
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 June 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1342624
This article is part of the Research Topic The Cellular and Molecular Mechanisms of Tumor Ecosystem Remodeling during Cancer Progression or Drug Treatment in Gastrointestinal and Respiratory Cancers View all 5 articles
Objective: Cytokines and cell subsets are important components of the tumor microenvironment. Previous research has revealed that there are differences in cytokines and cell subsets in the peripheral blood of lung cancer (LCA) patients before and after eradication. The purpose of this study is to explore the monitoring value of cytokines and cellular subpopulations as biomarkers in post-immunotherapy monitoring of patients with LCA after surgery
Methods: We conducted a case-control study using double-antibody sandwich magnetic microsphere flow cytometry with immunofluorescence technology and fluorescent monoclonal antibody multiparameter flow cytometry to detect differences in peripheral blood cytokines and cell subsets between LCA patients after immunotherapy and healthy controls.
Results: Our research results show that there are differences in the levels of IL-4, IL-6, IL-10, IL-17, IFN-γ, TNF-α in the peripheral blood of LCA patients (n=70) after immunotherapy compared to the healthy controls (n=55) (P<0.05), and there are differences in 10 cell subgroups including DP T Cells, AT cells, and NLR in the peripheral blood compared to the healthy controls (n=35) (P<0.05). Further analysis revealed significant differences in the detection data of IL-6, IL-10, IFN-γ, CD56dim NK cells, Total B cells, Total NE cells, CD15+M cells, and NLR between LCA deceased patients (n=25) and LCA surviving patients (n=27) during the same period (P<0.05). The continuous monitoring of cytokines and cell subsets is far more valuable than a single-time test, as abnormal fluctuations in the data of cytokines and cell subsets are often associated with poor prognosis. In addition, IL-6 and NLR showed the strongest discriminative ability between postoperative immunotherapy-treated LCA patients and healthy controls, with AUC values of 0.840 and 0.822, respectively. There was a significant association between IFN-γ and distant metastasis in LCA (P<0.05), as well as between CD56dim NK cells and lymph node infiltration (P<0.05).
Conclusion: This research results support peripheral blood cytokines and cell subsets as biomarkers for monitoring the postoperative immune status and predicting the prognosis of LCA patients after immunotherapy. The continuous monitoring of cytokines and cell subsets is far more valuable than a single-time detection.
Lung cancer (LCA) is a major contributor to cancer-related morbidity and mortality (1). Approximately 2.1 million new cases of LCA are diagnosed worldwide each year, with over 1.8 million deaths occurring annually (2). The current treatment strategy for sufficiently healthy LCA patients primarily revolves around surgical resection, supplemented by radiation therapy, chemotherapy, and the latest targeted immunotherapy (3). As the types of immunotherapy for LCA, such as therapeutic vaccines, immune modulators, autologous cell therapy, and monoclonal antibodies targeting checkpoint inhibitors signaling associated with activated T cells and/or cancer cells, continue to increase, the reliability and effectiveness of targeted immunotherapy have been validated (4). However, due to the individual variability among LCA patients and the uniqueness of each immune therapy’s target, the responsiveness of LCA patients to immunotherapy varies greatly (5). Therefore, a biomarker that can be repeatedly tested is needed to assess the immune status of LCA patients, evaluate treatment outcomes, and provide information for treatment decisions. Traditional LCA tumor markers, such as Carbohydrate Antigen 125 (CA125) and Neuron-Specific Enolase (NSE), are believed to be useful for monitoring the efficacy of immunotherapy in LCA patients. However, multiple studies have found that these tumor markers may not accurately reflect the treatment response of lung cancer patients (6, 7). In recent years, liquid biopsy markers such as Circulating Tumor Cells (CTC), Cell-Free DNA (cfDNA), and Exosomes have been considered as potential biomarkers for predicting immune efficacy after LCA immunotherapy. However, tumor heterogeneity and the high cost of detection may limit the clinical application of this technology (8). Peripheral blood cytokines and immune cells are considered intriguing targets for immunotherapy and clinical biomarker research, as they can reflect changes in the tumor microenvironment during treatment, patient treatment responsiveness, and provide crucial information about the progression of LCA disease (8–11). Compared to the tumor tissue obtained through surgery, which can only undergo immunoscore testing once (12), peripheral blood samples can be collected throughout the entire treatment of LCA patients, enabling dynamic monitoring of peripheral blood cytokines and immune cells (13, 14).
The peripheral blood contains various cytokines and immune cells, which play a particularly crucial role in the occurrence, prognosis, and treatment of LCA (15–17). Inflammatory cytokines are associated with the advanced stages of LCA, resistance to immunotherapy, and poor prognosis, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-8 increase with the progression of late-stage small cell LCA (18). IL-6 is an important cytokine in LCA that promotes epithelial-mesenchymal transition and tumor metastasis, facilitating the migration and invasion of LCA cells (19). The expression of the immunosuppressive cytokine IL-10 is correlated with the survival outcomes and treatment response rates of LCA patients. Multiple research findings indicate that patients whose serum IL-10 remained high levels during treatment have a worse prognosis (20). Interferon γ (IFN-γ) plays multiple roles in tumor immune responses. IFN-γ produced after the activation of T lymphocytes by tumor antigens can stimulate the proliferation and differentiation of tumor-infiltrating lymphocytes. It can also generate immunosuppressive factors, leading to direct negative feedback regulation of effector T cell function (21). Peripheral blood immune cells can dynamically reflect changes in the tumor microenvironment, the outcomes of anti-tumor immune responses, and predict clinical responses to immunotherapy (22), such as T-cell exhaustion associated with surgical complications and higher mortality rates, which can reflect the clinical treatment status (23). Natural killer (NK) cells, as a type of innate immune cell, can attack tumor cells without the need for activation (24). NK cells can be divided into CD56dim cell subsets, which express cytotoxicity, and CD56bright cell subsets, which primarily produce cytokines, based on the expression of CD56 (25). The special T-cell subset that simultaneously expresses the TCR receptor and CD56 receptor is called NKT cell subset, mainly involved in regulating the body’s responses to infections, tumor immunity, immune surveillance, and the perforin and granule enzymes expressed in their lytic granules can lyse tumor cells (26). The neutrophil-to-lymphocyte ratio (NLR) is associated with improved progression-free survival and overall survival in LCA patients (27).
Our hospital employs peripheral blood immune cells and a combination of seven cytokines for monitoring the immune status of LCA patients undergoing immunotherapy. However, due to the use of a clinical test kit commonly applicable to all cancers, it does not specifically indicate effectiveness for LCA, and there is also a lack of relevant research in this area. The study utilized flow cytometry to analyze changes in cytokines and immune cell phenotypes in the peripheral blood circulation of patients with LCA after targeted immunotherapy, and its correlation with the clinical characteristics of LCA patients. The aim was to comprehensively evaluate the value of using cytokines and cell subsets in postoperative immune monitoring of LCA patients and identify meaningful biomarkers for testing.
