
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol., 12 February 2024
Sec. Gynecological Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1343023
This article is part of the Research TopicOvarian Cancer Targeted Medication: PARP Inhibitors, Anti-Angiogenic Drugs, Immunotherapy, and More, volume IIView all 21 articles
 Sergei Krasny1†
Sergei Krasny1† Yauheni Baranau2†
Yauheni Baranau2† Sergey Polyakov1
Sergey Polyakov1 Ekaterina Zharkova1
Ekaterina Zharkova1 Olga Streltsova2
Olga Streltsova2 Aliona Filimonava2
Aliona Filimonava2 Volha Siarheyeva1
Volha Siarheyeva1 Sviatlana Kazlouskaya1
Sviatlana Kazlouskaya1 Anton Khorau1
Anton Khorau1 Vladimir Gabai3
Vladimir Gabai3 Alexander Shneider3,4*
Alexander Shneider3,4*Background: The purpose of this trial is to evaluate the safety and efficacy of ELENAGEN, a novel anticancer therapeutic DNA plasmid encoding p62/SQSTM1 protein, as an adjuvant to chemotherapy with gemcitabine (GEM) in patients with advanced platinum-resistant ovarian cancer.
Methods: This open-label prospective randomized study with two arms. GEM (1000 mg/m2) on days 1 and 8 every 3 weeks was administered in both arms: in the Chemo arm (n = 20), GEM was the only treatment, and in the ELENAGEN arm (n = 20), GEM was supplemented with ELENAGEN (2.5 mg i.m. weekly). The primary endpoint was progression-free survival (PFS), and the secondary endpoint was safety. Antitumor activity was assessed by RECIST 1.1, and criteria safety was assessed according to NCI CTCAE version 5.0.
Results: According to the cutoff data, the median follow-up was 13.8 months. There were no serious adverse events related to ELENAGEN treatment. The median PFS was 2.8 and 7.2 months in the Chemo and ELENAGEN arms, respectively (p Log-Rank = 0.03). Notably, at the time of cutoff, 9 patients (45%) in the ELENAGEN arm did not progress, with the longest PFS recorded thus far being 24 months. Subgroup analysis of patients in both arms demonstrated high efficacy of ELENAGEN in patients with worse prognostic factors: high pretreatment levels of CA125 and progression after platinum-free interval <3 months.
Conclusions: The addition of ELENAGEN to gemcitabine is effective in patients with platinum-resistant ovarian cancer, including those with a worse prognosis.
Clinical trial registration: https://www.clinicaltrials.gov/study/NCT05979298, identifier NCT05979298, 2023-08-07.
Approximately 20 000 new cases of ovarian cancer (OC) are diagnosed in the US every year, and its overall 5-year survival rate is about 50% (1). This high lethality occurs because patients are mainly diagnosed with OC at later stages, and, following front-line therapy, tumors eventually become chemoresistant (2). Combination of platinum-based chemotherapy with taxanes still remains the standard of care for advanced and recurrent OC, but recurrent OC remains difficult to treat due to chemotherapy resistance (2). Despite introduction of antiangiogenic and poly ADP-ribose polymerase I (PARP) inhibitors in recent years, they only modestly improved patient’s progression-free survival (3–5). Thus, novel OC therapeutics to improve long-term outcomes are urgently needed.
Recently, immunotherapy of cancer, especially with immune-checkpoint inhibitors (ICI), emerged as a novel treatment option for a number of solid tumors, and it was also tested in several clinical trials with OC (6). However, unlike other tumor types, the results of these trials were not encouraging. For instance, in patients with platinum-resistant OC, compared with standard chemotherapy with gemcitabine (GEM) or pegylated liposomal doxorubicin (PLD), PFS with the ICI nivolumab (anti-PDL1 antibody) was only 2.0 vs 3.8 months with GEM or PLD, and OS was 10.1 vs 12.1 months (7). Additionally, grade 3-related adverse events (AEs) occurred in 33% of patients in the nivolumab group (7). In the JAVELIN Ovarian 200 phase III trial of 566 patients with platinum-resistant OC, the addition of another anti-PD-L1 antibody, avelumab, to standard PLD treatment did not significantly increase PFS (3.7 vs 3.5 months) or OS (15.7 vs 13.1 months) (8). Furthermore, serious treatment-related adverse events occurred in 18% of patients in the combination group, compared with 11% in the PLD-only group (8). Thus, at present, the application of ICIs in the treatment of platinum-resistant OC does not appear encouraging.
