
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 12 February 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1343157
Castleman disease (CD), a distinct lymphoproliferative disorder, is infrequently encountered in clinical practice and poses significant diagnostic challenges. We present the case of a 48-year-old asymptomatic female, admitted for evaluation of a hepatic mass detected in the liver’s right lobe. Preoperative laboratory tests were within normal limits. Diagnostic imaging, including contrast-enhanced magnetic resonance imaging (MRI), was suggestive of hepatocellular carcinoma. Furthermore, contrast-enhanced abdominal computed tomography (CT) scans were indicative of hepatic malignancy. Subsequently, the patient underwent laparoscopic surgery targeting a retroperitoneal mass. During the surgical procedure, it was observed that the tumor was a retroperitoneal mass situated posterior to the liver, exhibiting localized adhesion to hepatic tissue. The postoperative histopathological analysis revealed the mass to be hyaline-vascular type Castleman disease (HV-CD), thereby refuting the initial diagnosis of a hepatic malignancy. This case underscores the complexity of diagnosing retroperitoneal Castleman disease, particularly when it masquerades as a hepatic tumor.
Castleman disease (CD) is an uncommon form of reactive lymphadenopathy with an unknown etiology. It most commonly presents as a mediastinal nodal mass. CD can be divided into two categories based on clinical presentation and disease course: unicentric CD (UCD) and multicentric CD (MCD). The prognostic outcomes for the two subtypes of CD differ significantly. MCD typically exhibits a poor prognosis and is not amenable to curative surgical intervention, necessitating pharmacological management to control the disease. In contrast, UCD, often of the hyaline-vascular (HV) subtype, is usually amenable to curative resection. Approximately 80% to 90% of cases are classified histologically as hyaline vascular (HV-CD). HV-CD typically lacks systemic symptoms, and laboratory examination results and radiological information often lack specificity (1). Therefore, in previous reports, when the disease occurs in or resembles other organs such as the kidney, parotid gland, or pancreas, CD may be mistaken for other solid tumors (2–4). However, there are scant reports in the literature regarding cases of retroperitoneal CD masquerading as malignant hepatic tumors. Herein, we present a case of a 48-year-old female patient who was initially diagnosed with liver malignancy based on preoperative imaging but was subsequently confirmed to have retroperitoneal HV-CD through postoperative pathological examination. Six months following the procedure, the patient’s enhanced abdominal CT scan demonstrated favorable postoperative recovery with no evidence of disease recurrence.
A 48-year-old female patient was admitted to the hospital for further diagnosis and treatment following the discovery of a tumor in the right lobe of her liver during a health checkup one week earlier. The patient had no clinical manifestations. Contrast-enhanced magnetic resonance imaging (MRI) revealed a nodular mass in the right lobe of the liver with an enhancement pattern that appeared as fast-in and fast-out, leading to a diagnosis of hepatocellular carcinoma (Figures 1D–F). However, upon admission to the hospital, contrast-enhanced abdominal computed tomography (CT) revealed a nodular enhancement measuring approximately 3.1 cm × 2.0 cm in the right lobe of the liver at the arterial phase. The degree of strengthening at the portal vein stage and the delayed stage was reduced, indicating hepatic cancer (Figures 1A–C). Preoperative laboratory examinations, including tumor markers for the digestive tract, were normal.