The study population included patients who underwent lung tumor resection surgery at the Luohu Hospital Group (Shenzhen, China) between January 2019 and December 2022 and were diagnosed with LCA. The inclusion criteria were patients with LCA who underwent R0 resection surgery and had histopathological confirmation. The exclusion criteria were patients with concurrent other cancers or immune-related diseases, those who received preoperative radiotherapy or chemotherapy, or those who had used immunomodulatory drugs in the past three years. Peripheral blood cytokine and immune cell testing data included all data from the first postoperative test for each patient until the end of the data collection period, with each patient receiving at least one peripheral blood cytokine and immune cell test after immunotherapy. Due to the limited number of patients undergoing simultaneous testing of peripheral blood cytokines and immune cell populations during their physical examinations, we selected 55 physical examination patients who were tested for cytokines at the same time and 35 physical examination patients who were tested for cell subsets as healthy controls. The specimens from LCA patients after immunotherapy were collected from those who received immunotherapy immediately after surgery for more than five months (average 9.4 months, range 5.0-18.5 months). The time of specimen collection for LCA patients before death is the time of the last specimen collection before the death report is issued (average 1.7 months, range 0.1-8.1 months). In addition, clinical data of patients with LCA were also collected.
The plasma separation method for LCA patients involves using peripheral blood samples with EDTA anticoagulant, centrifuging at 1500g for 15 minutes, which can be used for immediate experiments or stored at -20°C. Add 50 μL of capture microsphere suspension and 50 μL of plasma sample to the centrifuge tube, mix thoroughly, and incubate at room temperature in the dark for 1 hour. Perform magnetic separation to remove the supernatant, then add 100 μL of fluorescently labeled antibody working solution, mix thoroughly, and incubate at room temperature in the dark for 1 hour. Perform magnetic separation again to remove the supernatant, and wash the microspheres with 200 μL of magnetic bead dilution solution; repeat this step twice. Finally, resuspend the microspheres in 200 μL of magnetic bead dilution solution and detect using the EasySample flow cytometer. The reagents and equipment were purchased from Wellgrow Company (Shenzhen, China).
Add 5μL of labeled antibody to 100μL of EDTA anticoagulated blood, mix well, and incubate at room temperature in the dark for 15 minutes. Add 200-300μL of red blood cell lysis solution, mix well, and incubate in the dark for 10-15 minutes. Add 1.5ml of flow cytometry sheath fluid, mix, centrifuge at 400g for 5 minutes, discard the supernatant, and repeat this step twice. Finally, add 300μL of sheath fluid for Beckman Navios flow cytometry analysis. The cell concentration should not exceed 5*109. Based on the characteristics of cellular immune phenotypes, four multi-color flow cytometry panels were designed to identify immune cell subsets (Supplementary Table 1). The reagents and instruments were purchased from Beckman Coulter, Inc. (Brea, California, USA).
Use the operating software of the EasySample flow cytometer to analyze the cytokine dataset; use Kaluza Analysis software to analyze the dataset of cell subsets. The EasySample flow cytometer prepares a quantitative calibration standard (Supplementary Table 2) and constructs a standard curve before each use to ensure the comparability of instrument performance at different times. The Beckman Navios flow cytometry requires the addition of a control tube (isotype control antibody) each time a sample is tested, and daily instrument quality control is performed using the Cell Sorter & Tracker (CS&T) application to ensure that the detection results do not change over time. The above quality control materials are reagent accessories provided by a reagent company.
Statistical analysis was performed using SPSS software (IBM SPSS Statistics 26, Chicago, USA). In appropriate circumstances, use independent sample T-test and Mann-Whitney U-test to compare the differences in peripheral blood cytokines and cell subsets between LCA patients and healthy donors. In addition, Pearson χ2 test was used to analyze the correlation between certain cytokines, immune cells, and clinical features of tumors. Figures were created by GraphPad Prism 9.0. P-values ≤ 0.05 were considered statistically significant.
This study included a total of 70 LCA patients with data from peripheral blood cytokine and immune cell subset tests, as well as data from 55 healthy control individuals for cytokine testing and 35 healthy control individuals for immune cell subset testing. By the end of data retrieval, 25 out of the 70 LCA patients (35.71%) had passed away. The LCA patients were predominantly in the advanced stage, with 54 out of 70 (77.1%) having tumor grading primarily at stage IV, 39 out of 70 (55.7%) having poorly differentiated tumors, and distant lymph node metastasis and distant metastasis occurring in 57 out of 70 (81.4%) and 12 out of 70 (17.1%) cases, respectively. The age of LCA patients showed a higher trend compared to that of the healthy controls. Table 1 summarizes the clinical and pathological characteristics of LCA patients and healthy controls.
In this section of the study, we compared the differences in levels of 7 cytokines (IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, TNF-α) in peripheral blood plasma between LCA patients after targeted immunotherapy and healthy controls (Table 2). The levels of IL-2(P* = 0.367) were similar in LCA patients and healthy individuals, while the expression levels of IL-4 (P*=0.037), IL-6 (P* < 0.001), IL-10 (P* = 0.020), IL-17 (P* = 0.010), IFN-γ (P* = 0.018), and TNF-α (P* = 0.030) were increased.
Afterwards, we assessed the differences in peripheral blood immune cell profiles between LCA patients following immunotherapy and healthy controls (Table 3). In the T cell subsets, the percentage of double-positive T cells (DP T Cells/CD3+CD4+CD8+) (P* = 0.001) and γδ T cells (CD3+TCRγδ+%) (P* < 0.001) were decreased (Figures 1A, D), while the percentage of activated T cells (AT Cells/CD3+CD25+) (P* < 0.001) and regulatory T cells (Treg Cells/CD3+CD4+CD25+) (P* = 0.006) were increased (Figures 1B, C). The percentage of a specific T cell subset, NKT-like cells (CD3+CD56+), was also decreased (Figure 1E). The percentage of CD56dim NK cells (CD3-CD56+CD16+) in the NK cell subset was comparable between LCA patients and healthy individuals (P* = 0.022) (Figure 1F). The percentage of total B cells (CD3-CD19+) in lymphocytes is decreased (P* = 0.006), while the percentage of total neutrophils (NE/CD14-CD11B+) and total monocytes (M/CD14+) is increased (P* < 0.001 and P* = 0.005, respectively) (Figures 1G–I). Meanwhile, the CD15+ monocytes (CD14+CD15+) and intermediate monocytes (iMo/CD14+CD16+) in mononuclear cells both increased (P* = 0.010 and P* < 0.001, respectively) (Figures 1J, K). The NLR of LCA patients is elevated compared to healthy controls (P* < 0.001) (Figure 1L).
