- 1Clinical Genetics, Fox Chase Cancer Center, Philadelphia, PA, United States
- 2Health Economics, RTI Health Solutions, Research Triangle Park, NC, United States
- 3Pfizer Inc., Collegeville, PA, United States
- 4Pfizer Inc., Department of US Oncology Medical Affairs, New York, NY, United States
- 5Pfizer Inc., Department of HTA, Value and Evidence, New York, NY, United States
Aim: To examine clinical characteristics, real-world treatment patterns, and health outcomes among patients with germline BRCA1/2-mutated, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer (ABC).
Methods: A retrospective analysis was conducted using medical records from patients with HER2-negative ABC with BRCA1/2 mutation who received cytotoxic chemotherapy. Data were stratified into groups with triple-negative breast cancer (TNBC) or hormone receptor–positive (HR+)/HER2-negative diagnoses. Time-to-event outcomes (i.e., real-world progression-free survival [rwPFS] and overall survival [OS]) were calculated to summarize health outcomes.
Results: When diagnosed with ABC, most patients were younger than 60 years (mean age = 57.3 years), were white (76.4%), and had a family history of BRCA-related cancer (71.5%). A total of 305 patient records were examined; 194 patients (63.6%) had advanced TNBC, and 111 patients (36.4%) had HR+/HER2-negative ABC. Chemotherapy was primarily used as first-line treatment for both subgroups, but the TNBC subgroup received poly (ADP-ribose) polymerase (PARP) inhibitors at triple the rate as a second-line treatment and double the rate as a third-line treatment compared with the HR+/HER2-negative subgroup. Two-year OS rates were similar between the TNBC (73.9%) and the HR+/HER2-negative subgroups (77.0%), and anemia, nausea, and neutropenia were the most commonly reported toxicities across all treatments.
Conclusion: Clinicians should consider the use of targeted agents such as PARP inhibitors in earlier lines of therapy for ABC given the growing evidence that PARP inhibitors may improve PFS compared with chemotherapy while potentially offering a more manageable toxicity profile and improved quality of life.
Introduction
In the United States (US), 1 in 8 women will be diagnosed with breast cancer during their lifetime (1), and among those, 1 in 3 will develop metastatic disease (2, 3). The overall 5-year survival rate for women with localized or regional nonmetastatic breast cancer is approximately 86%-99%, but for those with metastatic breast cancer, the 5-year survival rate drops substantially to 29% (4). Women with a genetic predisposition, such as those with a germline mutation in the BRCA1/2 genes, have a significantly higher lifetime risk of developing breast cancer. Further, the cumulative lifetime risk of breast cancer for women who carry the BRCA1/2 genes was estimated to be 72% for women who carry the BRCA1 gene and 69% for women who carry the BRCA2 gene, and women who had either BRCA1 or BRCA2 mutations carried greater risk for developing breast cancer if they had a family history (5). BRCA1/2 mutations have been shown to impact not only breast cancer risk but also breast cancer outcomes. Women with a BRCA1 mutation and a breast cancer diagnosis have reduced time to progression and survival compared with BRCA2 mutation carriers and noncarriers of BRCA1/2 mutations (6). Additionally, treatment benefits from therapies such as adjuvant chemotherapy have been shown to be higher in patients without the BRCA1/2 genetic mutations compared with patients with the mutations (7).
Treatment for advanced breast cancer (ABC) is often tailored based on clinical stage, tumor histology, biomarker expression, prior therapy, and patient performance status (8). Treatment in earlier stages of breast cancer may include surgical resection, breast- conserving surgery, mastectomy (simple or double mastectomy, and modified radical mastectomy), or radiation therapy in women with BRCA1/2 mutation (9, 10). Historically, chemotherapy and/or endocrine therapy were the cornerstone of systemic treatment for patients with human epidermal growth factor receptor 2 (HER2)–negative ABC, but the treatment landscape is changing because of the recent introduction of therapies into the treatment paradigm. Recently, therapies such as poly (ADP-ribose) polymerase (PARP) inhibitors were approved in the US for the treatment of germline BRCA1/2-associated ABC (11–14). PARP inhibitors have been shown to improve the progression-free survival (PFS) compared with chemotherapy in patients with germline BRCA1/2-mutated, HER2-negative locally advanced and/or metastatic breast cancer (12, 15–17). Data from previous studies and the PRAEGNANT registry, which examines genetics and other molecular biomarkers in individuals with metastatic breast cancer, indicate that approximately 5% of patients with metastatic breast cancer carry germline BRCA1/2 mutations (18, 19) and thus could potentially derive benefit from a PARP inhibitor. The optimal treatment sequencing in this population has not yet been established. Patient characteristics, prescribing patterns, diverse health care systems, and variable access to care in the clinical practice setting differ from controlled clinical trial environments, which can cause variation in real-world outcomes for patients with germline BRCA1/2 mutations.
In this analysis, clinical characteristics, real-world treatment patterns, and health outcomes among patients with germline BRCA1/2-mutated, HER2-negative ABC in the US were evaluated in order to contextualize the results of relevant clinical trials and determine the extent of unmet need among these patients in the routine clinical-practice setting.