We have recently developed a novel anticancer therapeutic, ELENAGEN, based on plasmid DNA encoding the p62 (SQSTM1) protein (9). p62 is a multifunctional protein that participates in selective autophagy, signal transduction, the inflammatory response and other processes (10). p62 can be a good target for anticancer vaccines since its levels are elevated in almost all human tumors tested thus far, and it increases when tumors progress (see ref ( (11, 12) for review). While p62 is dispensable for normal cells, tumors require p62 for growth and metastasis (11). Importantly, p62 levels are also increased in OC and are associated with poor prognosis and platinum resistance, making p62 a good target for the immune response elicited by ELENAGEN (13, 14).
We conducted a preclinical study of the antitumor activity of ELENAGEN on several types of solid tumors in rodents. The drug showed its effectiveness on four types of solid tumors in mice (breast carcinoma, lung carcinoma, melanoma and sarcoma) as well as breast carcinoma in rats. Importantly, we observed suppression of metastasis in three different mouse models (9). Additionally, we conducted a pilot study of Elenagen in dogs with spontaneous mammary tumors, which are much closer to human breast tumors than transplantable tumors in rodents. We found that Elenagen in dogs exerted its effects in two ways: 1) in neoadjuvant settings, it made invasive and nonresectable tumors resectable, and 2) if mastectomy was impossible, tumors completely stopped growing during the period of observation (15, 16). Importantly, no toxicity of ELENAGEN was observed in either rodents or dogs (9, 15, 16).
Furthermore, we conducted a phase I/IIa clinical trial of ELENAGEN used as a monotherapy (17). In that study, ELENAGEN showed promise in treating patients with advanced disease for which all standard methods of treatment were exhausted. For example, the progression of OC was stopped for three or more months in 4 out of 6 patients. Importantly, in contrast to ICI (see above), AEs during ELENAGEN treatment were only Grade 1, and no severe AEs were observed (17). These data encouraged us to conduct a current clinical study of ELENAGEN with platinum-resistant OC.
In addition to evoking antitumor T- and B-cell immune responses (9, 15, 16), ELENAGEN can also alleviate chronic inflammation by suppressing the generation of proinflammatory cytokines such as TNF, IL-1, and IL-6 in different rodent disease models (18, 19). In contrast to acute inflammation, which is beneficial for the immune response to microbes and cancer cells, intratumoral chronic inflammation is detrimental since it disables immune cells, thus suppressing antitumor immunity (see ref (20) for review). Since most chemotherapeutics (at least partially) engage the immune system as part of their antitumoral mechanism of action (21), chronic inflammation decreases sensitivity to chemotherapy and prevents drug delivery to tumors (22), and alleviation of chronic inflammation can enhance the effect of chemotherapy.
Therefore, two mechanisms of ELENAGEN action, as an anticancer vaccine and anti-inflammatory drug, are complimentary and can make it a unique anticancer therapeutic in combination with chemotherapeutic agents for the treatment of OC.
This single-country open-label prospective randomized two-center study with two arms was performed from January 2020 until August 2022.
Eligible patients were ≥18 years old; had measurable ovarian cancer per RECIST 1.1 criterion that had progressed <6 months after completion of platinum-based therapy; had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and had adequate hematologic and organ functions.
The patients were randomly assigned in a 1:1 ratio. Forty patients underwent randomization, 20 were assigned to receive chemotherapy alone (GEM) 1000 mg/m2 days 1,8 every 3 weeks) and 20 were assigned to receive the same chemotherapy supplemented with ELENAGEN (2.5 mg i.m. weekly).
The primary end point was progression-free survival as assessed by investigators.
The secondary endpoints were overall response rate and safety.
According to the data cutoff, the median follow-up was 13.8 months.
In the safety analysis set and in the efficacy-evaluable set, all patients who received ≥ 1 dose (20 patients in each arm) were included. Safety was assessed on the basis of adverse events (AEs) and serious AEs (SAEs) according to NCI Common Terminology Criteria for Adverse Events version 5.0.