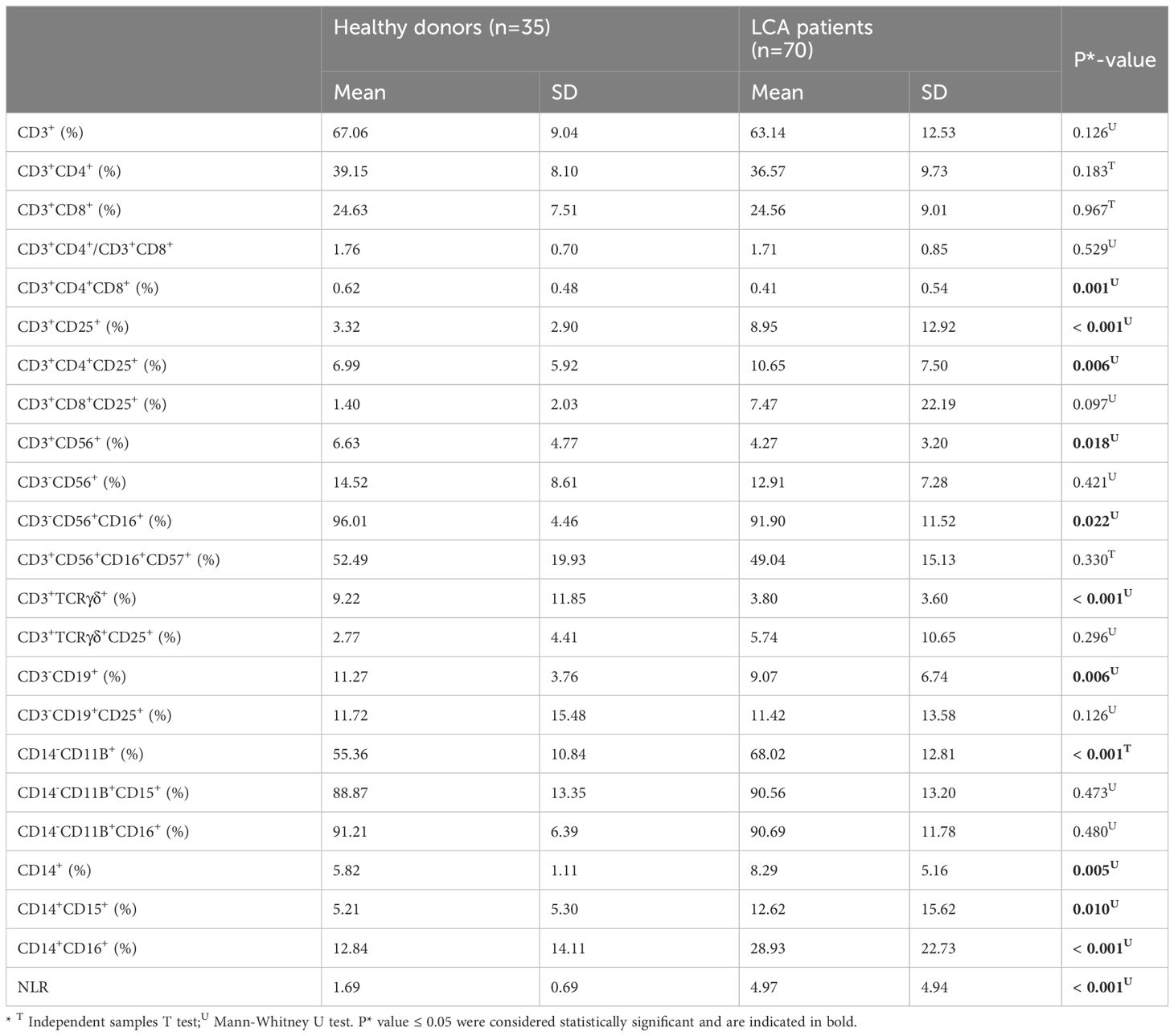
Table 3 Comparison between peripheral blood immune cell profiles in lung cancer patients and healthy controls.

Figure 1 The peripheral blood immune cell subset distribution in lung cancer patients compared to healthy controls. (A) The percentage of DP T cells. (B) Percentage of AT cells. (C) Percentage of treg cells. (D) Percentage of γδ T cells. (E) Percentage of NKT-like cells. (F) Percentage of CD56dim NK cells. (G) Percentage of total B cells. (H) Percentage of total NE cells. (I) Percentage of total M cells. (J) Percentage of CD15+ M cells. (K) Percentage of iMo cells. (L) Values of NLR.
In this section, we examined the differences in IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α values between 25 dying LCA patients’ last test and 27 LCA patients who were alive during the same period (Supplementary Table 3). The average survival time of dying LCA patients was 11.9 months, with a median of 10.2 months; therefore, the control group (survivors) specimen collection time was chosen as 10-12 months post-surgery. The levels of IL-4, IL-17, and TNF-α were similar in the plasma of LCA dying and living patients, while the levels of IL-6, IL-10, and IFN-γ were elevated in the plasma of LCA dying patients (Figures 2A–C). Further analyze the dynamic changes of plasma cytokines in LCA patients after targeted immunotherapy. Continuous monitoring curves were performed on 6 dying LCA patients who had been tested for cytokines more than 5 times and 10 living LCA patients, and the results are shown in Supplementary Figure 1. Additionally, due to independent IL-6 monitoring, there were two extra patients in both the dying and living LCA groups, totaling eight and twelve patients, respectively. In LCA patients, we observed an increased trend in the levels of certain plasma cytokines prior to death. For example, we observed elevated levels of IL-6 and IL-10 before death in five out of six LCA patients, and elevated levels of IFN-γ before death in four out of six LCA patients. However, the expression levels of plasma cytokines in living LCA patients remained relatively stable, even gradually decreasing. Only a few patients showed elevated levels, namely IL-6 (3/12), IL-10 (2/10), and IFN-γ (2/10).
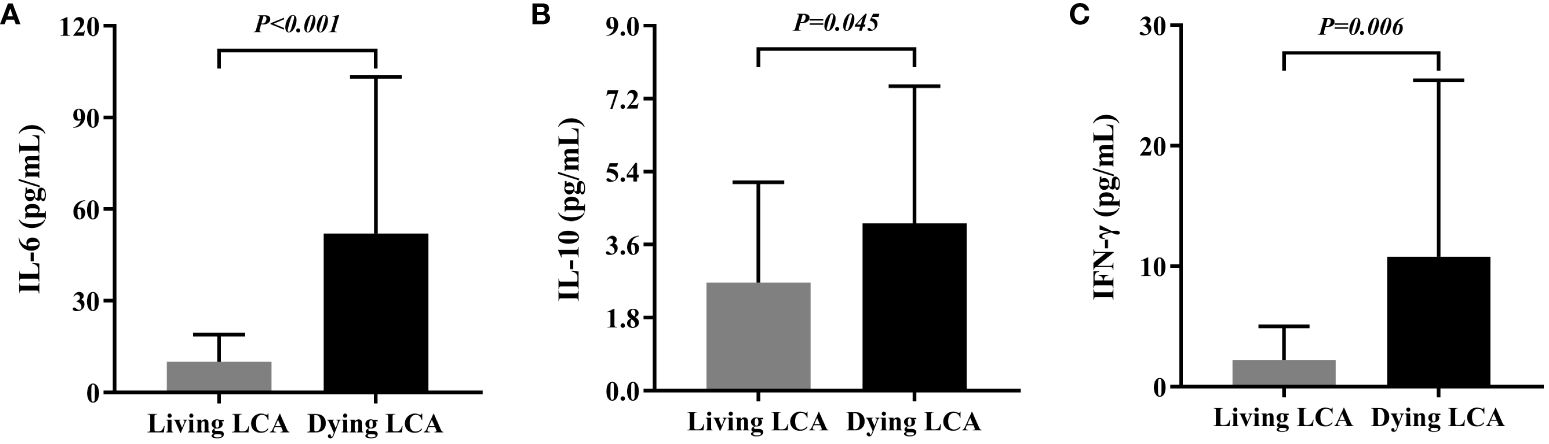
Figure 2 Comparison between peripheral Cytokines in living lung cancer patients and dying lung cancer. (A) Differential expression of IL-6 in living lung cancer patients and dying lung cancer. (B) Differential expression of IL-10 in living lung cancer patients and dying lung cancer. (C) Differential expression of IFN-γ in living lung cancer patients and dying lung cancer.
