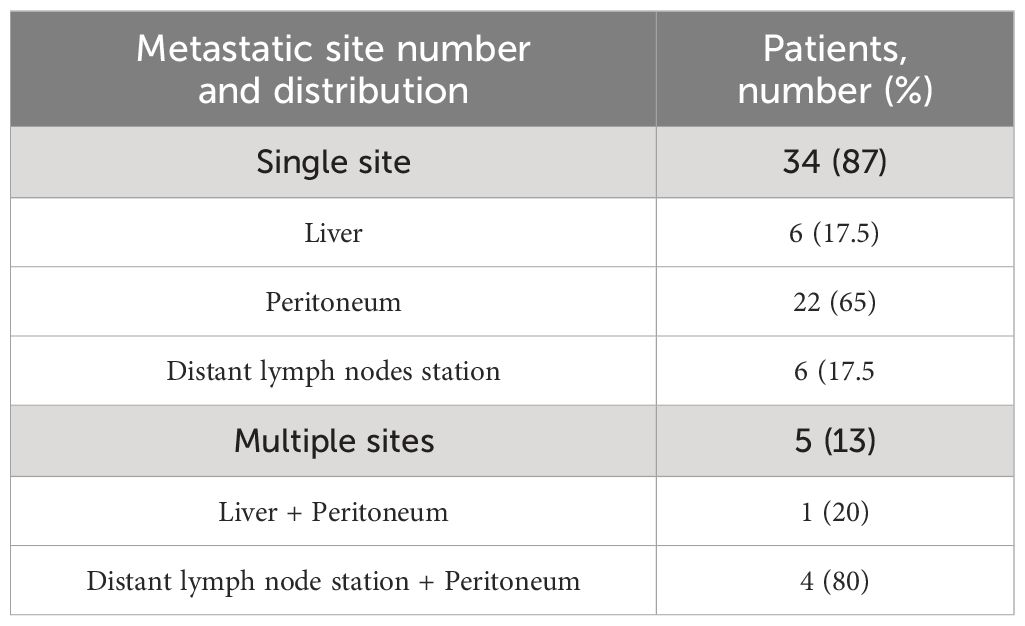
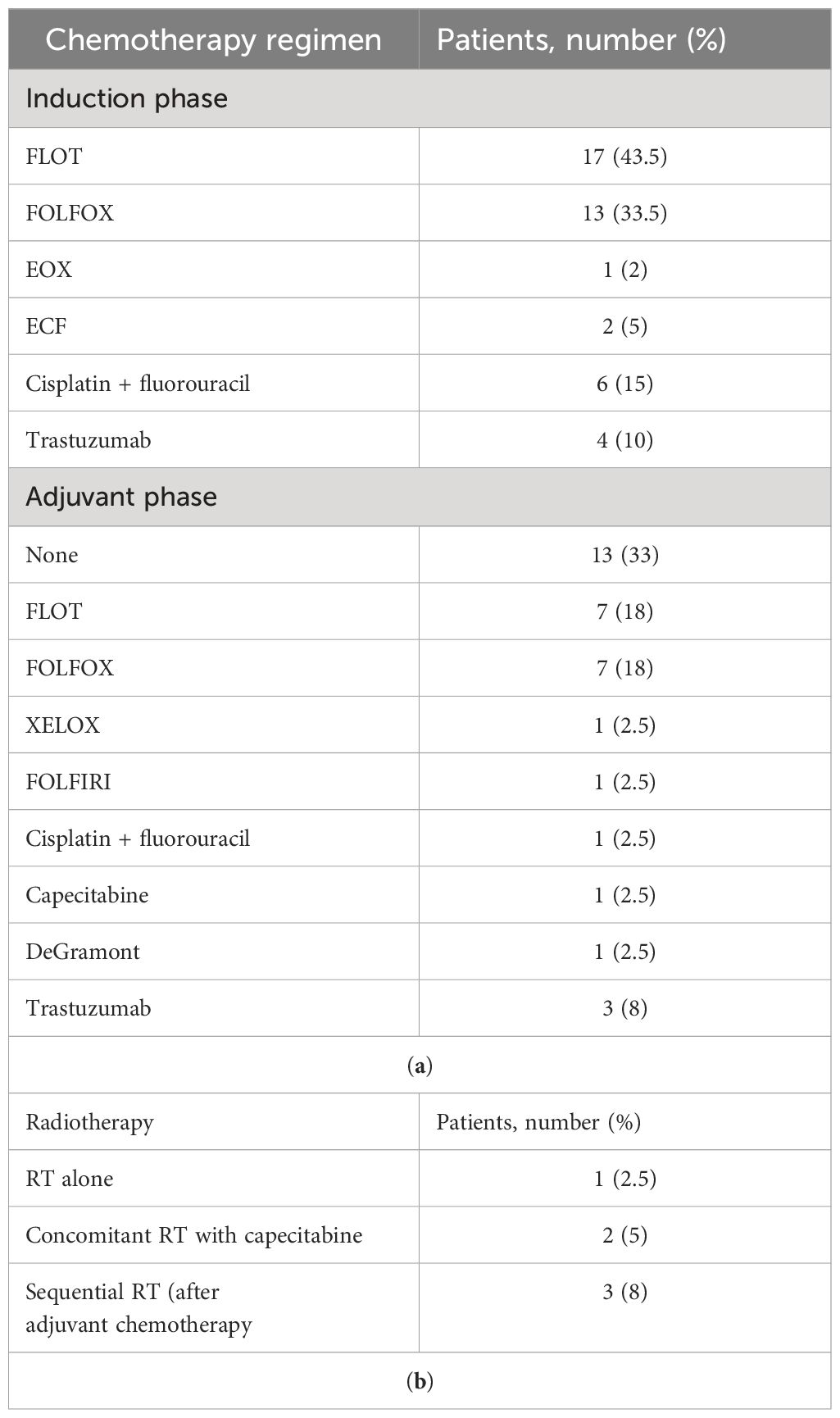
In the past 10 years, 39 patients with metastatic GC, 16 men and 23 women, matching the aforementioned criteria had undergone surgery with radical intent at our institution. Patients’ age at diagnosis ranged from 26 to 75 years, with a median age of 58 years. PS ECOG was 0–1 for 33 patients and 2 for 6 patients. Primary tumor site was located at the gastroesophageal junction in 8 cases, at the gastric body in 17 cases, and at the antrum/pylorus in 14 cases. All subjects had a histopathologically confirmed diagnosis of adenocarcinoma according to Lauren’s classification (22). The specimens of 14 patients were diagnosed as intestinal subtype, 15 were diffuse, 6 were mixed type, and 4 were indeterminate type. Signet ring cells have been detected in 13, all in patients with a diffuse subtype. Among the population, 18% of patients were found positive for HER2 amplification score ≥ 2+. All patients had a baseline clinical T4 stage except one T3, and most of them had a locoregional nodal involvement (N1–N2). A total of 34 study patients had only one metastatic site: 6 of them presented with only liver metastases, 22 presented with peritoneal metastases, and 6 presented with distant lymph nodes (Table 1). The others had ≥2 synchronous metastatic sites (Table 2). All of them were considered eligible to first-line chemotherapy and received 2–6 months of induction systemic platinum and fluoropyrimidine combination chemotherapy, with or without trastuzumab, according to histology and HER2 status (4, 5) (Table 3). Any case was referred to our MTB and considered amenable to radical surgery after the induction chemotherapy.
3.2 Surgical outcomes
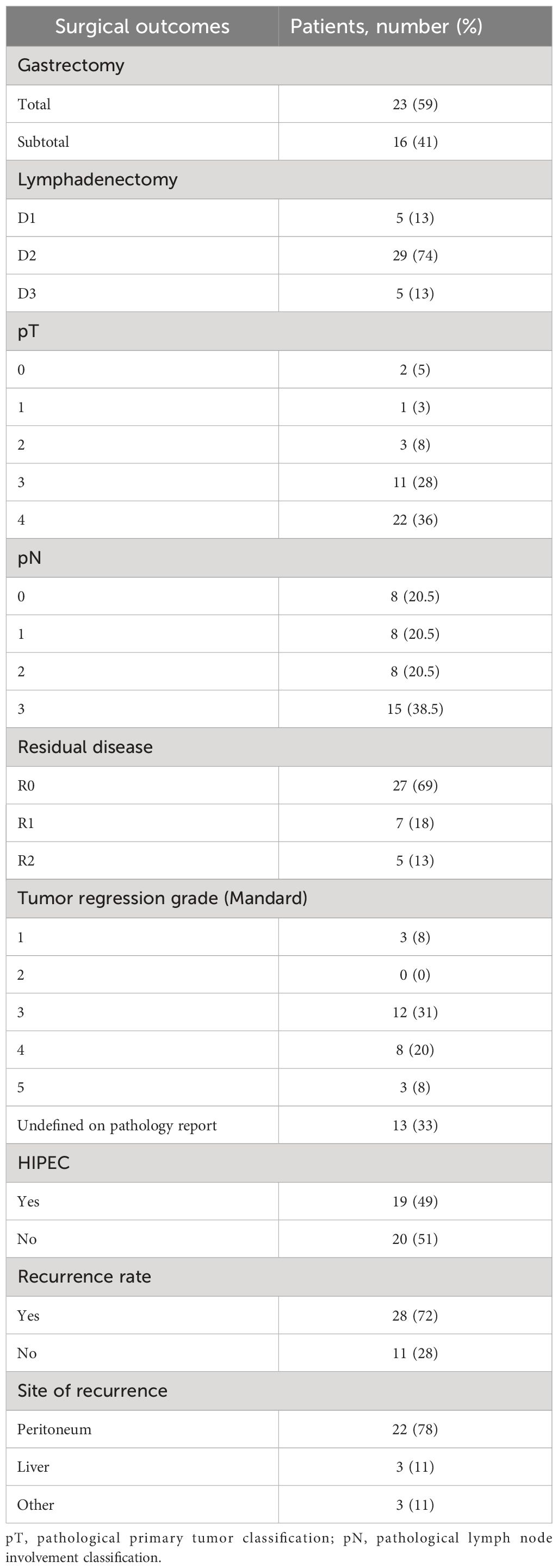
Surgery was conducted in both primitive tumor and metastases at the same time. Regarding surgical approach to primitive tumor, for 59% of patients, a total gastrectomy was performed, while in 41%, a subtotal gastrectomy was carried out. Four (10.2%) patients with GEJ adenocarcinoma underwent a thoraco-abdominal gastrectomy, while in all other cases, a laparoscopic abdominal surgical approach was used. In 28 (71.8%) cases, a laparoscopic technique was preferred, whereas in 11 (28.2%) cases, patients underwent a laparotomy. Up to 87% of cases underwent a lymphadenectomy ≥ D2; in five cases, a D1 lymphadenectomy was performed. A total of 19 patients (49%) presenting peritoneal-only metastatic spread underwent hyperthermic intraperitoneal chemotherapy (HIPEC). HIPEC was carried out according to the Coliseum technique (25) using mitomycin C (MMC) at a dose of 15 mg/m2 and cisplatin at a dose of 75 mg/m2 administered for 90 min with an inflow temperature of 41–42°C and an outflow temperature of 39–40°C (26). An R0 resection was achieved in 27 patients (69%), while microscopic (R1) or macroscopic (R2) residual disease was found in 7 (18%) and 5 (13%) cases, respectively (Table 4). TRG evaluated according to the Mandard criteria was available for 26 patients: 58% of them were pathologic responders (TRG 1–3) and 42% were non-responders (TRG 4–5). Combined surgery on both gastric primitive tumor and metastasis clearly affected operation duration but did not negatively affect patients’ outcome in terms of mortality and performance status. No major intraoperative complications or surgery-related deaths occurred in our series. During the hospitalization, up to 12 (30.7%) patients developed at least one postoperative complication. According to the Clavien–Dindo classification (27), the most frequent were grade I/II complications, while two patients (5.1%) reported a grade III complication. One patient reported an abdominal collection treated by percutaneous drainage plus antibiotic therapy; another experienced postoperative bleeding treated by angioembolization. Only one subject (2.5%) reported a grade IV complication: an acute renal failure requiring hemodialysis. A summary of postoperative complications is available in the Supplementary Materials (Supplementary Table S1). Actually, the greater surgical stress related to an extensive resection did not deteriorate prognosis in patients without postoperative complications (69.2%). After surgery, 26 patients (66.7%) were able to receive an adjuvant treatment (Table 3).
3.3 Survival outcomes