Antitumor activity was assessed by the investigator according to RECIST 1.1 criteria. Evaluation of the therapeutic effect was carried out by computer tomography (CT) every 9 weeks 19-20 days after each 3rd course of chemotherapy (before the 4th, 7th, and 10th courses, on a visit for follow-up and completion of treatment, and, if necessary, on unscheduled visits).
Tumor response was evaluated according to the RECIST criteria ver. 1.1. PFS was defined as the time from randomization to objective disease progression on imaging or death from any cause and was assessed using the Kaplan−Meier method. PFS in the two treatment arms was compared using an unstratified two-sided log-rank test. A P < 0.05 was considered statistically significant. For the subgroup analyses, a proportional Cox regression model was used.
Patient characteristics are summarized in Table 1. The most common histological type of platinum-resistant OC in both groups was high-grade serous adenocarcinoma. More than half of the patients in both groups progressed after only one line of platinum-based chemotherapy with platinum-free intervals of 3-6 months. Additionally, the majority of patients in both groups had high levels of CA125 as well as metastases in the peritoneum (75-85%) and elsewhere (Table 1). Figure 1 represents flow diagram of PROC patients included in the analysis
Safety was assessed in all 40 patients. During the study period, one death was registered in the ELENAGEN arm without any evidence of disease progression within 2 months after randomization, and its possible cause was venous embolism. Although autopsy was not performed and the final diagnosis was not determined, this adverse event was counted as thrombosis and unrelated to the disease. One patient in the ELENAGEN arm underwent surgery due to intestinal obstruction within one month after randomization, and the subsequent cycle of the treatment was delayed for three weeks. After recovery from the surgery, the patient continued treatment without evidence of progression to the cutoff date (up to 19 months).
The majority of adverse events in the GEM and ELENAGEN arms were caused by GEM and were presented by different types of hematological toxicity. No cases of febrile neutropenia or other life-threatening complications that required hospitalization occurred. The cases of intestinal obstruction and metabolic toxicity were caused by organ compression by gross tumor mass. Only skin rash, itching and redness at the injection site were considered to be related to ELENAGEN administration. At the same time, the number of adverse events with grade <= 3 and AEs of special interest (potentially related to plasmid administration) did not significantly differ between the groups (Table 2).
A slight increase in the number of hematological adverse events in the ELENAGEN arm was apparently related to the longer GEM exposure due to increased PFS.
The tumor response was assessed according to the RECIST 1.1 criteria. No complete responses were observed in either group. The objective response rate was higher in the ELENAGEN arm: partial response (PR) 5.9% and 26.7%, stable disease (SD) 35.3% and 53.3%, and disease progression 58.8% and 20.0% in the Chemo and ELENAGEN arms, respectively. In total, the disease control rate (PR and SD) was significantly higher in the ELENAGEN arm (80.0% vs 41.2% in the Chemo and ELENAGEN arms, respectively, p = 0,001). One patient in the ELENAGEN arm was able to undergo complete cytoreduction with no evidence of disease progression.
The median progression-free survival (PFS) was 2.8 and 7.2 months in the Chemo and ELENAGEN arms, respectively (p Log-Rank = 0.03) (Figure 2). For the lower 25th percentile (lower quartile), these numbers were 2.1 vs. 4.2 months, respectively, while for the upper quartile (75th percentile), 7.7 months, it was only possible to determine for the chemotherapy group alone.
Notably, at the time of cutoff, 9 patients (45%) in the ELENAGEN arm did not progress, with the longest PFS recorded thus far being 24 months.
We assessed the efficacy of ELENAGEN in subgroups with different basic characteristics.
The peritoneal effusion, CA125 level (normal or high), platinum-free interval (PFI), (up to 3 months vs 3-6 months), number of treatment lines for platinum-sensitive ovarian cancer and histological type of tumor (serous vs non-serous) were chosen as potential predictive factors. Cox proportional hazards regression analyses were performed (Table 3).
The CA125 level (normal or high), platinum-free interval (up to 3 months vs 3-6 months) and histological type of tumor (serous vs non-serous) were statistically significant in the Cox model.
However, due to the low number of patients with non-serous cancer (n=5 in both groups), additional analysis for histological type was not performed, but we performed pairwise comparisons of PFS in the Chemo and ELENAGEN arms according to the identified prognostic factors CA 125 level and PFI. The initial high CA-125 level and short PFI significantly affected PFS (Table 4; Figure 3).