Subsequently, we selected the immune cells that had previously been confirmed to exhibit differences between LCA patients and healthy individuals in the earlier part of the study and investigated their differences between dying and living LCA patients (Supplementary Table 4). The percentage of CD56dim NK cells in NK cells decreased, as did the percentage of Total B cells in lymphocytes (P = 0.005 and P = 0.044, respectively) (Figures 3A, B). Additionally, the percentage of Total NE cells in lymphocytes increased, as did the percentage of CD15+M cells in monocytes (P < 0.001 and P = 0.035, respectively) (Figures 3C, D). Furthermore, compared to living LCA patients, NLE significantly increased in LCA patients before death (P < 0.001) (Figure 3E). Similarly, select LCA patients who have undergone testing more than 5 times to plot continuous monitoring curves, investigating the dynamic trends in the percentage of cell subsets. A total of 7 LCA dead patients and 11 LCA alive patients met the requirements. Among the three critically dying LCA patients, there was a declining trend in the percentage of CD56dim NK cells, with one individual experiencing a sharp decline followed by a brief recovery. In contrast, among the eleven living LCA patients, the percentage of CD56dim NK cells fluctuated within a narrower range overall, with smaller overall variations compared to the critically dying LCA patients (Supplementary Figure 2A, B). The percentage of Total B cells gradually decreased in 5 cases of LCA dying patients, while the percentage of Total B cells remained relatively stable in 11 cases of LCA living patients, with no significant change in trend (Supplementary Figure 2C, D). Among the 7 dying LCA patients, there was a trend of increased total NE cell percentage before death, while the 11 surviving LCA patients showed interval fluctuations without a significant upward trend change (Supplementary Figures 2E, F). Among the 7 critically dying LCA patients, only two showed a relatively stable percentage of CD15+M cells before death; whereas among the 11 living patients, 7 exhibited relative stability (Supplementary Figures 2G, H). In both critically dying and living LCA patients, no clear trend change was observed in NLR, but the NLR extremes in critically dying LCA patients were higher than in living LCA patients (Supplementary Figure 2I, J).
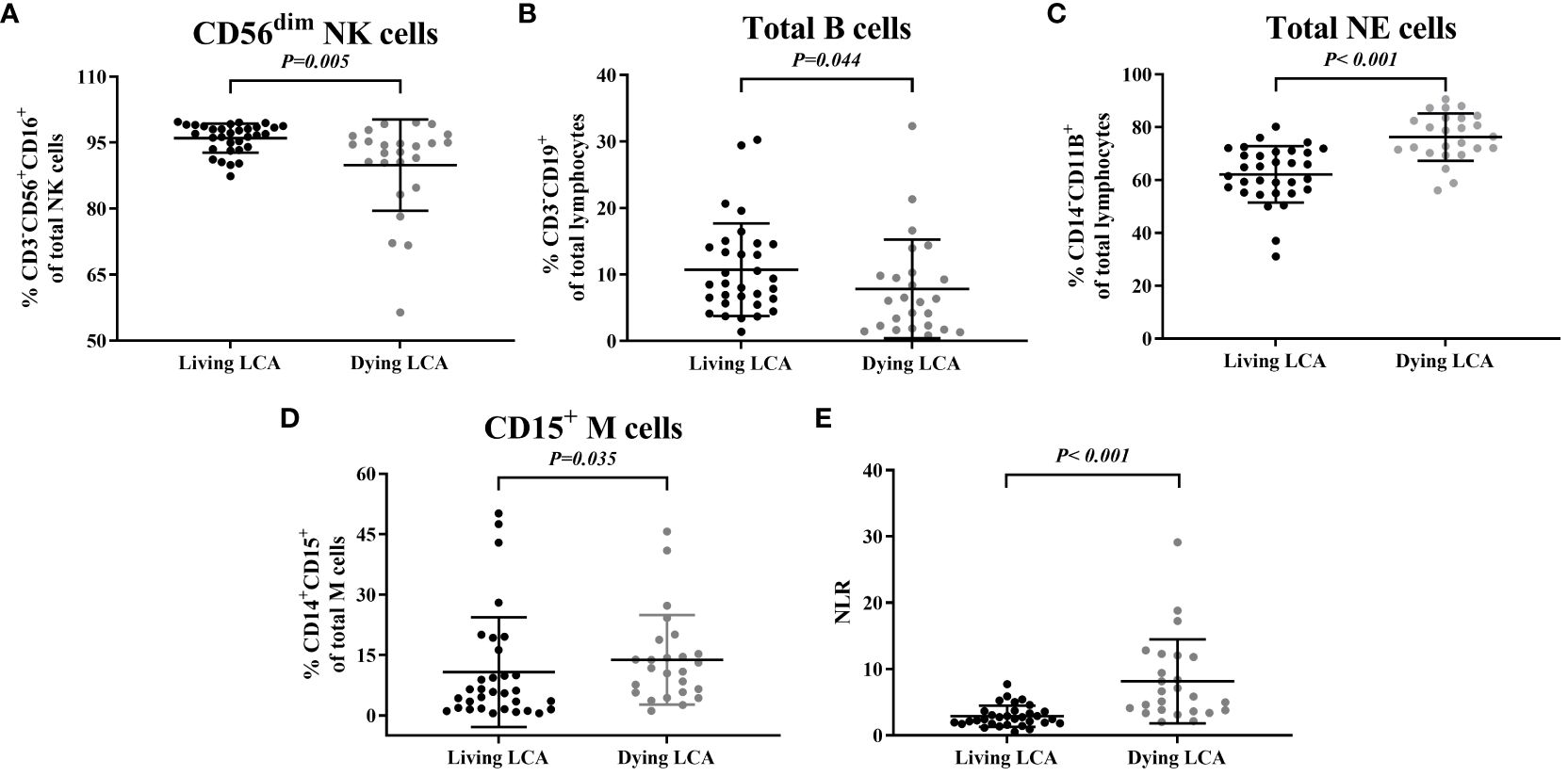
Figure 3 Comparison between peripheral blood immune cell profiles in living lung cancer. (A) Percentage of CD56dim NK cells. (B) Percentage of total B cells. (C) Percentage of total NE cells. (D) Percentage of CD15+ M cells. (E) Values of NLR.
After the research revealed differences in three cytokines and five cell subgroups between LCA patients and healthy controls, as well as between LCA dying patients and living patients, we studied their correlation with tumor characteristics. First, we analyzed the diagnostic capabilities of eight indicators, including three cytokines and five cell subsets, between LCA patients and healthy controls (Figure 4). Then, based on the parameters of the ROC curve, we calculated various indicators such as the Youden index, area under curve (AUC) area, sensitivity, and specificity for each indicator to identify the most suitable cutoff values for each indicator (Table 4). The AUC area for IL-6 and NLR are relatively high, with P < 0.001. IL-6 exhibits high sensitivity and specificity at the cutoff, while NLR has slightly lower sensitivity but the highest specificity. Both of them demonstrate strong discriminative ability between LCA patients and healthy individuals. Therefore, IL-6 and NLR may be effective biomarkers for postoperative immune therapy outcomes and patient prognosis in LCA patients.