At the data cutoff analysis of November 2022, 17 patients were alive and, among them, 7 were free from any disease recurrence. Recurrence rate (RR) after surgery was 72%: in 78% of cases, disease relapse occurred in the peritoneum (22 cases); 11% (3) of patients progressed with liver metastases; one patient had a recurrence on both sites; one had only distant lymph node relapse; one patient progressed with multiple metastasis also involving brain and lungs. After a median follow-up of 33.6 months, median OS was 26.6 months (95% CI 23.8–29.4) (Figure 1A) and median RFS was 10.6 months (95% CI 6.3–14.8) (Figure 2A). Estimated 3-year OS was 13%. At univariate analysis, factors that significantly influenced OS were a good pathologic response according to the Mandard criteria [TRG 1–3, not reached (NR) versus 20.5 months for TRG 4–5, HR 0.23, p = 0.019] (Figure 1B), PS ECOG ≤ 1 (26.7 versus 11.2 months, HR 0.3, p = 0.022) (Figure 1C), and the number of metastatic sites: 26.7 months for patients with a single site versus 12.9 months for those with ≥2 sites (HR 0.34, p = 0.039) (Figure 1D). Having a single metastatic site was also the only variable that statistically significantly influenced RFS (12.8 vs. 4.4 months, HR 0.10, p < 0.001) (Figure 2B). The multivariate analysis confirmed having a single metastatic site as the only factor that statistically significantly influenced OS (HR 0.08, p = 0.048) but not RFS (Supplementary Tables S2, S3). Other clinicopathological features such as age, sex, histological type, HER-2 status, T-stage, N-stage, and primitive tumor site did not influence the benefits seen in these patients. In a study by Samarasam et al., patients were stratified according to four prognostic factors, demonstrating how the survival advantage of patients who underwent surgical resection disappeared when ≥3 negative factors were present (28). Thus, we tried to test an analogue score adding a relevant fifth prognostic factor: (1) pathological tumor growth (T+) on surgical specimen; (2) presence of liver metastases (L+); (3) peritoneum (P+) or (4) non-locoregional lymph nodes (N+); and (5) R2 resection. Moreover, in our population, there was a statistically significant difference between patients with a score of 1–2 and patients with a score of ≥3 in terms of both OS (26.7 vs. 12.9 months, HR 0.31, p = 0.023) (Figure 1E) and RFS (12.8 vs. 4.4 months, HR 0.10, p < 0.001) (Figure 2C).