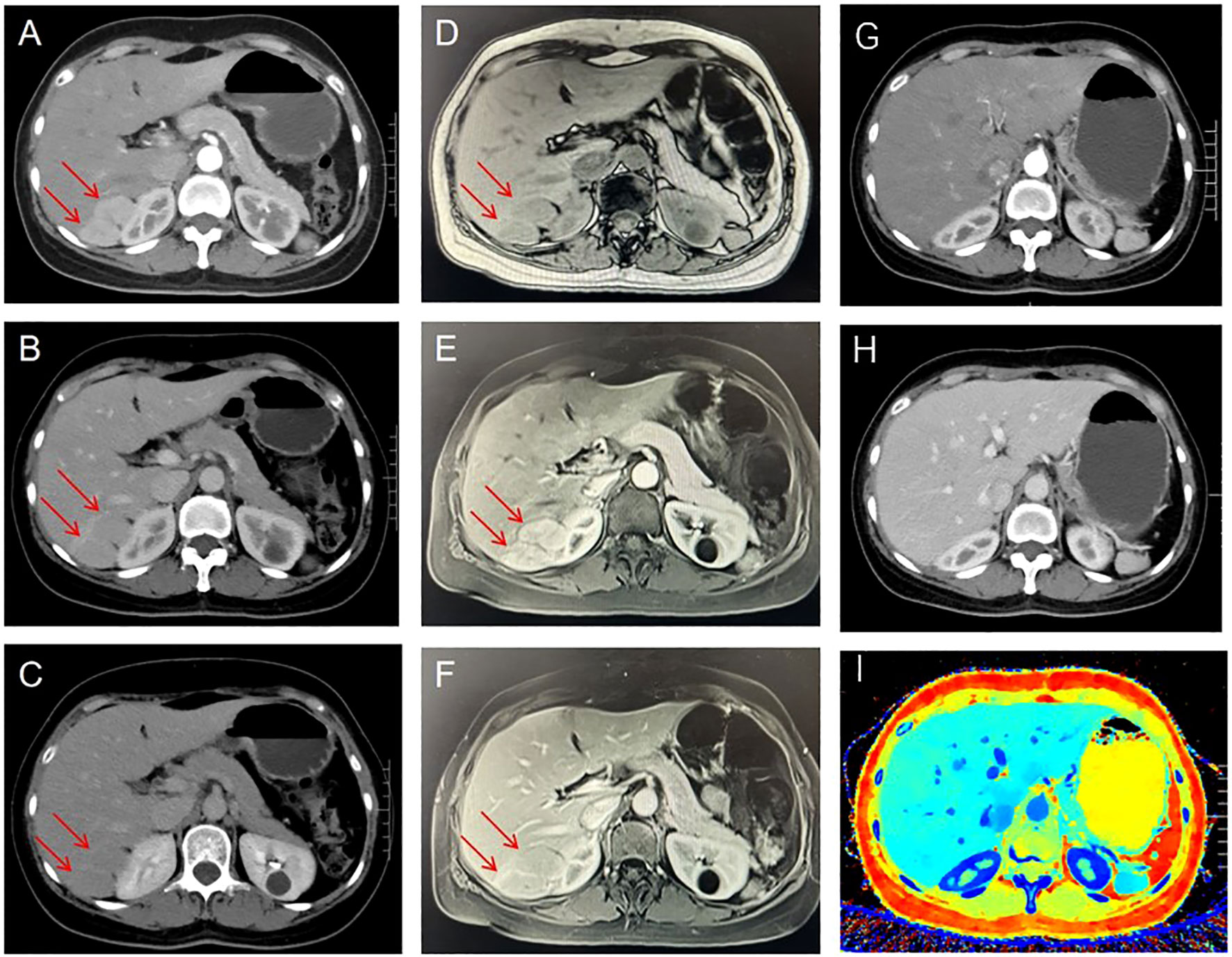
Figure 1 Preoperative contrast-enhanced CT showed a nodular and significantly enhanced lesion located in the right lobe of the liver during the arterial phase (A), with reduced enhancement in the portal and delayed phases (B, C). Preoperative Contrast-enhanced magnetic resonance imaging. Plain scan: T1W1 shows low signal intensity (D), Enhanced scan: obvious enhancement in arterial phase (E), less enhancement in portal phase and delayed phase than in surrounding liver parenchyma (F). Six months after surgery, the results of contrast-enhanced CT examination did not reveal any notable abnormalities: arterial phase (G); Venous phase (H); Z Effective (I). The tumor is indicated by red arrow.
After multi-disciplinary consultation, the tumor was considered most likely to be liver malignancy. Given the preoperative imaging findings suggesting tumor localization in segment VII of the liver, along with the inability to exclude a retroperitoneal origin of the tumor, a conventional laparoscopic right hepatectomy approach was proposed, designed to address both aforementioned scenarios and facilitate optimal exposure of the tumor. During the surgical intervention, an irregularly encapsulated tumor was identified adhering to the root of the right triangular ligament in the liver’s posterior lobe, in close proximity to the right renal capsule. (Figure 2A). The liver’s size and structure appeared normal. The adhesiolysis resulted in approximately 100 milliliters of hemorrhage. The tumor was completely resected and sent for pathological examination.
Upon gross examination, the tumor appears grayish-red in color, irregular in shape, and measures approximately 7cm x 4.6cm x 2.1cm. Two nodules, ranging in diameter from 3.6cm to 4.2cm, are visible on the section. The nodules are solid and soft, with a complete capsule covering their surface, and they appear grayish-red upon sectioning (Figure 2B). Histopathological examination of the mass, the tumor exhibits an increased number of lymphoid follicles with an expanded mantle zone consisting of small lymphocytes and atrophic germinal centers, which gives rise to an “onion skin-like” structure. Additionally, there is vascular proliferation and hyalinization present in the interfollicular zones, as well as scattered follicular dendritic cells and plasma cells (Figure 3).