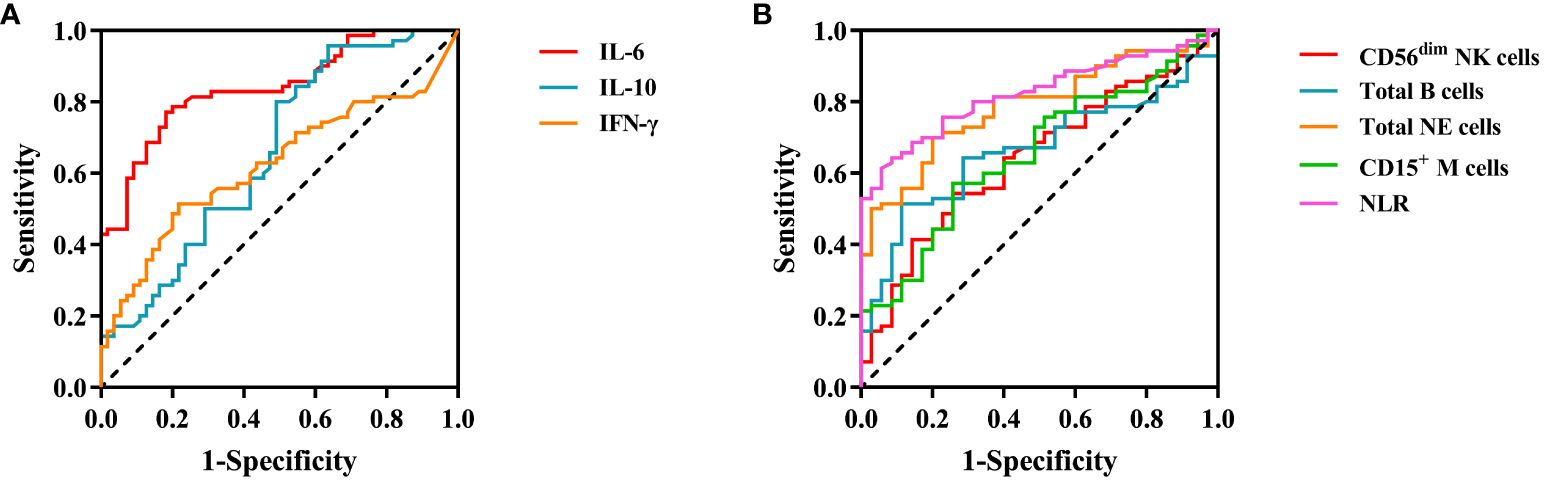
Figure 4 ROC curve for peripheral blood cytokines and immune cell in lung cancer. (A) Cytokines. (B) Immune cell.
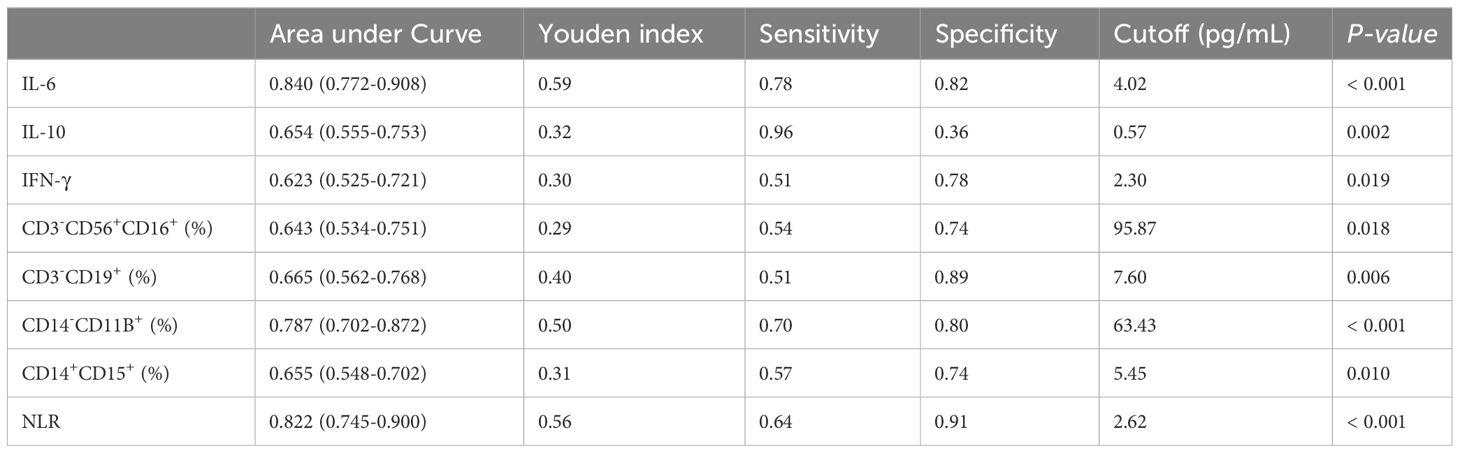
Table 4 ROC and cutoff values analysis of cytokines and immune cell in lung cancer patients after immunotherapy.
The differences in three cytokines and five cell subset markers were separately investigated in tumor grading, differentiation, lymph node infiltration, and distant metastasis (Supplementary Tables 5, 6). Only IFN-γ showed a difference in distant metastasis (P = 0.002), and CD3-CD56+CD16+ (%) showed a difference in lymph node infiltration (P = 0.028). In addition to these findings, we did not observe any correlation between other cytokines, immune cell subsets, and tumor characteristics.
Recently, some studies using the Immunoscore system to monitor the abundance of immune cells in cancer patient tumor tissues, predict survival outcomes, and assess immune therapy responses have confirmed the ability of the immune status of cancer patients as a predictive biomarker for tumor immunotherapy (28–30). The high infiltration of tumor immune cells and a higher score for the immune microenvironment are associated with favorable immune therapy efficacy and clinical outcomes in LCA (31). However, due to the significant trauma associated with tumor tissue biopsies and the insufficient repeatability of specimen collection, dynamic monitoring of the immune status of tumor patients cannot be achieved solely based on a single surgical specimen (32). The peripheral blood sample is easy to obtain and causes minimal trauma, making it very suitable for monitoring the immune function status of cancer patients dynamically (33). Over the years, despite the standard treatment strategies for LCA remaining surgery, chemotherapy, and radiation therapy, in recent years, tumor immunotherapy aimed at stimulating the host’s own immune system to eradicate cancer has been gradually gaining attention (34). In order to elucidate the correlation between the immune status of LCA patients after immunotherapy and disease progression, this study characterized the levels of peripheral blood cytokines and the distribution of cell subsets. We explored the differences in these cytokines and immune cell subsets between LCA patients and healthy individuals, as well as their varying changes in tumor patients with different responses to immunotherapy.
We observed changes in the peripheral blood cell cytokines in LCA patients after postoperative immunotherapy. Compared to healthy individuals, we found that the levels of IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α in the plasma increased in LCA patients after immunotherapy following surgery, consistent with previous studies (21, 35–38). The high levels of IL-4 can induce an increase in the activity of tissue proteases in macrophages, thereby promoting tumor growth, invasion, and tumor angiogenesis (39). In addition, the excessive production of vascular endothelial growth factor (VEGF) is positively correlated with elevated levels of IL-4, indicating that IL-4 may support tumor progression through different mechanisms (40). Patients with lower baseline IL-6 levels after immunotherapy are more likely to benefit from immunotherapy. This is consistent with a previous conclusion regarding PD-L1 treatment in small cell LCA (41). Research has shown that IL-10 inhibits the PD-1/PD-L1 pathway, leading to resistance of tumors to PD-1/PD-L1 immunotherapy. This suggests that IL-10 may have adverse effects in immunotherapy and also explains why individuals with higher levels of IL-10 in this study had a worse prognosis (42). A clinical retrospective study suggests that during treatment, the levels of IL-17 are associated with clinical benefits of immunotherapy, but are unrelated to baseline levels in peripheral blood before treatment (43). IFN-γ, on one hand, promotes the production of immunosuppressive molecules, thereby exerting direct negative feedback on the function of effector T cells. On the other hand, during the elimination phase of the tumor immune response, elevated levels of IFN-γ increase the cytotoxic activity against LCA tumor cells, and this mechanism of action leads to autoimmune-like side effects (43, 44). TNF-α plays a complex role in tumor immunity, often used as a tool to regulate immune cells and kill tumor cells. However, prolonged exposure to high concentrations of TNF-α levels can instead promote tumor progression and may lead to hemorrhagic necrosis (45). To further elucidate the value of cytokines in the dynamic monitoring of the immune status in LCA patients, we conducted additional analyses of changes in cytokines after postoperative immunotherapy. We compared the peripheral blood cytokines of LCA patients who died after immunotherapy with those of LCA patients who were alive during the same period. The average levels of IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α before the death of LCA patients were elevated, with statistically significant differences observed in IL-6, IL-10, and IFN-γ. Long-term surviving LCA patients often exhibit stable changes in plasma cytokine levels, while cytokine levels in deceased patients show a gradual or severe increase before death. Recent studies have also reached consistent conclusions, such as elevated IL-6 levels, suggesting a poor response to pembrolizumab therapy in advanced renal cell carcinoma treatment, as well as worse survival outcomes (46). Interrupting IL-10 signal transduction can induce the death of tumor cells in PD-1 treatment-resistant patients with colorectal cancer liver metastasis and activate the immune system’s anti-tumor response (47). The appropriate level of cytokines has a positive effect on the anti-tumor treatment of cancer patients, but high levels of cytokines often promote tumor development instead (37). Our research results indicate that a sharp increase in cytokines may be associated with a poor prognosis in LCA patients, while relatively stable cytokine levels are often associated with a longer survival period in LCA patients.