Materials and methods
Study design
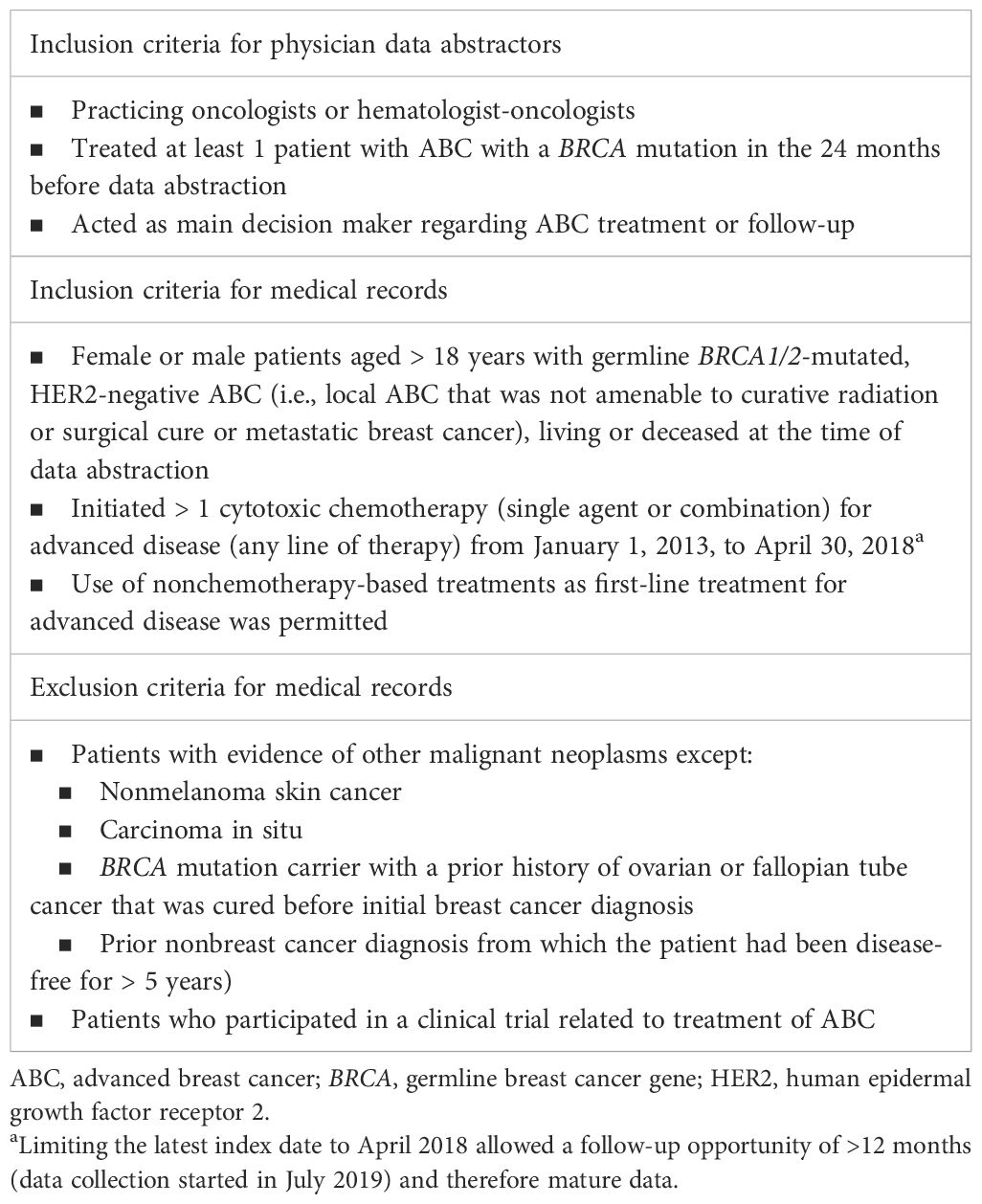
This study was a noninterventional, retrospective medical record review of adult patients with HER2-negative ABC and germline BRCA1/2 mutations who received cytotoxic chemotherapy. The RTI International Institutional Review Board determined that this study was not human subjects research (STUDY00020382), as the data were unidentifiable. ABC was defined as locally advanced breast cancer that was not amenable to curative radiation or surgery, or as metastatic disease. The sample was further stratified into two groups consisting of patients with advanced triple-negative breast cancer (TNBC) and patients with hormone receptor–positive (HR+)/HER2-negative ABC. Data describing patient and clinical characteristics, treatment patterns, and health outcomes were abstracted directly by eligible oncologists. A purposive, quasi-random sampling method was employed through the selection of medical records for patients whose last names began with a randomly generated letter. The inclusion criteria for oncologists, inclusion criteria for medical records, and exclusion criteria for medical records are presented in Table 1. Medical record abstraction occurred from July 2019 to May 2020, which allowed for extraction of follow-up data (when available) until May 2020.
Demographics and clinical characteristics
Demographic characteristics, including primary medical specialty, primary practice setting, and geographic region of practice, were collected for the recruited oncologists. For eligible patients, demographic data, as well as baseline clinical characteristics related to their initial breast cancer diagnosis and the diagnosis of ABC, were collected from medical records. Patient demographic data included sex, race, and family history of a BRCA-related cancer. Baseline clinical characteristics included age and Eastern Cooperative Oncology Group (ECOG) performance status at ABC diagnosis.
Treatment patterns
Data measures related to the treatment of breast cancer were collected before and after diagnosis of ABC (Figure 1). Treatment choices before the diagnosis of ABC included neoadjuvant treatment(s), surgery, adjuvant chemotherapy, adjuvant targeted therapy/biologics, adjuvant hormonal therapy, and radiation therapy. Types of treatment approaches, along with total number of systemic lines, collected after diagnosis of ABC included surgery, chemotherapy, targeted therapy/biologics, hormonal therapy, and radiation therapy.
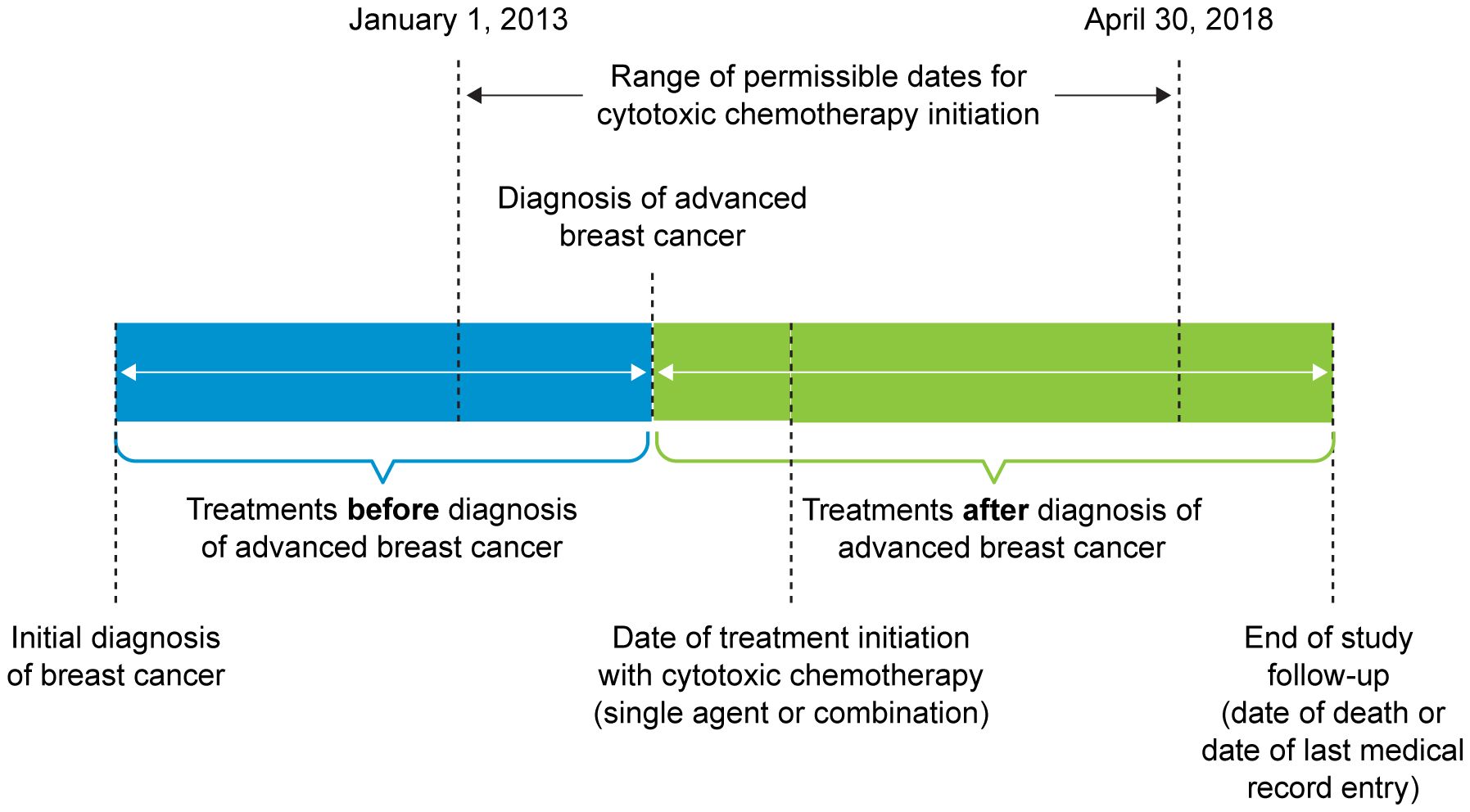
Figure 1 Treatment Pattern Time Period. Patients who were initially diagnosed with advanced breast cancer or were initially diagnosed at an earlier stage and experienced progression to advanced breast cancer were included in the study.
