
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 17 May 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1340865
Rhabdomyosarcoma (RMS) is a common soft tissue malignant tumor, especially in young patients. Alveolar rhabdomyosarcoma (ARMS) is a subtype of RMS that is prevalent in adolescents. This malignant tumor usually develops in the extremities and can also involve the trunk, perineum, and pelvis. Now, we report a rare case of pelvic lymph node metastatic alveolar RMS in a young patient, which was determined by fine needle aspiration cytology (FNAC). To the best of our knowledge, this is the first case in which the definite diagnosis of ARMS was initially made by FNAC.
RMS is the most common soft tissue sarcoma in children, accounting for about half of all soft tissue tumors, which are usually localized in the extremities, trunk, head, and neck (1, 2). It is rarely observed in adults and only accounts for 3% of adult soft tissue sarcomas, which has a significantly worse prognosis compared to children (3). RMS can be divided into two prevalent subtypes: embryonal rhabdomyosarcoma (ERMS) and ARMS. In addition, rare subtypes include pleomorphic rhabdomyosarcoma (PRMS) and spindle cell/sclerosing rhabdomyosarcoma (SRMS) (4). ARMS occurs most frequently in the deeper parts of the extremities and can also involve the trunk, perineum, and pelvis. In terms of age, ARMS accounts for approximately 20% of all pediatric RMS and is most common in adolescents and young adults between the ages of 10 and 25 (5).
FNAC is a minimally invasive way of obtaining diseased tissue and has achieved widespread application in the diagnosis of many sites and types of tumors (6–8). Combined with various assistive techniques, the correctness of the FNAC diagnosis is comparable to that of histopathological biopsy for some kinds of tumors (6, 9). FNAC of suspicious lymph nodes also provides additional information on tumor staging, which can better guide subsequent treatment (10, 11).
A 20-year-old male patient was admitted to the hospital with the chief complaint of “recurrent hematuria for 1 month, aggravated by fever and abdominal pain for 10 days”. A physical examination revealed that the patient had lower abdominal tenderness, bilateral scrotal swelling, and slight perianal swelling with squeezing pain. A digital rectal examination revealed that the patient’s prostate was enlarged with a soft mass nearby, had a fluctuating sensation, and was poorly defined. A small amount of discharge was present on the finger inspection.
A male urologic ultrasound was performed on the patient, which revealed bilateral hydronephrosis with uneven enlargement of the prostate, suggesting infectious disease. An enhanced CT scan of the lower abdomen and pelvis was performed subsequently, which suggested lymphoma or an infectious disease.
To further investigate the patient’s pelvic lesions, an MRI enhancement scan was carried out, which showed diffuse abnormal signals in the pelvis and pelvic extraperitoneal space. The prostate was enlarged with extensive swelling of the bladder, rectum, sigmoid colon bowel wall, pelvic floor muscle groups, pelvic peritoneum, and pelvic sacral fascia. Lymph nodes in the pelvic and bilateral inguinal regions were increased and enlarged. Lymphoma, or infection, was considered by the MRI (Figure 1).