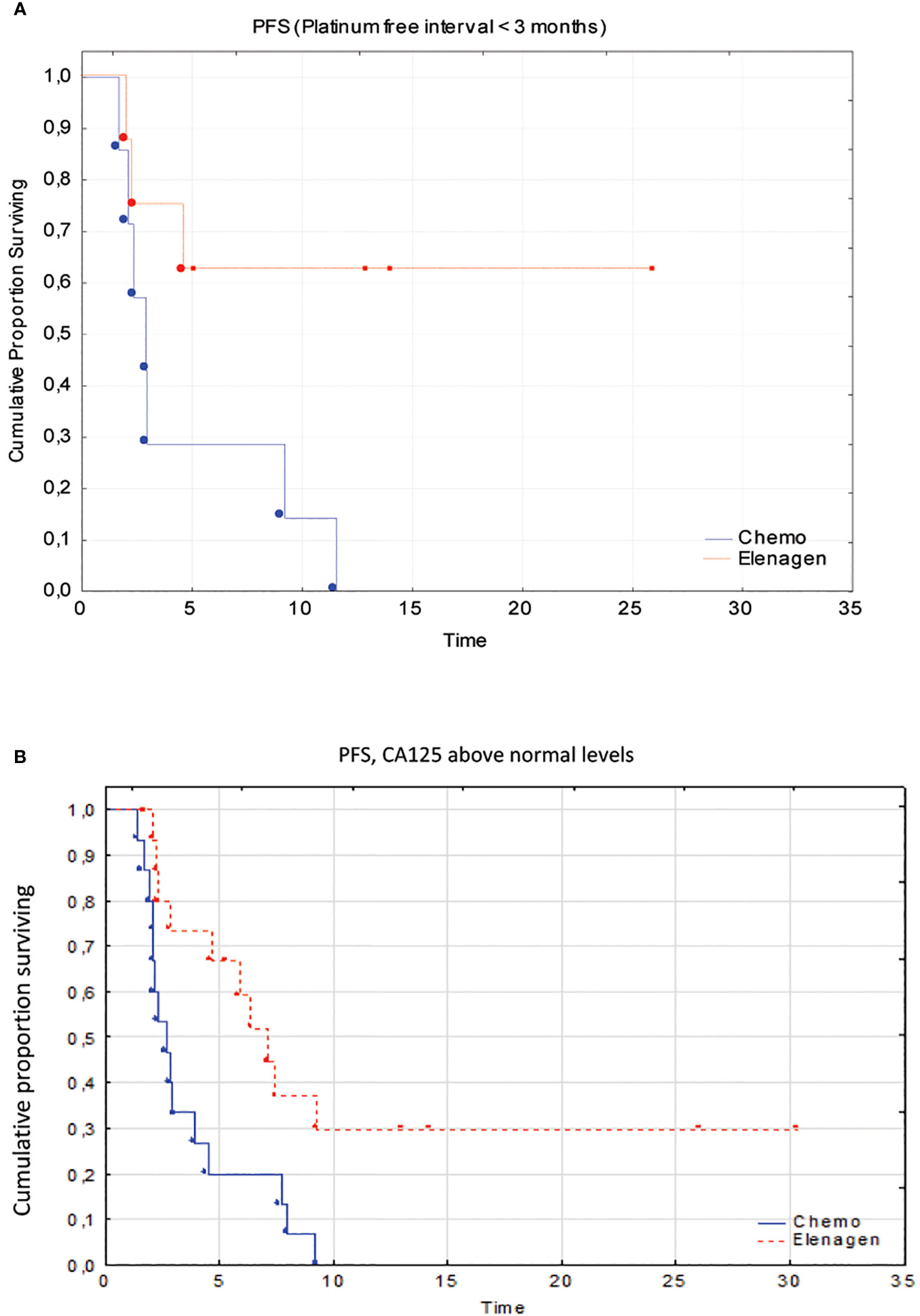
Figure 3 Subgroup analysis of patients with a platinum-free interval <3 months (A) and above normal CA125 levels (B).