Health outcomes
Health outcomes included time-to-event estimates (i.e., real-world PFS [rwPFS], overall survival [OS]) and prespecified treatment-related toxicities. Dates of progression and death (i.e., whether the patient died during the follow-up period and whether the death was related to breast cancer) were documented. Time-to-event estimates were calculated from the date of ABC diagnosis and the start of each treatment line (received after ABC diagnosis). Several treatment-related hematological (i.e., anemia, neutropenia, thrombocytopenia) and nonhematologic toxicities (i.e., hand-foot syndrome [or palmar-plantar erythrodysesthesia], nausea, vomiting) commonly observed in clinical trials and real-world studies (10, 20) were documented for each treatment line received after diagnosis of ABC.
Statistical analysis
Measures were summarized descriptively through tabular and graphical display of mean values, medians, ranges, and standard deviations for continuous variables of interest and frequency distributions for categorical variables. Time-to-event outcomes (i.e., rwPFS, OS) were calculated using Kaplan-Meier methods. All analyses were conducted using SAS (version 9.4 or higher) statistical software. This study was descriptive, with the primary goal of retrospectively summarizing existing treatment patterns and patient outcomes.
Results
Demographics and clinical characteristics
A total of 97 oncologists provided abstraction of 305 medical records of adult patients with germline BRCA1/2-mutated, HER2-negative ABC. The majority were medical oncologists (62.9%) and practiced in a private hospital or clinic (48.5%) (Supplementary Table 1). The geographic locations were approximately evenly distributed among the Southern (30.9%), Northeastern (26.8%), Midwestern (21.7%), and Western US (20.6%).
Patient demographics and clinical characteristics are presented in Table 2. Of the 305 included medical records, 194 (63.6%) patients had advanced TNBC and 111 (36.4%) patients had HR+/HER2-negative ABC. The median age at ABC diagnosis for the overall patient population with germline BRCA1/2-mutated, HER2-negative ABC was 57.3 (range, 33.3-79.8) years. Similar median ages at ABC diagnosis were found for the TNBC (57.2 years) and HR+/HER2-negative (58.2 years) subgroups. There was one male patient in the TNBC subgroup. The overall group was mostly white (76.4%); this composition decreased for the TNBC subgroup (71.7%) and increased for the HR+/HER2-negative subgroup (84.7%). The percentages of patients of Ashkenazi Jewish ancestry were similar across the overall group (11.5%), the TNBC subgroup (12.9%), and the HR+/HER2-negative subgroup (9.0%). The percentages of patients with no known family history of a BRCA-related cancer were 24.6%, 27.8%, and 18.9% for the overall group, the TNBC subgroup, and the HR+/HER2-negative subgroup, respectively. The percentage of patients with an ECOG performance status of 0 or 1 at ABC diagnosis was 74.6% for the overall group, 70.2% for the TNBC subgroup, and 83.2% for the HR+/HER2-negative subgroup.
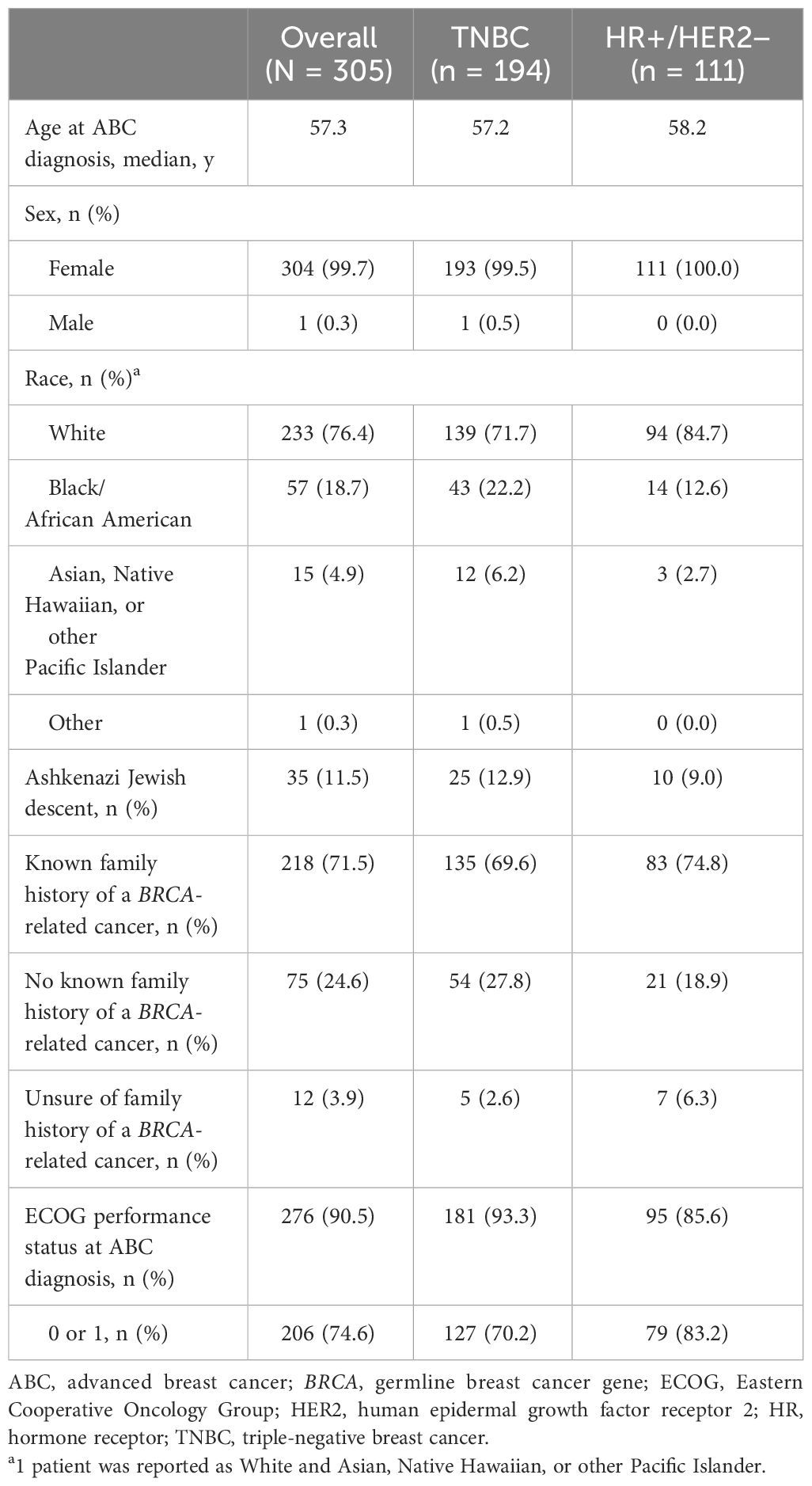
Table 2 Demographics and clinical characteristics in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer.