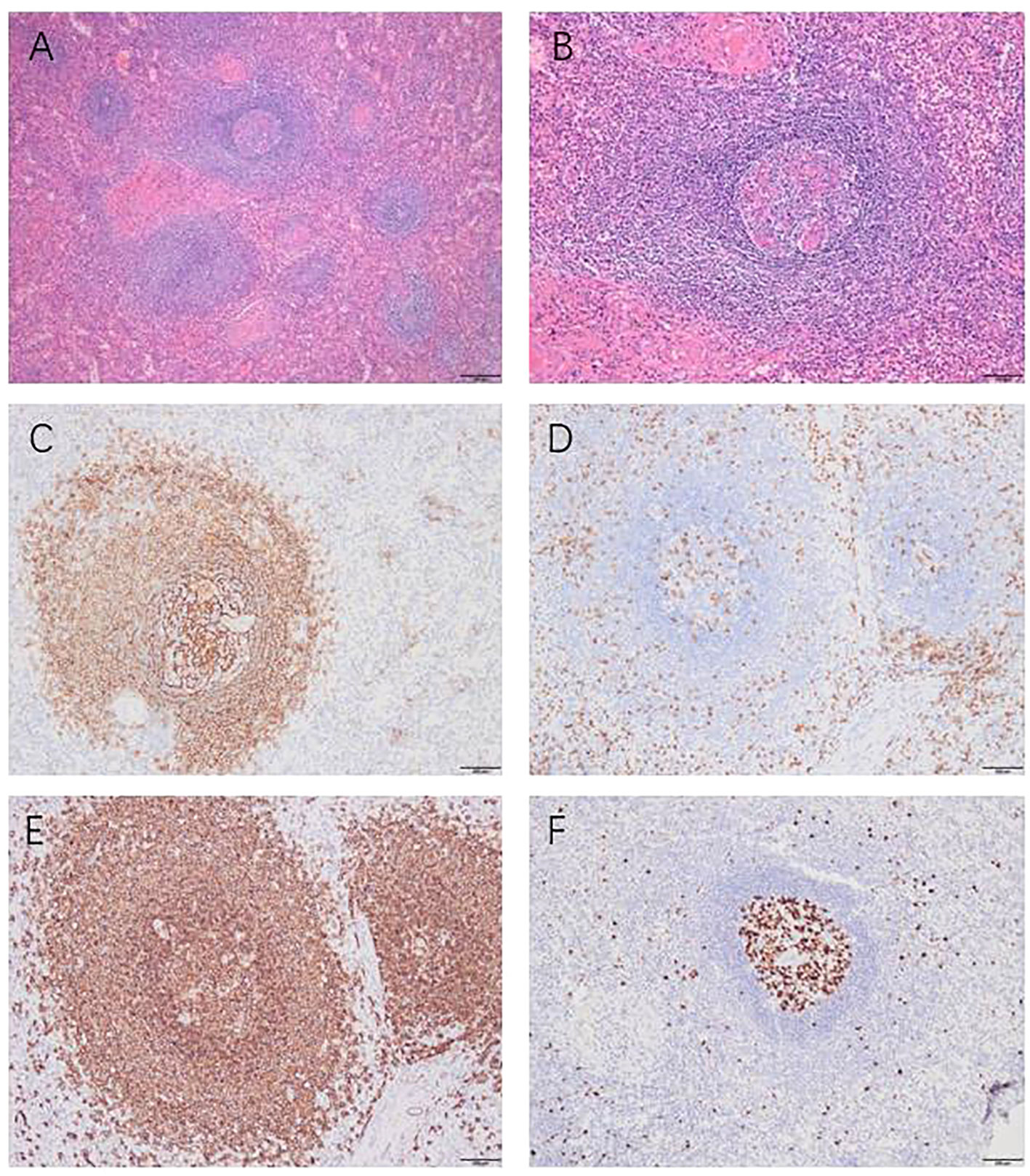
Figure 3 Histopathological and immunohistochemical findings, Histopathological examination (A, B): “onion skin-like” structure. Immunohistochemical examination: CD21 was present in the concentric follicular dendritic cell networks (C). CD3 was scattered positively among the T cells (D). CD20 was scattered positively among the B cells (E). KI67 showed high expression in the germinal center and low expression outside the germinal center (F). Magnification x100 (panels A); x200 (B–F).