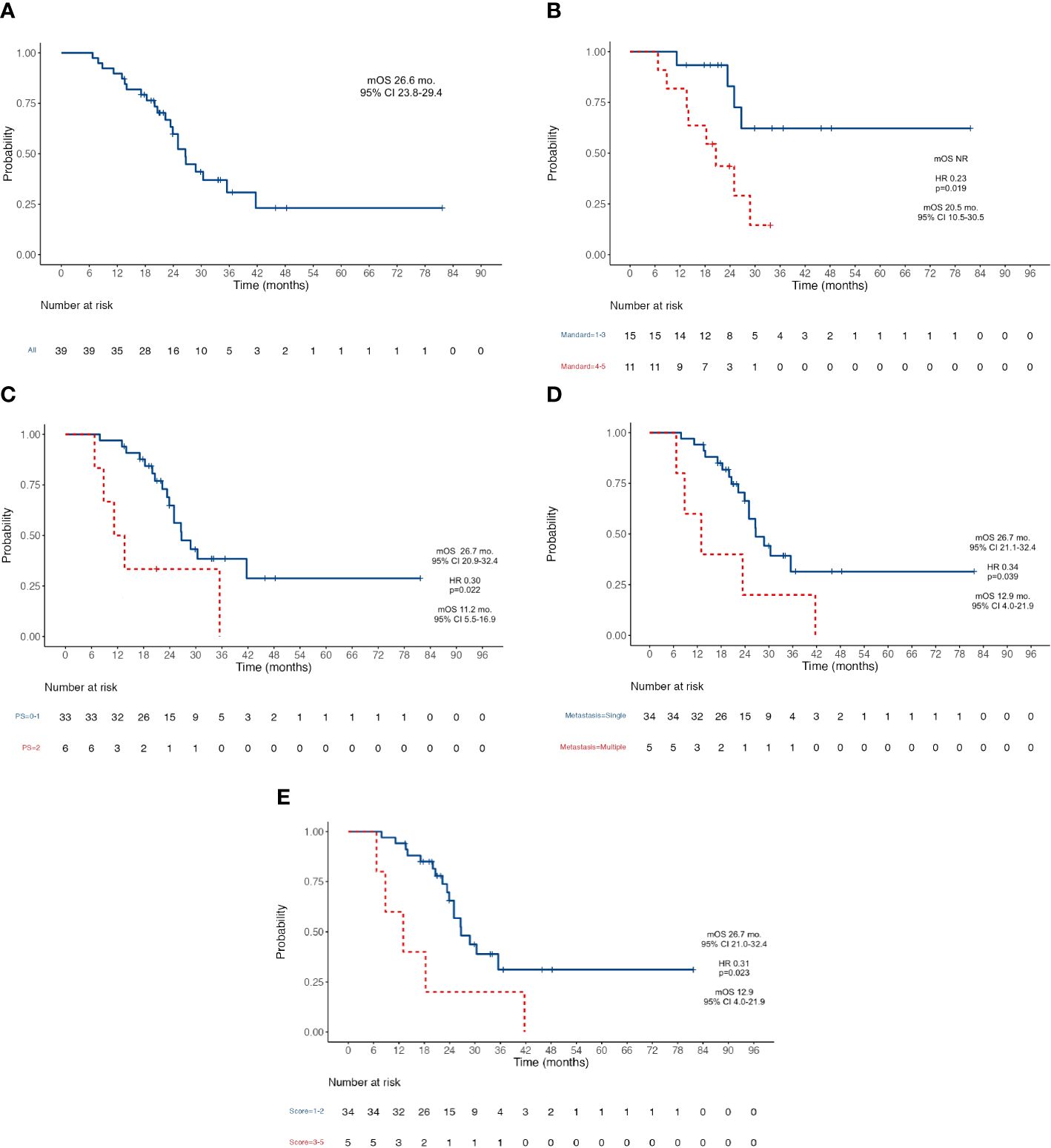
Figure 1 Kaplan-Meier survival curves for OS. (A) OS in the whole population; (B) OS according to Mandard Tumor Regression Grade after induction chemotherapy; (C) OS according to patient’s PS ECOG; (D) OS according to the number of metastatic sites; (E) OS according to the five-factors prognostic score. OS, Overall Survival; NR, Not Reached; PS, Performance Status; ECOG Eastern Cooperative Oncology Group; mOS, Median Overall Survival; mo, Months; CI, Confidence Interval; HR, Hazard ratio.