Platinum-resistant OC, even if treated with a standard therapy such as gemcitabine, PLD, paclitaxel, and topotecan, has a dismal prognosis: a medium PFS of 3-4 months and an OS of 12 months (23, 24). Therefore, a more effective therapy for this form of OC is urgently needed. Despite the success of immunotherapy with immune checkpoint inhibitors (ICIs) in some tumors (25)), such a combination of ICIs with chemotherapy in OC has not yet been successful, and this treatment was quite toxic (6, 8) (see Background). Thus, at present, the application of ICIs in the treatment of platinum-resistant OC does not appear encouraging.
Our study demonstrated that the addition of our novel plasmid drug ELENAGEN to a standard chemotherapy regimen with GEM had a profound effect on PFS, increasing it from 2.8 months to 7.2 months. Importantly, no signs of increased toxicity of this combined treatment compared to GEM alone were found. Remarkably, ELENAGEN in combination with GEM was also effective in patients with a dismal prognosis: progression after platinum therapy within 3 months and with high pretreatment levels of CA125. For instance, a recent meta-analysis of data from more than 10 000 patients demonstrated that the increased serum level of CA-125 before treatment correlated with poor progression-free survival (HR=1.59, 95% CI=1.44~1.76, p<0.001) and overall survival (HR=1.62, 95% CI=1.270-2.060, p<0.001) (26). We are aware that due to a low number of patients in our subgroup analysis, these observations should be evaluated in larger trials.
ELENAGEN operates through at least two complementary mechanisms. First, ELENAGEN can work as an immunotherapeutic by activating T- and B-cellular antitumor immune responses by inducing the generation of antibodies and T-lymphocytes to p62 (9, 16) and stimulating the accumulation of T-lymphocytes in tumors (15). Since OC, especially platinum-resistant OC, has higher levels of p62 than normal tissue (13, 14, 27, 28), such an immune response to p62 may contribute to the antitumor activity of ELENAGEN. Furthermore, it is reasonable to combine elenagen with chemotherapy since anticancer drugs are currently believed to engage, at least partially, the immune system (see ref (21) for review), which may increase the antitumor activity of ELENAGEN. Indeed, the combination of chemotherapy with ICI immunotherapy in some tumors had a greater effect than either treatment alone, and such combinations are approved by the FDA (25). Accordingly, in our previous study, we found that patients with breast and ovarian cancers achieved additional tumor stabilization for 3-7 months when subjected to chemotherapy following ELENAGEN treatment even if the tumors were initially chemoresistant (17, 29).
Second, ELENAGEN was shown to decrease chronic inflammation (30), which may hamper the effect of chemotherapy (22). Elevated levels of the proinflammatory cytokine IL-6 in the serum or ascites of OC patients correlated with chemoresistance, particularly platinum resistance (31), and higher ascites levels of IL-6 and TNF predict worse PFS in patients with OC (32). Thus, decreasing chronic inflammation ELENAGEN may promote the effect of chemotherapy in OC. Last but not least, in dogs with mammary tumors, we found that ELENAGEN treatment results in tumor shrinkage, changes in the structure of the tumor matrix and lowering the grade of the tumors (15, 16). Such tumor “normalization” may also contribute to sensitization to chemotherapy. Finally, Elenagen treatment dramatically changes the expression of collagen isoforms (16), making it easier for tumor-infiltrating lymphocytes (TILs) to enter the tumor and harder for metastatic cells to exit. Thus, these effects of elenagen make it a unique anticancer therapeutic.
In conclusion, the addition of ELENAGEN to gemcitabine is effective in patients with ovarian cancer, including those with a worse prognosis. Future studies of ELENAGEN with various tumors and chemotherapy regimens are warranted.
All data generated or analyzed during this study are included in this Article. Data not shown in the manuscript are available from the corresponding author on reasonable request.
The study was approved by the Ministry of Health of Belarus (#03-19) and ethical review boards of N. Alexandrov National Cancer Centre of Belarus and Minsk City Cancer Center. Informed consent were signed by all study participants.
SKr: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. YB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – review & editing. SP: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. EZ: Data curation, Formal Analysis, Validation, Writing – review & editing. OS: Investigation, Methodology, Project administration, Resources, Writing – review & editing. AF: Formal Analysis, Investigation, Resources, Writing – review & editing. VS: Investigation, Methodology, Writing – review & editing. SKa: Investigation, Software, Validation, Writing – review & editing. AK: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. VG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing. AS: Conceptualization, Supervision, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Ministry of Health of Belarus and CureLab Oncology.