Treatment patterns
For both the TNBC (Figure 2) and HR+/HER2-negative (Figure 3) subgroups, first-line treatments were similar, with chemotherapy being the most commonly used treatment, but differences in treatments between these subgroups emerged in the second-line setting and beyond. First-line treatment for the TNBC subgroup was composed of mostly nonplatinum-based chemotherapy (60.8%) and platinum-based chemotherapy (39.2%). In second-line treatment, the TNBC subgroup had nearly equivalent rates of nonplatinum-based chemotherapy (40.2%) and PARP inhibitors (42.0%). Third-line treatment in the TNBC subgroup again had similar rates for nonplatinum-based chemotherapy (39.1%) and PARP inhibitors (43.5%). In the HR+/HER2-negative subgroup, first-line treatment consisted of chemotherapy for 78.4% of patients and endocrine-based therapy (EBT) for 19.8% of patients. In second-line treatment, most of the HR+/HER2-negative subgroup received EBT (51.2%), with lower percentages of chemotherapy (31.0%) and PARP inhibitors (13.1%). In third-line treatment for the HR+/HER2-negative subgroup, chemotherapy again became the dominant treatment (50.0%), with decreased use of EBT (27.8%) and increased use of PARP inhibitors (19.4%).
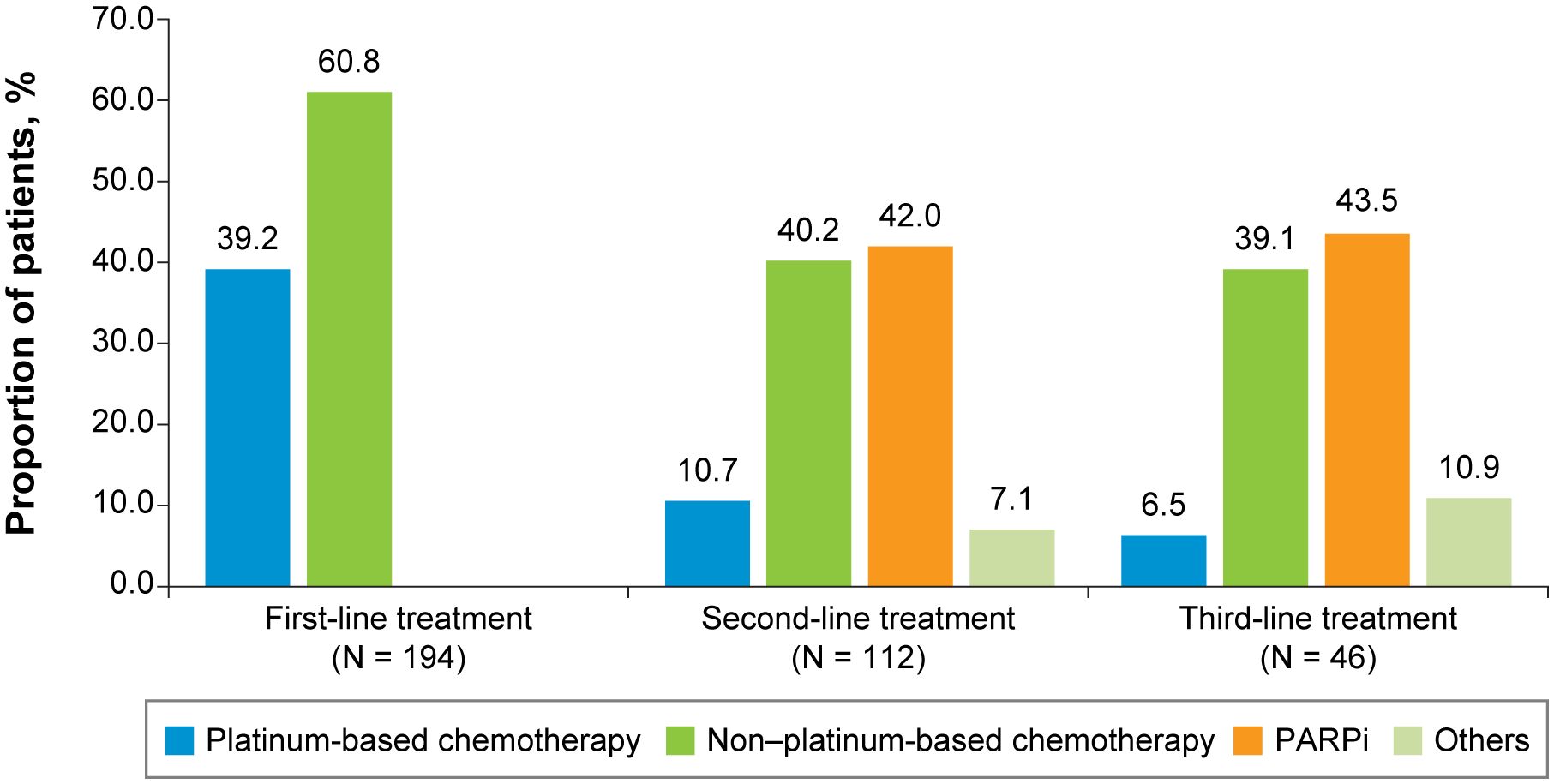
Figure 2 First-, Second-, and Third-Line Treatments Among Patients With Advanced Triple-Negative Breast Cancer. PARPi, poly (ADP-ribose) polymerase inhibitors.