Our research has found differences in the percentages of cell subsets between LCA patients and healthy controls, and these findings are consistent with previous studies in multiple tumor research. DP T cells are a multifunctional T cell subset derived from single-positive T cells (SP T cells) following antigen stimulation, and their function may be related to the ability to enhance T cell memory levels (48). In this study, the percentage of DP T cells in LCA patients is decreased, which is inconsistent with the results of studies on other cancers, and further cohort validation of this result is needed in the future (49). Compared to healthy individuals, LCA patients have a lower percentage of peripheral blood γδ T cells and NKT-like cells (50, 51), and a higher percentage of AT cells, Treg cells, and Total M cells (52–54). These findings have been reported in multiple cancer studies. In addition, we found that the percentages of CD56dim NK cells, Total B cells, Total NE cells, CD15+ M cells, and the NLR not only showed differences between LCA patients and healthy individuals but also exhibited differential characteristics within the cell subsets tested concurrently in LCA deceased patients and surviving patients. In this study, the percentage of CD56dim NK cells is inversely correlated with the course of cancer in LCA patients, indicating a negative association between the levels of CD56dim NK cells after immunotherapy and the prognosis of LCA patients. CD56dim NK cells are primarily involved in cell lysis and target cell killing, while also being a significant source of pro-inflammatory and chemotactic factors. Therefore, a decrease in the levels of CD56dim NK cells leads to a reduced ability of LCA patients to eliminate tumor cells (55). More and more evidence suggest that B cells play a crucial synergistic role in tumor control. A decrease in the overall levels of B cells leads to a reduced B cell-mediated antigen presentation, diminished capacity of the body to produce cytokines, decreased antibody-dependent cellular cytotoxicity, and reduced phagocytic activity (56). As the most abundant cell type in the human body, neutrophils serve as important regulators of cancer. The elevation of total NE cells levels is closely associated with cancer development, tumor angiogenesis, and tumor immune suppression. Previous studies have confirmed the increase in neutrophil levels in various types of tumors (57). The clinical research on CD15+ monocytes in LCA is limited, considering that CD15+ is a characteristic marker of monocyte differentiation, it may be related to the anti-tumor chemotactic activity of monocytes (58). A study on the correlation between NLR and LCA prognosis found that the NLR values of patients who died during the follow-up period significantly increased, while the NLR values of patients who were still alive remained stable. There was a significant correlation between NLR values and the follow-up time for both LCA patients who died and those who survived, and their conclusions align with ours (59). In summary, the changes in the percentage of LCA cell subsets are correlated with the post-immunotherapy immune status of LCA patients. Continuous monitoring of changes in cell subsets can provide a better understanding of the immune function and therapeutic efficacy of LCA patients and assist in predicting the prognosis of LCA patients.
We also compared the discriminative abilities of cytokines and cell subsets after immunotherapy between LCA patients and healthy controls. IL-6 and NLR showed the best performance, with AUC values of 0.840 and 0.822, respectively. This suggests that IL-6 and NLR have more significant value in postoperative monitoring of LCA patients and tumor recurrence. After immunotherapy, a higher NLR was positively correlated with an increased risk of death in LCA patients, while a lower NLR was positively correlated with a longer overall survival period (60). Subsequently, based on the calculated cutoff value, the clinical correlation between LCA tumor features and postoperative immune therapy-related cytokines and immune subsets was assessed. Only IFN-γ showed statistical significance with distant tumor metastasis, while CD56dim NK cells exhibited significant differences in lymph node infiltration. The other cytokines and cell subsets after post-LCA surgery immunotherapy do not show any clinical relevance. There are reports that IFN-γ can promote the metastasis of colorectal cancer through MACC1, so IFN-γ may promote the metastasis of LCA through some yet undefined mechanism (61). The results of a clinical correlation study on NK cells indicate that the distribution of CD56dim NK cells is correlated with tumor metastasis, staging, and distribution (60). In addition, this study did not find any other cytokines or cell subsets related to the clinical features of LCA. Of course, there is currently controversy in related research, with some studies showing correlations while others do not, which will require further clinical studies for validation.
However, our study also has some limitations. Firstly, it is a retrospective study, and due to the rarity of checking cytokines and cell subsets in healthy populations, there are age differences between the enrolled LCA patients and the healthy control group. Secondly, because there are few LCA patients who are simultaneously tested for cytokines and cell subsets, the number of specimens included in the analysis in this study is relatively small. Finally, due to patient loss to follow-up, the survival data for some patients were lost.
In conclusion, we have confirmed that there are changes in cytokines and cell subsets in LCA patients after postoperative immunotherapy. Continuous monitoring of cytokines and cell subsets is more valuable than a single-time assessment. Furthermore, IL-6 and NLR, as two indicators, exhibit the strongest discriminatory ability between postoperative immunotherapy-treated LCA patients and healthy controls and should receive sufficient attention. The peripheral blood cytokines and immune cell profile have the potential value to become biomarkers for monitoring the immune status after LCA immunotherapy. For future research, we advocate designing prospective randomized controlled clinical trial protocols, further enriching the research cohort, exploring the changes in cytokines and cell subsets in patients with LCA after receiving targeted therapy, and their correlation with clinical characteristics of LCA, revealing their potential application in immune therapy monitoring after LCA surgery, and improving the survival rate and quality of life of LCA patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The application for the use of clinical data in this study was approved by the Ethics Committee of Shenzhen Luohu District People's Hospital (2023-LHQRMYY-KYLL-047). This study was a retrospective study, and written informed consent was not required.