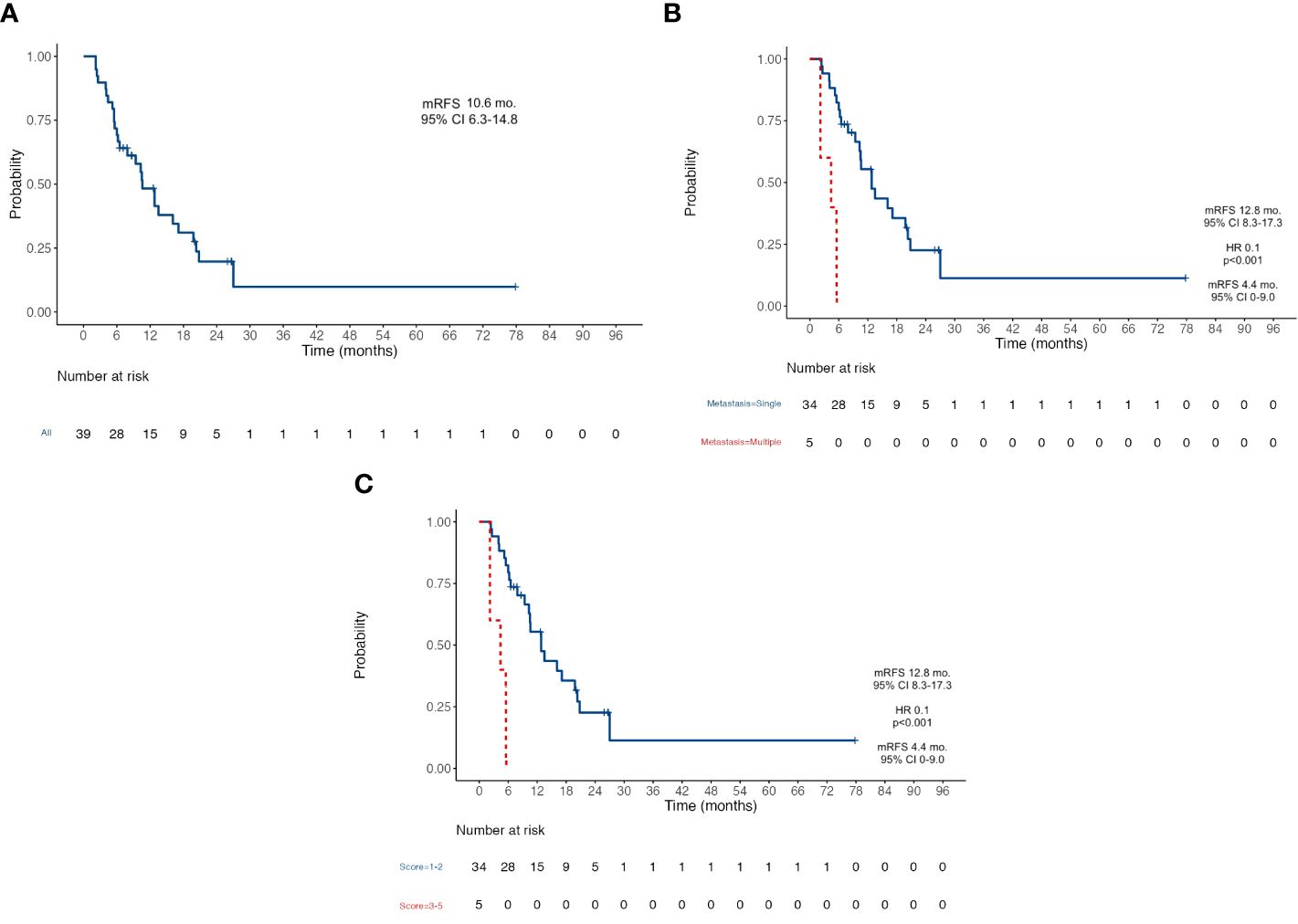
Figure 2 Kaplan-Meier survival curves for Recurrence-Free Survival RFS. (A) RFS in the whole population; (B) RFS according to the number of metastatic sites; (C) RFS according to the five-factors prognostic score. RFS, Recurrence-Free Survival; mFRS, Median Recur-rence-Free Survival; mo, Months; CI, Confidence Interval; HR Hazard ratio.