The immunohistochemical analysis conducted on the excised specimen revealed a distinctive pattern: CD20 and CD79ɑ were positively expressed among B cells, while CD3 and CD5 showed positive expression among T cells. In the germinal centers, there was positive expression of CD10 and Bcl-6, whereas Bcl-2 staining was notably absent. A marked elevation in KI67 expression was observed within the germinal centers, contrasting with lower expression levels in surrounding areas. Additionally, the specimen tested negative for IgG4 and CyclinD1 (Figure 3). Collectively, these immunohistochemical findings corroborate the diagnosis of HV-CD.
Postoperatively, the patient’s recovery was uneventful, with no adverse complications reported. One week following the surgical intervention, the patient demonstrated satisfactory recovery, warranting discharge from the hospital. A six-month postoperative follow-up involving an enhanced CT scan of the abdomen revealed no evidence of disease recurrence, confirming the effectiveness of the treatment (Figures 1G–I).
CD is a rare non-neoplastic lymphoproliferative disorder first described in 1954 by Castleman and Towne as a benign localized lump or mass. The disease has two subtypes: UCD and MCD. MCD is characterized by systemic inflammatory symptoms, organ system dysfunction, and laboratory abnormalities such as fever, weight loss, low protein levels, systemic lymphadenopathy, renal function damage, and sometimes the development of POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, protein and skin changes). In contrast, UCD has almost no clinical manifestation (5, 6). Lesions can appear anywhere in the body but are most commonly found in the mediastinum (7). This case details a retroperitoneal, asymptomatic UCD. The initial imaging findings closely mimicked those of malignant hepatic tumors. Ultimately, the lesion was successfully excised through localized surgical resection.
CD is often under-recognized due to its rarity, rendering the diagnostic process challenging. For a definitive diagnosis of UCD, lymphadenopathy must be limited to a single lymph node or a localized nodal region (1). This necessitates a comprehensive diagnostic work-up that includes not only histological analysis of an excised lymph node but also radiological evaluation, typically involving whole-body CT or FDG-PET. However, CT imaging often lacks distinctive features (8).
Existing literature documents instances of CD manifesting in the retroperitoneal or perirenal regions. Notably, Fu et al. (9) described a case of CD located posterior to the right lower abdominal peritoneum, presenting as an isolated heterogeneous soft tissue mass on CT scans. Additionally, S Okada et al. (10) reported on a perirenal retroperitoneal CD case, where T1-weighted MRI revealed signal intensity of the mass akin to muscle tissue, and T2-weighted MRI showed heterogeneous internal signal characteristics with peripheral enhancement. Diverging from these cases, the tumor in this case demonstrated rapid enhancement and washout patterns in both contrast-enhanced CT and MRI.
UCD typically demonstrates pronounced enhancement in the arterial phase on contrast-enhanced CT/MRI scans. Some cases may exhibit enhancement in the delayed phase, with visible blood supply vessels within or around the lesion being a common feature (8). Conversely, hepatic carcinoma characteristically presents with rapid enhancement and washout in contrast-enhanced imaging. In this case report, the preoperative imaging findings of the patient’s tumor bore similarities to those typical of hepatic carcinoma.
The metabolic activity in CD makes PET-CT a valuable diagnostic tool (11), though its high cost limits its widespread use. In a previous case report (12), UCD was accurately diagnosed preoperatively via endoscopic ultrasound-guided fine needle biopsy (EUS-FNB). In this case, a preoperative biopsy was not performed due to the high likelihood of a malignant hepatic tumor diagnosis and concerns about needle tract cancer metastasis. Additionally, the tumor’s deep location posed challenges for a safe and effective biopsy. Regardless of the hypothetical preoperative biopsy results, the tumor was amenable to complete surgical removal.
Histological examination is the gold standard for diagnosing CD, requiring lesion resection or biopsy. Most UCD cases undergo immediate surgical resection (13). To determine this CD case’s specific subtype and differentiate it from other differential diagnoses, including lymphoma and autoimmune disorders, additional immunohistochemical analyses were conducted. These findings also provide prognostic insights and guide therapeutic strategies for CD with varied immunophenotypes (14).