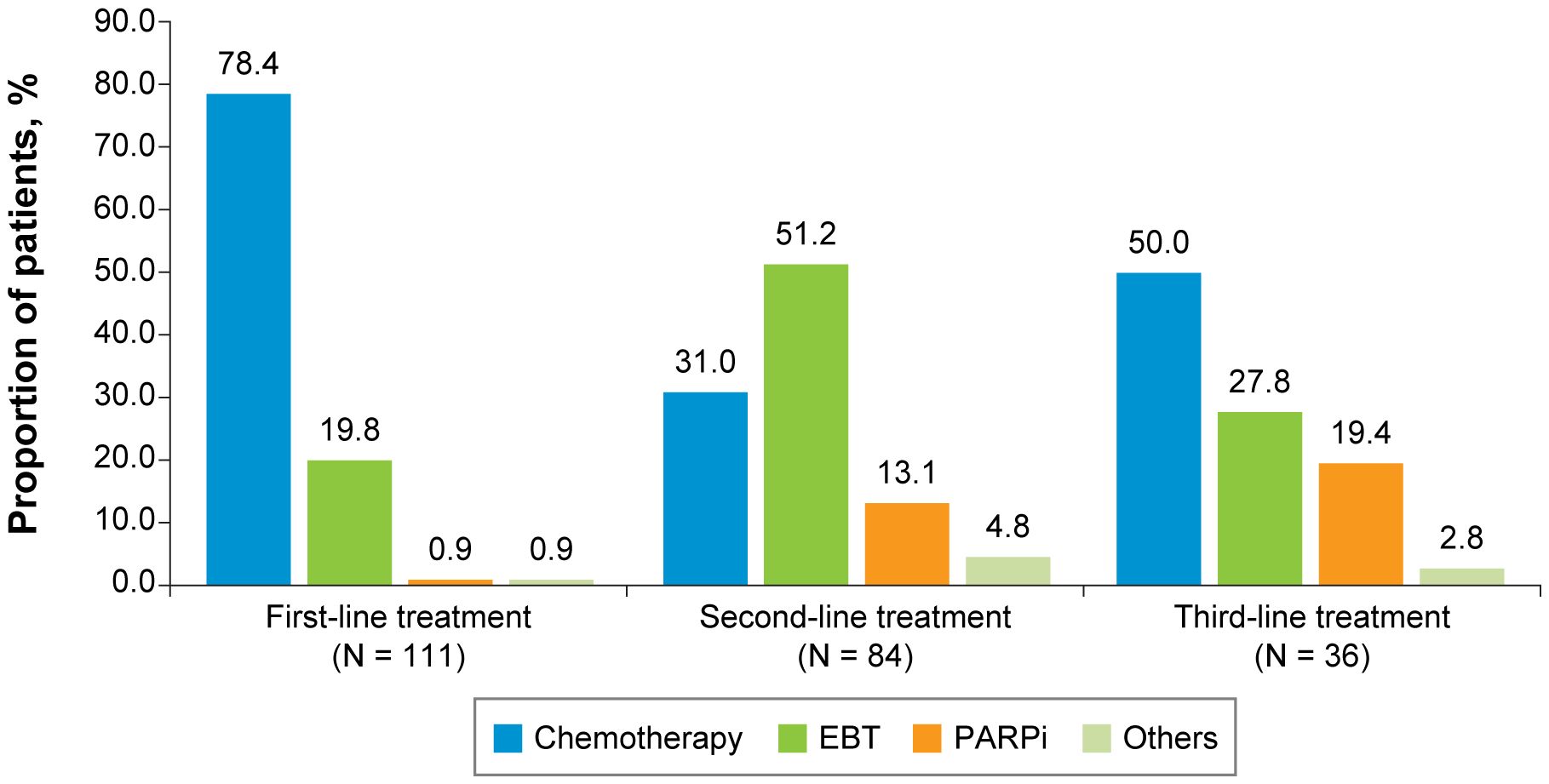
Figure 3 First-, Second-, and Third-Line Treatments Among Patients With HR+/HER2-Negative ABC. ABC, advanced breast cancer; EBT, endocrine-based therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PARPi, poly (ADP-ribose) polymerase inhibitors.
Efficacy and safety outcomes
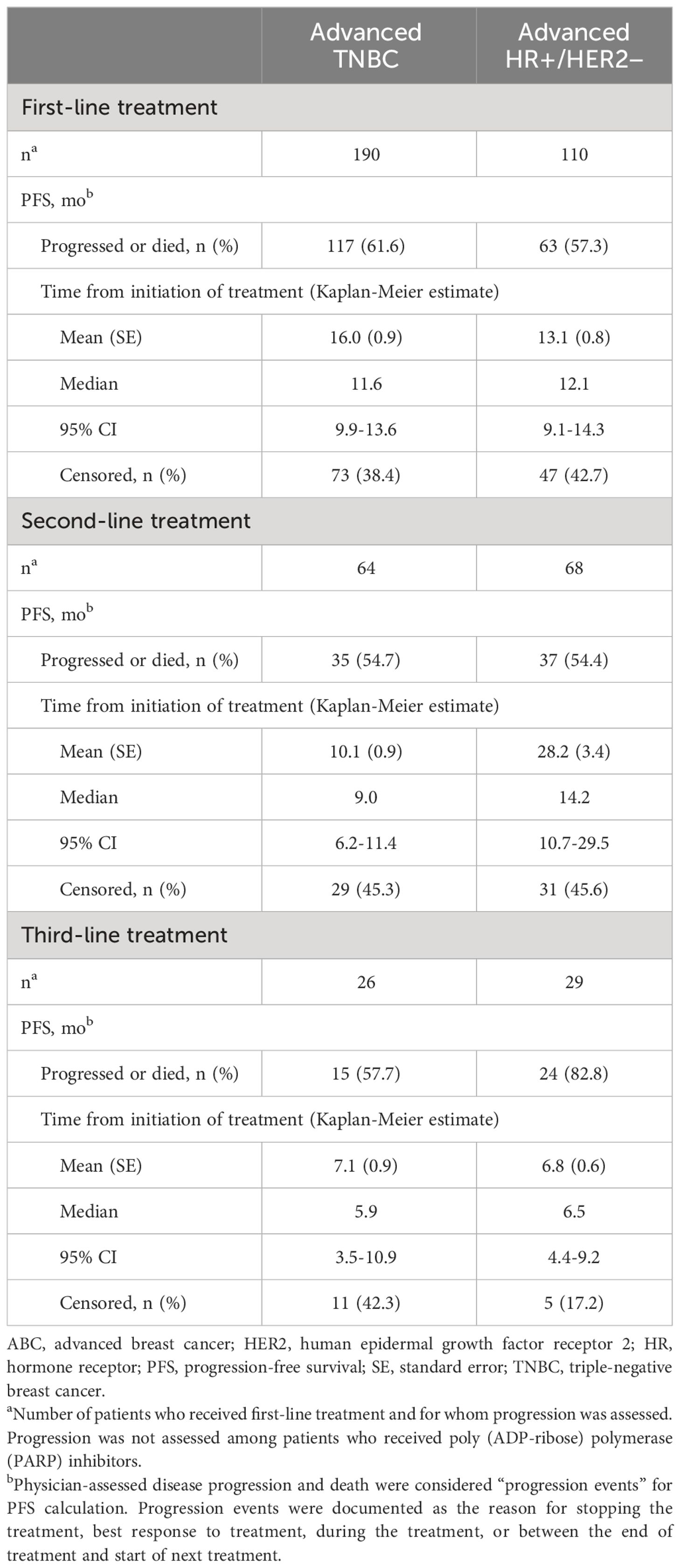
During the first-, second-, and third-line treatments, rwPFS was assessed for the TNBC and HR+/HER2-negative ABC subgroups (Table 3). Among patients with TNBC, the median PFS for first-line treatment (n = 190) was 11.6 months (95% confidence interval [CI], 9.9-13.6); for second-line treatment (n = 64), the median PFS was 9.0 months (95% CI, 6.2-11.4); and for third-line treatment (n = 26), the median PFS was 5.9 months (95% CI, 3.5-10.9). Among patients who were HR+/HER2 negative, the median PFS for first-line treatment (n = 110) was 12.1 months (95% CI, 9.1-14.3); for second-line treatment (n = 68), the median PFS was 14.2 months (95% CI, 10.7-29.5); and for third-line treatment (n = 29), the median PFS was 6.5 months (95% CI, 4.4-9.2). Overall survival from index chemotherapy was assessed for the TNBC and HR+/HER2-negative ABC subgroups (Table 4). Patients in the TNBC subgroup had a 2-year OS rate of 73.9%, which decreased to 53.1% at 5 years. The median OS was not estimable during the study follow-up. Patients in the HR+/HER2-negative subgroup had a 2-year OS rate of 77.0%, which decreased to 44.2% at 5 years, and a median OS of 45.9 months.