4 Discussion
In recent years, OGC has become a recognized entity in clinical practice although a formal definition is still lacking (29). Despite chemotherapy treatment being the standard of care for advanced disease, increasing data from several series suggest that selected patients with limited metastatic spread might achieve long-term disease control and prolonged survival thanks to an aggressive multimodal strategy including surgery (11, 14, 28, 30–36). In the REGATTA trial, the authors explored the role of surgery for OGC with a single non-curable metastatic site, and results did not show any advantage for the surgical resection of the primary tumor (32). To note, up to 75% of the enrolled patients presented with peritoneal involvement such as non-curable metastasis, an independent poor prognostic factor (37). Instead, Markar et al. reported how combining metastasectomy and gastrectomy might be associated with improved survival without increasing postoperative mortality (34). Recently, the role of induction chemotherapy gained increasing attention, following early results from both clinical trials and real-world experience (12, 14, 31, 32, 38, 39). Han et al. found that patients with metastatic GC who were good responders to induction chemotherapy and who underwent curative R0 resection achieved an impressive median survival of 22.9 months (31). In our series, we collected real-world data from the repository of a high-volume tertiary referral institution (40). We strictly followed inclusion criteria comparable to the previous studies demonstrating a median OS extent to 26.6 months (95% CI 23.8–29.4) and a median RFS of 10.6 months (95% CI 6.3–14.8), overcoming any historical OS reported in first-line chemotherapy pivotal trials, which ranges approximately 9–11 months (9, 41, 42). In our study, how correct patient selection plays a key role clearly emerged, as revealed by the significant impact of the number of metastatic organs involved on OS (HR 0.34, p = 0.031) and RFS (HR 0.101, p < 0.001) rather than the specific site (L+, P+, or N+), provided that surgery was performed in a high-flow center after MTB discussion, as in our cases. Furthermore, the pivotal role of an experienced GC-dedicated MTB and the need for a repeated multidisciplinary evaluation have to be emphasized, not only at diagnosis but also during the treatment phase, owing to the fact that each patient deserves individual recommendations at any disease time point. Surgical radicality, defined as R0 resection, was achieved in 69% of patients, whereas in a minority of cases, a complete removal of all sites of metastasis was not technically feasible, mainly due to a greater extent of disease to the peritoneum than what we expected based on preoperative staging. Nevertheless, residual disease (R1–R2) seems not to affect OS as a single independent prognostic factor. In the same way, lymphadenectomy extent did not influence the benefit on OS. In each case, the best possible surgical procedure was performed in order to obtain the maximal cytoreduction and leave the patient free from macroscopical disease. In the majority of cases, a D2 lymphadenectomy was done, whereas a lymphadenectomy D3 was performed in a minority of patients based on the extent of disease in order to reach a radical resection as aforementioned. In contrast, patients who underwent only perigastric (D1) lymphadenectomy might be considered patients with a suboptimal surgical treatment, but their presence in the study, as well as the presence of R1–R2 resections, reflects a real-world surgical outcome and emphasizes how even these patients could obtain survival advantage from a combinatory strategy gaining a meaningful benefit from induction chemotherapy. Undoubtedly, the surgeon’s expertise significantly influenced the postoperative outcome of patients, revealing the gastrectomy plus lymphadenectomy combined with metastasectomy to be a safe procedure without significant improved morbidity. An emerging factor that also influenced OS was the efficacy of the preoperative antineoplastic systemic treatment; indeed, a good pathologic response such as Mandard TRG ≤ 3 was associated with better prognosis. In Oyama et al.’s retrospective study, comparing neoadjuvant versus adjuvant treatment in patients with GC and para-aortic lymph node metastasis who underwent surgical resection, 87.5% of patients in the first arm had a pathological response with 2-year OS and RFS rates of 93.8% and 75.0%, respectively (39). Despite the fact that pathological complete response (pCR) was not significantly associated with OS and RFS in the univariate analysis in our population, it is encouraging that patients who reached a pCR (TRG 1) on the surgical specimen had no evidence of disease relapse or death at data cutoff. These results identified whether surgery in a patient with OGC might be considered as another effective therapeutic line in a sequencing strategy. The major limitation of our study is its retrospective nature, which may entail selection bias and potential confounders. In our real-world experience, the indication to surgery was discussed in MTB for each patient at diagnosis. The indication to surgery was assessed again by our MTB after the completion of induction chemotherapy and on treatment radiological evaluation. Thus, we considered only patients who underwent surgery, not evaluating the rate of OGC that is potentially resectable at diagnosis and then becomes lost because of disease progression. Furthermore, the small sample size might have affected the results, limiting the power of our study and the statistical impact of some validated prognostic factors that appear not to have a significant role in survival outcome in our analyses. However, the small sample size reflects the reality of a monoinstitutional experience and the low rate of patients among the metastatic setting that might match the OGC definition criteria. Eventually, the final OS might have been influenced by chemotherapy protocols and number of cycles received during the neoadjuvant and adjuvant phases and all the subsequent systemic therapies administered on progressive disease. The variety in treatment regimens was an unavoidable bias as a consequence of the dramatic changes in the treatment landscape for patients with GC over the last 10 years, but due to this heterogeneity, it is not possible to estimate their impact on survival outcomes. In spite of these limits, our results send a clear message about the importance of a multimodal approach in patients with OGC and contribute to extend the evidence on current treatment options in this setting, which is still a matter of debate. The combined approach of induction chemotherapy followed by subsequent radical surgery on primitive tumor and synchronous metastases requires confirmation from large prospective randomized trials to build a new evidence-based standard of care. A first effort in this direction was the phase II FLOT-3 trial, which exploited the efficacy of induction chemotherapy followed by surgical resection in both limited and extensive metastatic GC with an overall median OS of 31.3 months for patients who underwent surgery, with a benefit of up to 1 year for those with limited metastatic spread compared to others (12). The ongoing phase III FLOT 5-RENAISSANCE trial further investigates this topic by enrolling patients with untreated OGC to be randomized 1:1 to undergo chemotherapy or surgical resection of primary tumor and metastases after induction chemotherapy (NCT02578368) (15). Likewise, the French SURGIGAST study compares the continuation of chemotherapy against a radical surgical approach in OGC (NCT03042169) (43).