We reported a rare case of UCD located in the retroperitoneum of the right hepato-renal space that was misdiagnosed as a liver malignant tumor due to the lack of clinical symptoms and imaging features. Paying attention to the patient’s history and conducting comprehensive comparisons of various types of imaging results may assist with the diagnosis. Preoperative biopsy also holds a pivotal role in the diagnostic process for this disease. CD has a good prognosis with surgical removal of the lesion. In cases where UCD is suspected, complete surgical resection may successfully treat the patient without an unnecessarily extensive resection.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Clinical Trial Ethics Committee of Southwest Medical University Affiliated Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
M-JK: Data curation, Writing – original draft. J-CZ: Data curation, Writing – original draft. CF: Conceptualization, Writing – review & editing. BL: Conceptualization, Writing – review & editing. SS: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Key Research and Development Project of Science & Technology Department of Sichuan Province (grant number:20ZDYF1129).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dispenzieri A, Fajgenbaum DC. Overview of castleman disease. Blood (2020) 135(16):1353–64. doi: 10.1182/blood.2019000931
2. Li Y, Zhao H, Su B, Yang C, Li S, Fu W. Primary hyaline vascular castleman disease of the kidney: case report and literature review. Diagn Pathol (2019) 14(1):94. doi: 10.1186/s13000-019-0870-9
3. Bollig C, Moon S, Sujoy V, Younis R. Castleman disease of the parotid in childhood: A case report. Am J Otolaryngol (2014) 35(4):517–9. doi: 10.1016/j.amjoto.2014.03.005
4. Xu J, Zhou BO, Cao HL, Wang BO, Yan S, Zheng SS. Surgical management of isolated retroperitoneal Castleman's disease: A case report. Oncol Lett (2016) 11(3):2123–6. doi: 10.3892/ol.2016.4177
5. Carbone A, Borok M, Damania B, Gloghini A, Polizzotto MN, Jayanthan RK, et al. Castleman disease. Nat Rev Dis Primers (2021) 7(1):84. doi: 10.1038/s41572-021-00317-7
6. Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: where are we now? Semin Diagn Pathol (2016) 33(5):294–306. doi: 10.1053/j.semdp.2016.05.006
7. Abramson JS. Diagnosis and management of Castleman disease. J Natl Compr Canc Netw (2019) 17(11.5):1417–9. doi: 10.6004/jnccn.2019.5037
8. Zhao S, Wan Y, Huang Z, Song B, Yu J. Imaging and clinical features of Castleman disease. Cancer Imaging (2019) 19(1):53. doi: 10.1186/s40644-019-0238-0
9. Fu Z, Liu M, Chen X, Yang X, Li Q. Paraneoplastic pemphigus associated with Castleman disease detected by 18f-Fdg Pet/Ct. Clin Nucl Med (2018) 43(6):464–5. doi: 10.1097/RLU.0000000000002072
10. Okada S, Maeta H, Maeba T, Goda F, Mori S. Castleman disease of the pararenal retroperitoneum report of a case. Surg Today (1999) 29(2):178–81. doi: 10.1007/BF02482246
11. Koa B, Borja AJ, Aly M, Padmanabhan S, Tran J, Zhang V, et al. Emerging role of 18f-Fdg Pet/Ct in Castleman disease: A review. Insights Imaging (2021) 12(1):35. doi: 10.1186/s13244-021-00963-1
12. Nayak HK, Pattnaik B, Patra S, Panigrahi C, Kumar C, Dutta T, et al. Pancreatic Castleman's disease: ring of fire sign and endoscopic ultrasonography guided fine-needle biopsy helps preoperative diagnosis. Pancreatology (2022) 22(3):443–5. doi: 10.1016/j.pan.2022.03.011
13. van Rhee F, Oksenhendler E, Srkalovic G, Voorhees P, Lim M, Dispenzieri A, et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv (2020) 4(23):6039–50. doi: 10.1182/bloodadvances.2020003334
Keywords: Castleman disease, retroperitoneal, hepatic malignant tumor, contrast-enhanced computed tomographic, contrast-enhanced magnetic resonance imaging
Citation: Kang M-J, Zhang J-C, Fang C, Li B and Su S (2024) Retroperitoneal Castlemans disease mimicking a liver cancer: a case report. Front. Oncol. 14:1343157. doi: 10.3389/fonc.2024.1343157
Received: 23 November 2023; Accepted: 15 January 2024;
Published: 12 February 2024.
Edited by:
Cheng Xue, Second Military Medical University, ChinaReviewed by:
Dragos Eugen Georgescu, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2024 Kang, Zhang, Fang, Li and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Su, MTM4ODI3Nzg1NTRAMTYzLmNvbQ==
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.