CZ: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft. HM: Investigation, Project administration, Supervision, Writing – review & editing. ML: Data curation, Formal analysis, Writing – review & editing. SW: Methodology, Writing – review & editing. XD: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing. XZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Shenzhen Basic Research Project (Nos. JCYJ20190812171215641); Shenzhen Key Medical Discipline (SZXK054).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1342624/full#supplementary-material
1. Hussain SH, Huertas CS, Mitchell A, Deman A, Laurenceau E. Biosensors for circulating tumor cells (ctcs)-biomarker detection in lung and prostate cancer: trends and prospects. Biosens Bioelectron. (2022) 197:113770. doi: 10.1016/j.bios.2021.113770
2. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. (2021). doi: 10.1002/ijc.33588
3. Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (London England). (2018) 18:s41–6. doi: 10.7861/clinmedicine.18-2-s41
4. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. (2023) 22:40. doi: 10.1186/s12943-023-01740-y
5. Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. (2019) 2:e196879. doi: 10.1001/jamanetworkopen.2019.6879
6. Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. (2019) 17:74. doi: 10.1186/s12967-019-1828-0
7. Yang X, Xiao Y, Zhou Y, Deng H, Yuan Z, Dong L, et al. Dynamic monitoring of serum tumor markers as prognostic factors in patients with advanced non-small-cell lung cancer treated with first-line immunotherapy: a multicenter retrospective study. Ther Adv Med Oncol. (2023) 15:2651018. doi: 10.1177/17588359231206282
8. Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. (2017) 169:750–65. doi: 10.1016/j.cell.2017.04.014
9. Brownlie D, von Kries A, Valenzano G, Wild N, Yilmaz E, Säfholm J, et al. Accumulation of tissue-resident natural killer cells, innate lymphoid cells, and cd8(+) t cells towards the center of human lung tumors. Oncoimmunology. (2023) 12:2233402. doi: 10.1080/2162402X.2023.2233402
10. Phillips JD, Fay KA, Bergeron AJ, Zhang P, Mielcarz DW, Calkins AM, et al. The effect of lung resection for nsclc on circulating immune cells: a pilot study. Curr Oncol (Toronto Ont.). (2023) 30:5116–34. doi: 10.3390/curroncol30050387
11. Abolfathi H, Sheikhpour M, Shahraeini SS, Khatami S, Nojoumi SA. Studies in lung cancer cytokine proteomics: a review. Expert Rev Proteomics. (2021) 18:49–64. doi: 10.1080/14789450.2021.1892491
12. Chi K, Sun W, Yang X, Wu J, Wang H, Liu X, et al. A prognostic classification based on the international association for the study of lung cancer histologic grading and immunoscore in kras-mutant invasive non-mucinous adenocarcinoma. Thorac Cancer. (2022) 13:1050–8. doi: 10.1111/1759-7714.14360
13. Schindler H, Lusky F, Daniello L, Elshiaty M, Gaissmaier L, Benesova K, et al. Serum cytokines predict efficacy and toxicity, but are not useful for disease monitoring in lung cancer treated with pd-(l)1 inhibitors. Front Oncol. (2022) 12:1010660. doi: 10.3389/fonc.2022.1010660
14. Pai JA, Hellmann MD, Sauter JL, Mattar M, Rizvi H, Woo HJ, et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific t cells during immune checkpoint blockade. Cancer Cell. (2023) 41:776–90. doi: 10.1016/j.ccell.2023.03.009
15. Lee H, Lee H, Chang J. Inflammatory cytokine: an attractive target for cancer treatment. Biomedicines. (2022) 10(9):2116. doi: 10.3390/biomedicines10092116
16. Brueckl NF, Wirtz RM, Reich FPM, Veltrup E, Zeitler G, Meyer C, et al. Predictive value of mrna expression and dynamic changes from immune related biomarkers in liquid biopsies before and after start of pembrolizumab in stage iv non-small cell lung cancer (nsclc). Transl Lung Cancer Res. (2021) 10:4106–19. doi: 10.21037/tlcr-21-587
17. Kim CG, Hong MH, Kim KH, Seo I, Ahn B, Pyo K, et al. Dynamic changes in circulating pd-1(+) cd8(+) t lymphocytes for predicting treatment response to pd-1 blockade in patients with non-small-cell lung cancer. Eur J Cancer (Oxford England: 1990). (2021) 143:113–26. doi: 10.1016/j.ejca.2020.10.028
18. Song X, Zhou S, Xiao N, Li Y, Zhen D, Su C, et al. Research on the relationship between serum levels of inflammatory cytokines and non-small cell lung cancer. Asian Pacific J Cancer Prevention: Apjcp. (2013) 14:4765–8. doi: 10.7314/APJCP.2013.14.8.4765
19. Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, et al. Il-6 promotes metastasis of non-small-cell lung cancer by up-regulating tim-4 via nf-κb. Cell Prolif. (2020) 53:e12776. doi: 10.1111/cpr.12776
20. Zeng L, O’Connor C, Zhang J, Kaplan AM, Cohen DA. Il-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine. (2010) 49:294–302. doi: 10.1016/j.cyto.2009.11.015
21. Lim JU, Yoon HK. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine. (2021) 138:155363. doi: 10.1016/j.cyto.2020.155363
22. Hwang M, Canzoniero JV, Rosner S, Zhang G, White JR, Belcaid Z, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer. (2022) 10(6):e004688. doi: 10.1136/jitc-2022-004688
23. Kim CG, Kim G, Kim KH, Park S, Shin S, Yeo D, et al. Distinct exhaustion features of t lymphocytes shape the tumor-immune microenvironment with therapeutic implication in patients with non-small-cell lung cancer. J Immunother Cancer. (2021) 9(12):e002780. doi: 10.1136/jitc-2021-002780
24. Chiossone L, Dumas P, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
25. Hamilton G, Plangger A. The impact of nk cell-based therapeutics for the treatment of lung cancer for biologics: targets and therapy. Biologics: Targets Ther. (2021) 15:265–77. doi: 10.2147/BTT.S290305
26. Terabe M, Berzofsky JA. Tissue-specific roles of nkt cells in tumor immunity. Front Immunol. (2018) 9:1838. doi: 10.3389/fimmu.2018.01838
27. Russo A, Russano M, FranChina T, Migliorino MR, Aprile G, Mansueto G, et al. Neutrophil-to-lymphocyte ratio (nlr), platelet-to-lymphocyte ratio (plr), and outcomes with nivolumab in pretreated non-small cell lung cancer (nsclc): a large retrospective multicenter study. Adv Ther. (2020) 37:1145–55. doi: 10.1007/s12325-020-01229-w
28. Mezheyeuski A, Backman M, Mattsson J, Martín-Bernabé A, Larsson C, Hrynchyk I, et al. An immune score reflecting pro- and anti-tumoural balance of tumour microenvironment has major prognostic impact and predicts immunotherapy response in solid cancers. Ebiomedicine. (2023) 88:104452. doi: 10.1016/j.ebiom.2023.104452
29. Liu Y, Liu Z, Yang Y, Cui J, Sun J, Liu Y. The prognostic and biology of tumour-infiltrating lymphocytes in the immunotherapy of cancer. Br J Cancer. (2023) 129(7):1041–9. doi: 10.1038/s41416-023-02321-y
30. Zuo S, Wei M, Wang S, Dong J, Wei J. Pan-cancer analysis of immune cell infiltration identifies a prognostic immune-cell characteristic score (iccs) in lung adenocarcinoma. Front Immunol. (2020) 11:1218. doi: 10.3389/fimmu.2020.01218
31. Yang L, Wei S, Zhang J, Hu Q, Hu W, Cao M, et al. Construction of a predictive model for immunotherapy efficacy in lung squamous cell carcinoma based on the degree of tumor-infiltrating immune cells and molecular typing. J Transl Med. (2022) 20:364. doi: 10.1186/s12967-022-03565-7