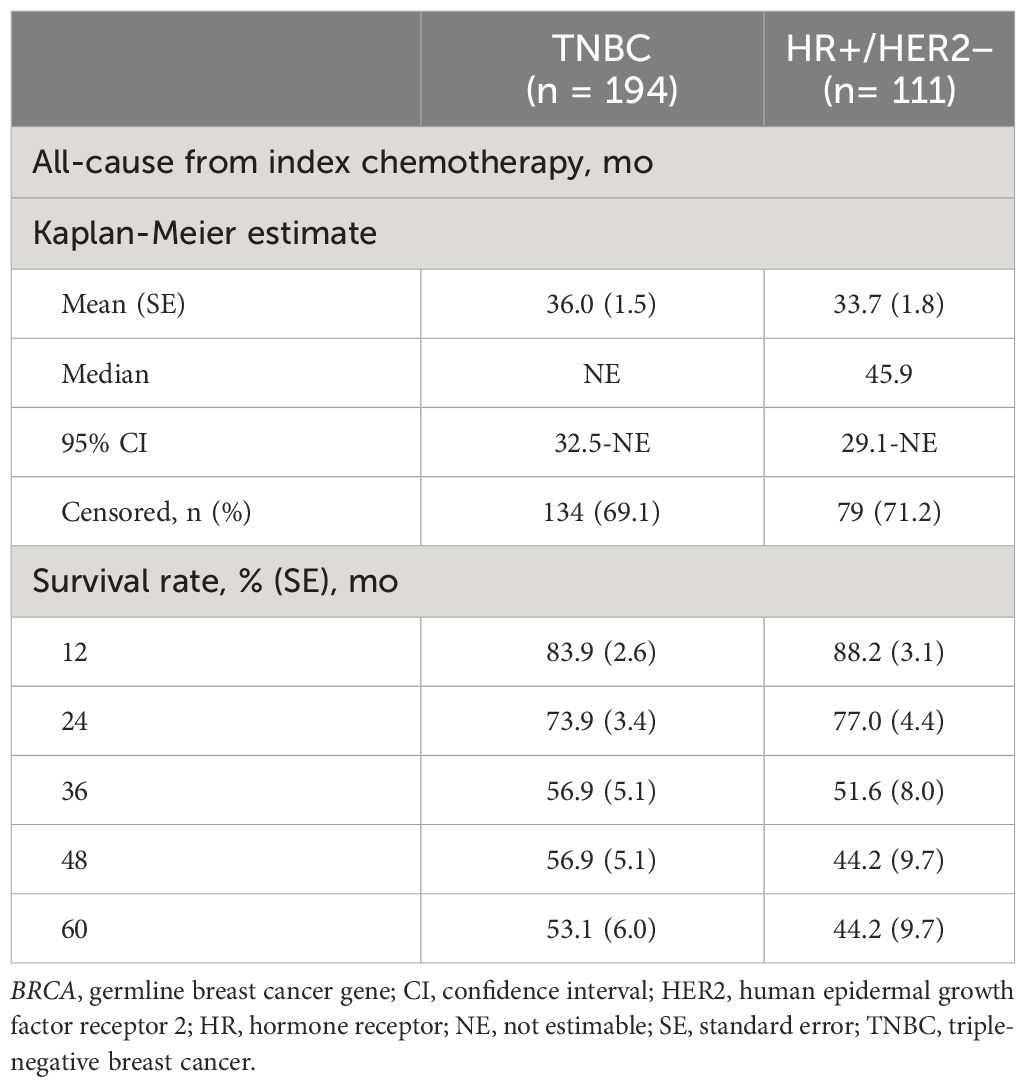
Table 4 Overall survival and mortality among patients with germline BRCA1/2-mutated, HER2-negative advanced breast cancer.
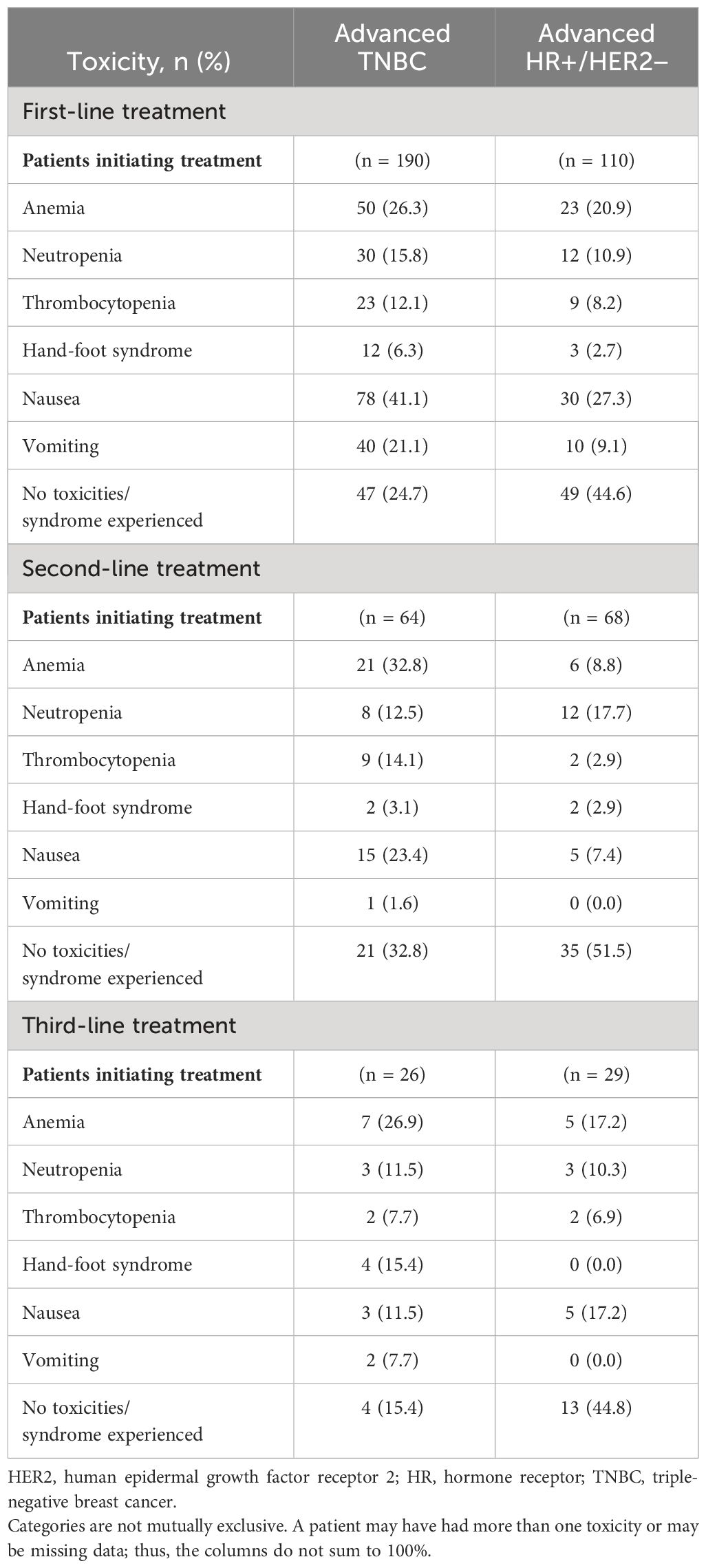
The prespecified treatment-related hematological toxicities and evidence of hand-foot syndrome, nausea, and vomiting during the first-, second-, and third-line treatments were documented for the TNBC and HR+/HER2-negative ABC subgroups (Table 5). For first-line treatment, the most commonly experienced toxicities for both the TNBC (n = 190) and HR+/HER2-negative (n = 110) subgroups were nausea (41.1% and 27.3%, respectively) and anemia (26.3% and 20.9%, respectively). Toxicities were not documented for 24.7% of the patients in the TNBC subgroup and 44.6% of patients in the HR+/HER2-negative subgroup in the first-line setting. For second-line treatment, the TNBC subgroup (n = 64) documented anemia (32.8%) and the HR+/HER2-negative subgroup (n = 68) documented neutropenia (17.7%) as the most commonly experienced toxicities. Toxicities were not documented for 32.8% of the TNBC subgroup and 51.5% of the HR+/HER2-negative subgroup in the second-line setting. For third-line treatment, nearly half (44.8%) of the 29 patients in the HR+/HER2-negative subgroup did not document toxicities, compared with 15.4% of patients who did not document toxicities in the TNBC subgroup (n = 26). However, the small sample sizes for third-line treatment warrant caution in interpretation of the toxicities experienced.