The authors thank the patients who participated in this study and their families and the investigators at N. N. Alexandrov National Cancer Centre of Belarus and Minsk City Cancer Center.
VG and AS are employees of CureLab Oncology, which holds the intellectual property (IP) to the Elenagen treatment. The clinical trial registration was retrospective.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Pignata S, Cecere S C, Du Bois A, Harter P, Heitz F. Treatment of recurrent ovarian cancer. Ann Oncol (2017) 28:viii51–viii6. doi: 10.1093/annonc/mdx441
3. Rossi L, Verrico M, Zaccarelli E, Papa A, Colonna M, Strudel M, et al. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget (2017) 8(7):12389–405. doi: 10.18632/oncotarget.13310
4. Ibrahim EM, Refae AA, Bayer AM, Sagr ER. Poly(ADP-ribose) polymerase inhibitors as maintenance treatment in patients with newly diagnosed advanced ovarian cancer: a meta-analysis. Future Oncol (2020) 16(10):585–96. doi: 10.2217/fon-2020-0057
5. Arora S, Narayan P, Ison G, Berman T, Suzman DL, Wedam S, et al. U.S. FDA drug approvals for gynecological Malignancies: A decade in review. Clin Cancer Res (2022) 28(6):1058–71. doi: 10.1158/1078-0432.CCR-21-2599
6. James NE, Woodman M, DiSilvestro PA, Ribeiro JR. The perfect combination: enhancing patient response to PD-1-based therapies in epithelial ovarian cancer. Cancers (2020) 12(8):2150. doi: 10.3390/cancers12082150
7. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol (2015) 33(34):4015–22. doi: 10.1200/JCO.2015.62.3397
8. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22(7):1034–46. doi: 10.1016/S1470-2045(21)00216-3
9. Venanzi F, Shifrin V, Sherman MY, Gabai V, Kisilev O, Komissarov A, et al. Broad-spectrum anti-tumor and anti-metastatic DNA vaccine based on p62-encoding vector. Oncotarget (2013) 4(10):1829–35. doi: 10.18632/oncotarget.1397
10. Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci (2012) 37(6):230–6. doi: 10.1016/j.tibs.2012.02.008
11. Gabai V, Shifrin V. Feasibility analysis of p62 (SQSTM1) – encoding DNA vaccine as a novel cancer immunotherapy. Int Rev Immunol (2014) 33(5):375–82. doi: 10.3109/08830185.2014.954699
12. Tang J, Li Y, Xia S, Li J, Yang Q, Ding K, et al. Sequestosome 1/p62: A multitasker in the regulation of Malignant tumor aggression (Review). Int J Oncol (2021) 59(4). doi: 10.3892/ijo.2021.5257
13. Iwadate R, Inoue J, Tsuda H, Takano M, Furuya K, Hirasawa A, et al. High expression of SQSTM1/p62 protein is associated with poor prognosis in epithelial ovarian cancer. Acta Histochemica et cytochemica (2014) 47(6):295–301. doi: 10.1267/ahc.14048
14. Xia M, Yu H, Gu S, Xu Y, Su J, Li H, et al. 62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int J Oncol (2014) 45:2341–8. doi: 10.3892/ijo.2014.2669
15. Gabai V, Venanzi FM, Bagashova E, Rud O, Mariotti F, Vullo C, et al. Pilot study of p62 DNA vaccine in dogs with mammary tumors. Oncotarget (2014) 5(24):12803–10. doi: 10.18632/oncotarget.2516
16. Venanzi FM, Gabai V, Mariotti F, Magi GE, Vullo C, Sufianov AA, et al. p62-DNA-encoding plasmid reverts tumor grade, changes tumor stroma, and enhances anticancer immunity. Aging (2019) 11(22):10711–22. doi: 10.18632/aging.102486
17. Ponomarenko DM, Klimova ID, Chapygina YA, Dvornichenko VV, Zhukova NV, Orlova RV, et al. Safety and efficacy of p62 DNA vaccine ELENAGEN in a first-in-human trial in patients with advanced solid tumors. Oncotarget (2017) 8(34):56030–40. doi: 10.18632/oncotarget.16574