5 Conclusions
Despite the limitations of the study due to its retrospective nature, it confirms the survival benefit of a sequential strategy of preoperative chemotherapy and radical surgical excision of both primary tumor and metastases in patients with OCG, providing a new treatment option for patients who are currently treated only with palliative chemotherapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of Fondazione Policlinico Agostino Gemelli IRCSS - Catholic University of Sacred Heart (protocol code 42028/19-ID2825). The study was conducted in accordance with the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Author contributions
MM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MB: Conceptualization, Writing – review & editing. RV: Conceptualization, Writing – review & editing. ED: Conceptualization, Writing – review & editing. AB: Conceptualization, Writing – review & editing. AD: Conceptualization, Writing – review & editing. FR: Conceptualization, Writing – review & editing. VT: Conceptualization, Writing – review & editing. AA: Conceptualization, Writing – review & editing. GT: Conceptualization, Writing – review & editing. AS: Conceptualization, Writing – review & editing. CP: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1343596/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Marte G, Tufo A, Steccanella F, Marra E, Federico P, Petrillo A, et al. Efficacy of surgery for the treatment of gastric cancer liver metastases: A systematic review of the literature and meta-analysis of prognostic factors. J Clin Med. (2021) 10(5):1141. doi: 10.3390/jcm10051141
4. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33(10):1005–20. doi: 10.1016/j.annonc.2022.07.004
5. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
6. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376(9742):687–97. doi: 10.1016/S0140–6736(10)61121-X
7. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398(10294):27–40. doi: 10.1016/S0140–6736(21)00797–2
8. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. (2006) 24(18):2903–9. doi: 10.1200/JCO.2005.05.0245
9. Okines AFC, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. (2009) 20(9):1529–34. doi: 10.1093/annonc/mdp047
10. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19(2):329–38. doi: 10.1007/s10120-015-0575-z
11. Jin P, Ji X, Tian Y. Surgical management of oligometastatic disease in gastric cancer. Clin Res Hepatol Gastroenterol. (2020) 44(5):638–45. doi: 10.1016/j.clinre.2020.02.003
12. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. (2017) 3(9):1237–44. doi: 10.1001/jamaoncol.2017.0515
13. Ministrini S, Solaini L, Cipollari C, Sofia S, Marino E, D’Ignazio A, et al. Surgical treatment of hepatic metastases from gastric cancer. Updates Surg. (2018) 70(2):273–8. doi: 10.1007/s13304–018-0536–2
14. Carmona-Bayonas A, Jiménez-Fonseca P, Echavarria I, Sánchez Cánovas M, Aguado G, Gallego J, et al. Surgery for metastases for esophageal-gastric cancer in the real world: Data from the AGAMENON national registry. Eur J Surg Oncol. (2018) 44(8):1191–8. doi: 10.1016/j.ejso.2018.03.019
15. Al-Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen S, et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer. (2017) 17(1):893. doi: 10.1186/s12885–017-3918–9
16. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PloS Med. (2015) 12(10):e1001885. doi: 10.1371/journal.pmed.1001885
17. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67(2):93–9. doi: 10.3322/caac.21388
18. Stein HJ, Feith M, Siewert JR. Cancer of the esophagogastric junction. Surg Oncol. (2000) 9:35–41. doi: 10.1016/s0960–7404(00)00021–9
19. Schena CA, Laterza V, De Sio D, Quero G, Fiorillo C, Gunawardena G, et al. The role of staging laparoscopy for gastric cancer patients: current evidence and future perspectives. Cancers. (2023) 15:3425. doi: 10.3390/cancers15133425
20. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
21. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. (1994) 73(11):2680–6. doi: 10.1002/1097–0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c
22. Lauren P. The two histological main types of Gastric Carcinoma: Diffuse and so-called Intestinal-Type Carcinoma. An attempt at a histo-clinical cassification. Acta Pathol Microbiol Scand. (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
23. Leocata P, Ventura L, Giunta M, Guadagni S, Fortunato C, Discepoli S, et al. Carcinoma gastrico: studio istopatologico di 705 casi [Gastric carcinoma: a histopathological study of 705 cases]. Ann Ital Chir. (1998) 69:331–7