Discussion
The current study involved a relatively large review of medical records that described the clinical characteristics, treatment patterns, rwPFS, and OS of adult patients with HER2-negative ABC with germline BRCA1/2 mutation who had received cytotoxic chemotherapy. At the time of ABC diagnosis, most patients in this sample were younger than 60 years (mean age = 57.3 years), were white (76.4%), and had a family history of BRCA-related cancer (71.5%). Nearly 25% of the patients who were germline BRCA1/2 mutation carriers in this study had no known family history of BRCA-related cancers, thus emphasizing the importance of not limiting BRCA1/2 genetic testing to those who have family history. Two-year OS rates were similar between the advanced TNBC subgroup (73.9%) and the HR+/HER2-negative subgroup (77.0%). Anemia, nausea, and neutropenia were among the most commonly documented toxicities across all treatment lines, although some patients had no documentation of experiencing the prespecified toxicities. Because of our selection criteria, chemotherapy was primarily used as first-line treatment for both TNBC and HR+/HER2-negative subgroups. However, compared with the HR+/HER2-negative subgroup, the TNBC subgroup received PARP inhibitors at triple the rate in the second-line setting and double the rate in the third-line setting. The introduction of PARP inhibitors for second-line treatment of TNBC could be linked to the increased proportion of patients who did not experience toxicities, from 24.7% in the first line to 32.8% in the second line. The relatively higher use of PARP inhibitors in the TNBC subgroup compared with the HR+/HER2-negative subgroup likely reflects that there are limited nonchemotherapy treatment options for patients with triple-negative disease, whereas patients with HR+/HER2-negative ABC have other nonchemotherapy-targeted treatment options, such as CDK4/6 inhibitors, PIK3CA inhibitors, and mTOR inhibitors, combined with endocrine therapy that can be utilized in the second and third lines.
Recent clinical trials have demonstrated that the use of PARP inhibitors—a nonchemotherapy-targeted treatment option—has significantly improved clinical and patient-reported outcomes with a manageable side effect profile in patients with HER2-negative ABC carrying a germline BRCA1/2 mutation (12, 15, 16). OlympiAD, a phase 3 clinical trial of patients with germline BRCA1/2-mutated, HER2-negative ABC, showed that a significantly longer median PFS was observed in patients who received olaparib (7.0 months; n = 205 patients), a PARP inhibitor treatment, compared with patients who received single-agent chemotherapy (either capecitabine, eribulin, or vinorelbine), for whom PFS was 4.2 months (n = 97 patients, P < 0.001) (12). Similarly, EMBRACA, a phase 3 clinical trial of patients with germline BRCA1/2-mutated, HER2-negative ABC, demonstrated significantly longer median PFS in patients who received talazoparib (8.6 months; n = 287 patients), a PARP inhibitor treatment, compared with patients who received 1 of 4 physician’s choice standard chemotherapy options, for whom PFS was 5.6 months (n = 144, P < 0.001) (15). A safety analysis using the EMBRACA trial data evaluated the safety profile of talazoparib in 286 patients compared with chemotherapy in 126 patients with germline BRCA1/2, HER2-negative mutation and ABC (16). While anemia and nausea were commonly experienced adverse events, talazoparib participants experiencing these events had more favorable patient-reported outcomes compared with participants on chemotherapy.
Therefore, an accumulating body of evidence suggests patients who receive PARP inhibitors have better PFS, more favorably reported outcomes, and fewer instances of discontinuation secondary to side effects compared with patients treated with chemotherapy. To date, the sequencing of treatment options, particularly when to introduce PARP inhibitors into the treatment of patients with HER2-negative germline BRCA1/2 ABC, has yet to be explored. Targeted therapy with PARP inhibitors may need to be considered in earlier lines of treatment in patients with ABC. This consideration is especially relevant among patients with TNBC whose alternative treatment options will consist of chemotherapy alone or chemotherapy combined with immunotherapy, which are associated with significant toxicities and limited improvement in breast cancer outcomes (i.e., PFS and/or OS).
Study limitations
This study represents the first dataset to examine real-world treatment patterns and outcomes exclusively among HER2-negative patients with germline BRCA1/2-mutated ABC and provides valuable findings related to how targeted therapies, such as PARP inhibitors, are being incorporated into real-world practice in this select patient population. However, given that data abstraction occurred between July 2019 and May 2020 (maximum follow-up until May 2020), the incidence of PARP inhibitor therapy may have changed, and PARP inhibitors might be prescribed more often. A limitation of this study is that patients selected for study inclusion represented a purposive sample of medical records obtained from oncologists who were willing to participate in the study. Therefore, study findings may not be generalizable to the overall population of patients with germline BRCA1/2-mutated ABC. To minimize this limitation and improve generalizability of findings, oncologists were recruited from a variety of geographic regions and practice types. Another limitation was that data were limited to medical records to which oncologists had access. Information on health care services received outside the physician’s care setting that was not recorded in the medical record was unavailable for this study (e.g., treatment for adverse events received through care rather than the abstracting physician specialist). Accordingly, some adverse events may not have been included because they were reported elsewhere and not collected in the medical record. Also, while not all known treatment-related toxicities were evaluated in this study, toxicities commonly observed in clinical trials and real-world studies were assessed. Lastly, to homogenize the sample, patients in this study were required to have initiated at least one cytotoxic chemotherapy for ABC, which may have resulted in selection bias and could be the reason for relatively lower utilization of endocrine or endocrine-based therapies and PARP inhibitors observed in our study population.