32. Casagrande GMS, Silva MDO, Reis RM, Leal LF. Liquid biopsy for lung cancer: up-to-date and perspectives for screening programs. Int J Mol Sci. (2023) 24(3):2505. doi: 10.3390/ijms24032505
33. Kunimasa K, Goto T. Immunosurveillance and immunoediting of lung cancer: current perspectives and challenges. Int J Mol Sci. (2020) 21(2):597. doi: 10.3390/ijms21020597
34. Aldarouish M, Wang C. Trends and advances in tumor immunology and lung cancer immunotherapy. J Exp Clin Cancer Research: Cr. (2016) 35:157. doi: 10.1186/s13046-016-0439-3
35. Tan N, Song J, Yan M, Wu J, Sun Y, Xiong Z, et al. Association between il-4 tagging single nucleotide polymorphisms and the risk of lung cancer in China. Mol Genet Genomic Med. (2019) 7:e00585. doi: 10.1002/mgg3.585
36. Liu Y, Gao Y, Lin T. Expression of interleukin-1 (il-1), il-6, and tumor necrosis factor-α (tnf-α) in non-small cell lung cancer and its relationship with the occurrence and prognosis of cancer pain. Ann Palliat Med. (2021) 10:12759–66. doi: 10.21037/apm-21-3471
37. Pan YW, Zhou ZG, Wang M, Dong JQ, Du KP, Li S, et al. Combination of il-6, il-10, and mcp-1 with traditional serum tumor markers in lung cancer diagnosis and prognosis. Genet Mol Research: Gmr. (2016) 15(4). doi: 10.4238/gmr15048949
38. Liu L, Liu R, Wei C, Li D, Gao X. The role of il-17 in lung cancer growth. Cytokine. (2023) 169:156265. doi: 10.1016/j.cyto.2023.156265
39. Gocheva V, Wang H, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. Il-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. (2010) 24:241–55. doi: 10.1101/gad.1874010
40. Zhao P, Bu X, Wei X, Sun W, Xie X, Li C, et al. Dendritic cell immunotherapy combined with cytokine-induced killer cells promotes skewing toward th2 cytokine profile in patients with metastatic non-small cell lung cancer. Int Immunopharmacol. (2015) 25:450–6. doi: 10.1016/j.intimp.2015.02.010
41. Liu C, Yang L, Xu H, Zheng S, Wang Z, Wang S, et al. Systematic analysis of il-6 as a predictive biomarker and desensitizer of immunotherapy responses in patients with non-small cell lung cancer. BMC Med. (2022) 20:187. doi: 10.1186/s12916-022-02356-7
42. Vahl JM, Friedrich J, Mittler S, Trump S, Heim L, Kachler K, et al. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br J Cancer. (2017) 117:1644–55. doi: 10.1038/bjc.2017.336
43. Zhao N, Yi Y, Cao W, Fu X, Mei N, Li C. Serum cytokine levels for predicting immune-related adverse events and the clinical response in lung cancer treated with immunotherapy. Front Oncol. (2022) 12:923531. doi: 10.3389/fonc.2022.923531
44. Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol. (2018) 10:1960364788. doi: 10.1177/1758834017749748
45. Benoot T, Piccioni E, De Ridder K, Goyvaerts C. Tnfα and immune checkpoint inhibition: friend or foe for lung cancer? Int J Mol Sci. (2021) 22(16):8691. doi: 10.3390/ijms22168691
46. Sang YB, Yang H, Lee WS, Lee SJ, Kim S, Cheon J, et al. High serum levels of il-6 predict poor responses in patients treated with pembrolizumab plus axitinib for advanced renal cell carcinoma. Cancers (Basel). (2022) 14(23):5985. doi: 10.3390/cancers14235985
47. Sullivan KM, Jiang X, Guha P, Lausted C, Carter JA, Hsu C, et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. (2023) 72:325–37. doi: 10.1136/gutjnl-2021-325808
48. SChad SE, Chow A, Mangarin L, Pan H, Zhang J, Ceglia N, et al. Tumor-induced double positive t cells display distinct lineage commitment mechanisms and functions. J Exp Med. (2022) 219(6):e20212169. doi: 10.1084/jem.20212169
49. Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias S, Dartiguenave F, Burruni R, et al. Double positive cd4(+) cd8(+) t cells are enriched in urological cancers and favor t helper-2 polarization. Front Immunol. (2019) 10:622. doi: 10.3389/fimmu.2019.00622
50. Cheng M, Hu S. Lung-resident γδ t cells and their roles in lung diseases. Immunology. (2017) 151:375–84. doi: 10.1111/imm.12764
51. Lin X, Chen X, Long X, Zeng C, Zhang Z, Fang W, et al. New biomarkers exploration and nomogram construction of prognostic and immune-related adverse events of advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Respir Res. (2023) 24:64. doi: 10.1186/s12931-023-02370-0
52. Zhang D, Chen Z, Wang DC, Wang X. Regulatory t cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev. (2015) 34:277–90. doi: 10.1007/s10555-015-9566-0
53. Szlasa W, Wilk K, Knecht-Gurwin K, Gurwin A, Froń A, Sauer N, et al. Prognostic and therapeutic role of cd15 and cd15s in cancer. Cancers (Basel). (2022) 14(9):2203. doi: 10.3390/cancers14092203
54. Bauernhofer T, Kuss I, Friebe-Hoffmann U, Baum AS, Dworacki G, Vonderhaar BK, et al. Role of prolactin receptor and cd25 in protection of circulating t lymphocytes from apoptosis in patients with breast cancer. Br J Cancer. (2003) 88:1301–9. doi: 10.1038/sj.bjc.6600860
55. Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, et al. Characterization of circulating t-, nk-, and nkt cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunology Immunotherapy: Cii. (2019) 68:1011–24. doi: 10.1007/s00262-019-02343-7
56. Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating b cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer. (2022) 22:414–30. doi: 10.1038/s41568-022-00466-1
57. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:173. doi: 10.1186/s13045-021-01187-y
58. Nakayama F, Nishihara S, Iwasaki H, Kudo T, Okubo R, Kaneko M, et al. Cd15 expression in mature granulocytes is determined by alpha 1,3-fucosyltransferase ix, but in promyelocytes and monocytes by alpha 1,3-fucosyltransferase iv. J Biol Chem. (2001) 276:16100–6. doi: 10.1074/jbc.M007272200
59. Bar-Ad V, Palmer J, Li L, Lai Y, Lu B, Myers RE, et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin Trans Oncology: Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico. (2017) 19:711–7. doi: 10.1007/s12094-016-1593-y
60. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage iv non-small cell lung cancer (nsclc): neutrophil to lymphocyte ratio (nlr), lymphocyte to monocyte ratio (lmr), platelet to lymphocyte ratio (plr) and advanced lung cancer inflammation index (ali). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.16
Keywords: lung cancer, cytokines, cell subsets, immunotherapy, tumor progression
Citation: Zhang C, Mo H, Li M, Wang S, Dou X and Zhang X (2024) The effects of postoperative targeted immunotherapy on peripheral blood cytokines and immune cell profile in lung cancer patients. Front. Oncol. 14:1342624. doi: 10.3389/fonc.2024.1342624
Received: 08 February 2024; Accepted: 02 May 2024;
Published: 05 June 2024.
Edited by:
Michael R. Shurin, University of Pittsburgh Medical Center, United StatesReviewed by:
Suresh Kalathil, University at Buffalo, United StatesCopyright © 2024 Zhang, Mo, Li, Wang, Dou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowen Dou, ZG91eGlhb3dlbjU3M0AxNjMuY29t; Xiuming Zhang, enhtMDc2MEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.