18. Sabbieti MG, Agas D, Capitani M, Marchetti L, Concetti A, Vullo C, et al. Plasmid DNA-coding p62 as a bone effective anti-inflammatory/anabolic agent. Oncotarget (2015) 6(6):3590–9. doi: 10.18632/oncotarget.2884
19. Halenova T, Savchuk O, Ostapchenko L, Chursov A, Fridlyand N, Komissarov AB, et al. P62 plasmid can alleviate diet-induced obesity and metabolic dysfunctions. Oncotarget (2017) 8(34):56030–40. doi: 10.18632/oncotarget.19840
20. Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer (2022) 8(11):901–14. doi: 10.1016/j.trecan.2022.07.002
21. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell (2015) 28(6):690–714. doi: 10.1016/j.ccell.2015.10.012
22. Gkretsi V, Zacharia LC, Stylianopoulos T. Targeting inflammation to improve tumor drug delivery. Trends Cancer (2017) 3(9):621–30. doi: 10.1016/j.trecan.2017.07.006
23. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24:vi24–32. doi: 10.1093/annonc/mdt333
24. Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol (2014) 6(5):229–39. doi: 10.1177/1758834014544121
25. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) (2020) 12(3). doi: 10.3390/cancers12030738
26. Wang Q, Feng X, Liu X, Zhu S. Prognostic value of elevated pre-treatment serum CA-125 in epithelial ovarian cancer: A meta-analysis. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.868061
27. Li S, Wei Y. Association of HMGB1, BRCA1 and P62 expression in ovarian cancer and chemotherapy sensitivity. Oncol Lett (2018) 15(6):9572–6. doi: 10.3892/ol.2018.8482
28. Yan X-Y, Qu X-Z, Xu L, Yu S-H, Tian R, Zhong X-R, et al. Insight into the role of p62 in the cisplatin resistant mechanisms of ovarian cancer. Cancer Cell Int (2020) 20(1):128. doi: 10.1186/s12935-020-01196-w
29. Ponomarenko DM, Gabai VL, Sufianov AA, Kolesnikov SI, Shneider AM. Response of a chemo-resistant triple-negative breast cancer patient to a combination of p62-encoding plasmid, Elenagen, and CMF chemotherapy. Oncotarget (2019) 11(3):294–9. doi: 10.18632/oncotarget.27323
30. Sabbieti MG, Marchegiani A, Sufianov AA, Gabai VL, Shneider A, Agas D. P62/SQSTM1 beyond autophagy: physiological role and therapeutic applications in laboratory and domestic animals. Life (2022) 12(4):539. doi: 10.3390/life12040539
31. Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine (2012) 58(2):133–47. doi: 10.1016/j.cyto.2012.01.015
32. Kolomeyevskaya N, Eng KH, Khan ANH, Grzankowski KS, Singel KL, Moysich K, et al. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-alpha; and interleukin-6 in predicting reduced progression-free survival in epithelial ovarian cancer. Gynecologic Oncol (2015) 138(2):352–7. doi: 10.1016/j.ygyno.2015.05.009
Keywords: chemotherapy, DNA vaccine, immunotherapy, chemoresistance, platinum
Citation: Krasny S, Baranau Y, Polyakov S, Zharkova E, Streltsova O, Filimonava A, Siarheyeva V, Kazlouskaya S, Khorau A, Gabai V and Shneider A (2024) Clinical efficacy of plasmid encoding p62/SQSTM1 (Elenagen) in combination with gemcitabine in patients with platinum-resistant ovarian cancer: a randomized controlled trial. Front. Oncol. 14:1343023. doi: 10.3389/fonc.2024.1343023
Received: 22 November 2023; Accepted: 12 January 2024;
Published: 12 February 2024.
Edited by:
Zhaoqian Liu, Central South University, ChinaReviewed by:
Debasish Kumar Dey, University of Oklahoma, United StatesCopyright © 2024 Krasny, Baranau, Polyakov, Zharkova, Streltsova, Filimonava, Siarheyeva, Kazlouskaya, Khorau, Gabai and Shneider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Shneider, YXNobmVpZGVyQGN1cmVsYWIuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.