Key findings
Given the growing evidence that PARP inhibitors may improve PFS better than chemotherapy while potentially offering a more manageable toxicity profile and improved quality of life, clinicians should consider the use of targeted agents such as PARP inhibitors in earlier lines of therapy for ABC. Further prospective trials examining the sequencing of targeted agents in ABC are needed. Ultimately, the results of this study may contextualize the results of ongoing clinical trials, inform routine clinical-practice, and improve patient lives.
Data availability statement
The datasets presented in this article are not readily available because they are not public. Requests to access the datasets should be directed to rparikh@rti.org.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
EO: Investigation, Supervision, Visualization, Writing – review & editing. RCP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft. EE: Formal analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. BA: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review & editing. AH: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. LSA: Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. AN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. KW: Investigation, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funding from Pfizer Inc. The funder had the following involvement in the study: employees of the funder (Pfizer Inc.) were involved in the research and contributed to this article as authors. RTI Health Solutions, an independent nonprofit research organization, received funding under a research contract with Pfizer Inc. to conduct this study and provide publication support in the form of manuscript writing, styling, and submission.
Acknowledgments
The authors thank Brian Samsell, PhD, and Melissa Mehalick, PhD, of RTI Health Solutions for medical writing assistance. Pfizer Inc. provided funding for publication support in the form of manuscript writing, styling, and submission.
Conflict of interest
EO was employed at Fox Chase Cancer Center at the time of study and is presently employed at John Theurer Cancer Center and Hackensack Meridian Health; has a consulting or advisory role with Pfizer, Foundation Medicine, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Ethos Immunomedics, Novartis, and OncLive/MJH Life Sciences; has received honoraria from Guidepoint Global; has received research funding from Merck and Genentech/Roche; has no relationships to disclose for the speakers’ bureau, patents, royalties, or other intellectual property; expert testimony; leadership, or travel accommodations, expenses; and has an immediate family member who holds stock and other ownership interests in Bristol-Myers Squibb and is a Bristol-Myers Squibb/Celgene recipient. RCP, EE, and AH are employees of RTI Health Solutions, an independent nonprofit research organization, which received funding to conduct the study presented here. BA, LSA, and AN are employees of Pfizer Inc., which funded the study presented here. KW was employed at Fox Chase Cancer Center at the time of study, is presently employed at Medstar Georgetown Cancer Institute at Washington Hospital Center, and consults for Novartis.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1341665/full#supplementary-material.
References
1. The American Cancer Society medical and editorial content team. How common is breast cancer? The American Cancer Society (2021). Accessed on May 23, 2021. Available at: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html.
2. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. (2005) 10 Suppl 3:20–9. doi: 10.1634/theoncologist.10-90003-20
3. Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. (2013) 274:113–26. doi: 10.1111/joim.12084
4. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Accessed on May 3, 2021. Available at: https://seer.cancer.gov/statfacts/html/breast.html.
5. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. (2017) 317:2402–16. doi: 10.1001/jama.2017.7112
6. Bayraktar S, Gutierrez-Barrera AM, Lin H, Elsayegh N, Tasbas T, Litton JK, et al. Outcome of metastatic breast cancer in selected women with or without deleterious BRCA mutations. Clin Exp Metastasis. (2013) 30:631–42. doi: 10.1007/s10585-013-9567-8
7. Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. (2016) 122:1178–84. doi: 10.1002/cncr.29903
8. Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med (Lausanne). (2017) 4:227. doi: 10.3389/fmed.2017.00227
9. Evron E, Ben-David AM, Goldberg H, Fried G, Kaufman B, Catane R, et al. Prophylactic irradiation to the contralateral breast for BRCA mutation carriers with early-stage breast cancer. Ann Oncol. (2019) 30:412–7. doi: 10.1093/annonc/mdy515
10. Society AC. Treating breast cancer 2021. Available online at: https://www.cancer.org/cancer/breast-cancer/treatment.html.
11. US Food and Drug Administration. Talazoparib prescribing information. Pfizer Labs. (2018), Accessed on April 22, 2021.
12. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
13. Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. (2015) 66:455–70. doi: 10.1146/annurev-med-050913-022545
14. Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. (2016) 8:362ps17. doi: 10.1126/scitranslmed.aaf9246
15. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. (2018) 379:753–63. doi: 10.1056/NEJMoa1802905
16. Hurvitz SA, Gonçalves A, Rugo HS, Lee KH, Fehrenbacher L, Mina LA, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: Detailed safety analyses from the phase III EMBRACA trial. Oncologist. (2020) 25:e439–e50. doi: 10.1634/theoncologist.2019-0493
17. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology Version 6. 2020. National Comprehensive Cancer Network (3025 Chemical Road, Suite 100, Plymouth Meeting, PA 19462), Accessed on October 14, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
18. Armstrong N, Ryder S, Forbes C, Chalker A, Ross J, Quek R. An international systematic review (SR) of breast cancer (BC) BRCA mutation (BRCAm) prevalence. Ann Oncol. (2018) 29:viii97–viii8. doi: 10.1093/annonc/mdy272.297
19. Fasching PA, Hu C, Hart S, Hartkopf AD, Taran FA, Janni W, et al. Germline BRCA1and BRCA2 mutations in patients with HER2-negative metastatic breast cancer (mBC) treated with first-line chemotherapy: Data from the German PRAEGNANT registry. J Clin Oncol. (2019) 37:1048. doi: 10.1200/JCO.2019.37.15_suppl.1048
Keywords: metastatic breast cancer, BRCA1/2 gene mutations, poly (ADP-ribose) polymerase (PARP) inhibitors, human epidermal growth factor receptor 2 (HER2)-negative, hormone receptor-positive (HR+), triple-negative breast cancer (TNBC)
Citation: Obeid E, Parikh RC, Esterberg E, Arondekar B, Hitchens A, Arruda LS, Niyazov A and Whitaker K (2024) Clinical characteristics, treatment patterns, and outcomes in adult patients with germline BRCA1/2-mutated, HER2-negative advanced breast cancer: a retrospective medical record review in the United States. Front. Oncol. 14:1341665. doi: 10.3389/fonc.2024.1341665
Received: 20 November 2023; Accepted: 08 April 2024;
Published: 16 May 2024.
Edited by:
Norikazu Masuda, Nagoya University, JapanReviewed by:
J. Guilherme Gonçalves – Nobre, University of Lisbon, PortugalLuo Hai, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2024 Obeid, Parikh, Esterberg, Arondekar, Hitchens, Arruda, Niyazov and Whitaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elias Obeid, ZWxpYXMub2JlaWRAaG1obi5vcmc=
†Present addresses: Elias Obeid, John Theurer Cancer Center, Hackensack, NJ, United States and Hackensack Meridian School of Medicine, Totowa, NJ, United States
Kristen Whitaker, Medstar Georgetown Cancer Institute at Washington Hospital Center, Washington D. C., United States
 Elias Obeid
Elias Obeid Rohan C. Parikh
Rohan C. Parikh Elizabeth Esterberg2
Elizabeth Esterberg2