24. Derieux S, Svrcek M, Manela S, Lagorce-Pages C, Berger A, André T, et al. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard’s Tumor Regression Grade (TRG) in gastric adenocarcinoma. Dig Liver Dis. (2020) 52(1):107–14. doi: 10.1016/j.dld.2019.07.010
25. Sugarbaker PH. Management of peritoneal-surface Malig- nancy: the surgeon’s role. Langenbeck’s Arch Surg. (1999) 384:576–87. doi: 10.1007/s004230050246
26. Rosa F, Galiandro F, Ricci R, Di Miceli D, Longo F, Quero G, et al. Survival advantage of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced gastric cancer: experience from a Western tertiary referral center. Langenbecks Arch Surg. (2021) 406:1847–57. doi: 10.1007/s00423–021-02102–2
27. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
28. Samarasam I, Chandran BS, Sitaram V, Perakath B, Nair A, Mathew G. Palliative gastrectomy in advanced gastric cancer: is it worthwhile? ANZ J Surg. (2006) 240(2):205–13. doi: 10.1111/j.1445–2197.2006.03649.x
29. Kroese TE, van Laarhoven HWM, Nilsson M, Lordick F, Guckenberger M, Ruurda JP, et al. Definition of oligometastatic esophagogastric cancer and impact of local oligometastasis-directed treatment: A systematic review and meta-analysis. Eur J Cancer. (2022) 166:254–69. doi: 10.1016/j.ejca.2022.02.018
30. Hioki M, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg. (2010) 34(3):555–62. doi: 10.1007/s00268–010-0396–5
31. Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol. (2013) 107(5):511–6. doi: 10.1002/jso.23284
32. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17(3):309–18. doi: 10.1016/S1470–2045(15)00553–7
33. Martella L, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for liver metastases from gastric cancer: A meta-analysis of observational studies. Med (Baltimore). (2015) 94(31):e1113. doi: 10.1097/MD.0000000000001113
34. Markar SR, Mackenzie H, Mikhail S, Mughal M, Preston SR, Maynard ND, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. (2017) 20(2):379–86. doi: 10.1007/s10120–016-0604–6
35. Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford). (2010) 12(9):589–96. doi: 10.1111/j.1477–2574.2010.00224.x
36. Kodera Y, Fujitani K, Fukushima N, Ito S, Muro K, Ohashi N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. (2014) 17(2):206–12. doi: 10.1007/s10120-013-0299-x
37. Ye Z, Yu P, Cao Y, Chai T, Huang S, Cheng X, et al. Prediction of peritoneal cancer index and prognosis in peritoneal metastasis of gastric cancer using NLR-PLR-DDI score: A retrospective study. Cancer Manag Res. (2022) 14:177–87. doi: 10.2147/CMAR.S343467
38. Li ZY, Tang L, Zhang LH, Bu ZD, Wu AW, Wu XJ, et al. Weekly docetaxel and cisplatin plus fluorouracil as a preoperative treatment for gastric cancer patients with synchronous multiple hepatic metastases: a pilot study. Med Oncol. (2010) 27(4):1314–8. doi: 10.1007/s12032-009-9381-y
39. Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. (2012) 105(6):535–41. doi: 10.1002/jso.22125
40. Rosa F, Alfieri S, Tortorelli AP, Fiorillo C, Costamagna G, Doglietto GB. Trends in clinical features, postoperative outcomes, and long-term survival for gastric cancer: a Western experience with 1,278 patients over 30 years. World J Surg Oncol. (2014) 12:217. doi: 10.1186/1477–7819-12–217
41. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. (2008) 358(1):36–46. doi: 10.1056/NEJMoa073149
42. Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. (2017) 8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4
Keywords: gastric cancer, induction chemotherapy, metastasectomy, surgical oncology, cancer survival
Citation: Maratta MG, Vitale A, Basso M, Vivolo R, Di Monte E, Biondi A, Di Giorgio A, Rosa F, Tondolo V, Agnes A, Tortora G, Strippoli A and Pozzo C (2024) Benefit of a multimodal approach combining chemotherapy and surgery in oligometastatic gastric cancer: experience from a tertiary referral center. Front. Oncol. 14:1343596. doi: 10.3389/fonc.2024.1343596
Received: 23 November 2023; Accepted: 17 May 2024;
Published: 07 June 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Dragos Eugen Georgescu, Carol Davila University of Medicine and Pharmacy, RomaniaBirendra Kumar Sah, Shanghai Jiao Tong University, China
Copyright © 2024 Maratta, Vitale, Basso, Vivolo, Di Monte, Biondi, Di Giorgio, Rosa, Tondolo, Agnes, Tortora, Strippoli and Pozzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Grazia Maratta, bWFyaWFncmF6aWEubWFyYXR0YUBndWVzdC5wb2xpY2xpbmljb2dlbWVsbGkuaXQ=
†These authors share first authorship
‡These authors share senior authorship
 Maria Grazia Maratta1*†
Maria Grazia Maratta1*† Antonio Vitale
Antonio Vitale Andrea Di Giorgio
Andrea Di Giorgio Fausto Rosa
Fausto Rosa Antonia Strippoli
Antonia Strippoli