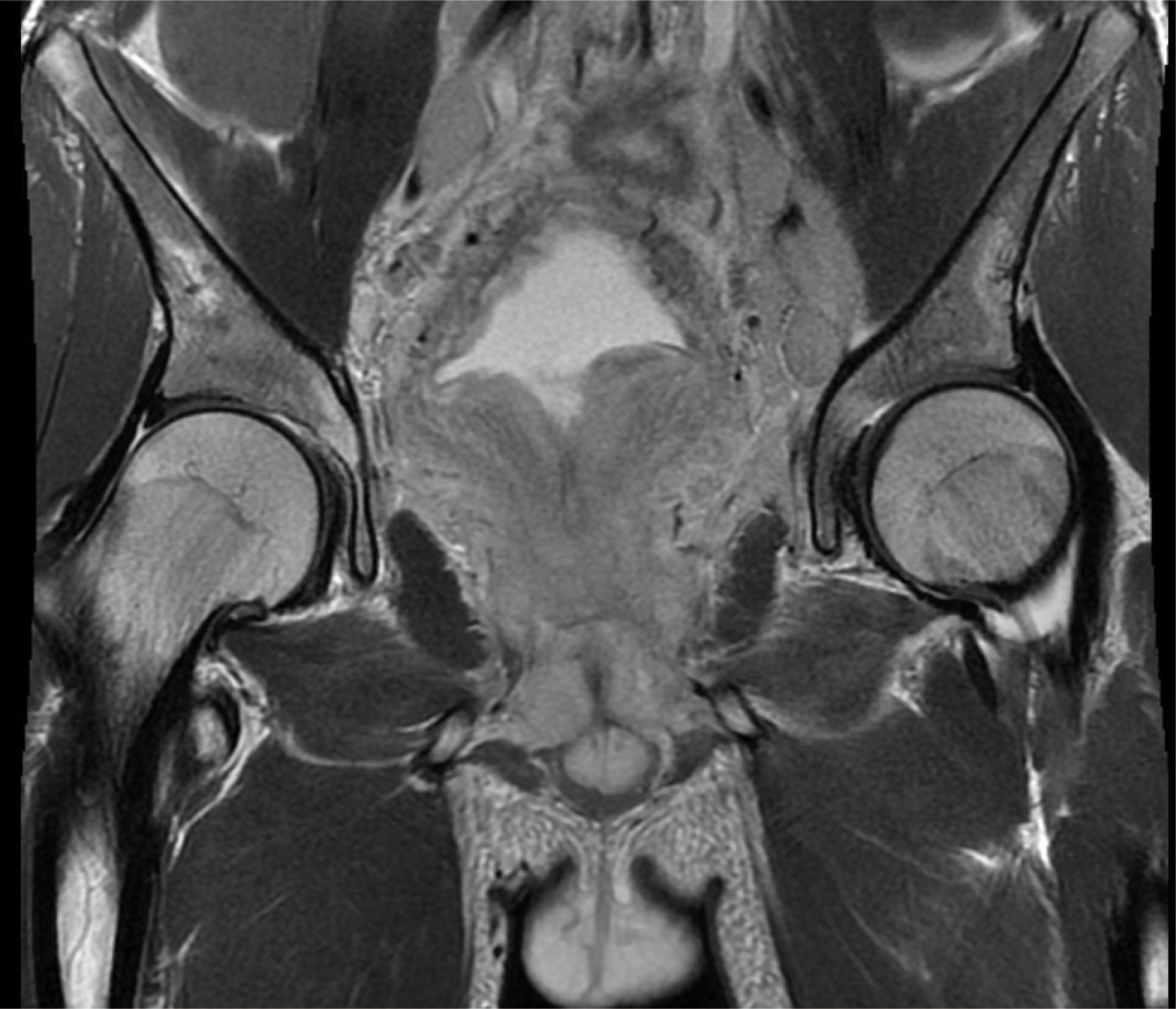
Figure 1 The MRI figure showed diffuse abnormal signals in the pelvis and pelvic extraperitoneal space. The prostate was enlarged, and there was extensive swelling of the bladder, rectum, sigmoid colon bowel wall, pelvic floor muscle groups, pelvic peritoneum, and pelvic sacral fascia. Lymph nodes in the pelvic and bilateral inguinal regions were increased and partly enlarged.
Fine needle aspiration (FNA) was performed for this patient with ultrasound guidance. Ultrasound showed an enlarged lymph node measuring about 33 mm × 16 mm × 16 mm, adjacent to the left iliac vessel, which had unclear corticomedullary demarcation and a rich internal blood flow signal. After local anesthesia, multipoint aspiration of a left iliac perivascular lymph node was conducted by using a 23-G needle under ultrasonic monitoring. Two smears were made and fixed with 95% alcohol immediately. The residue material in the needle was collected and fixed in 10% neutral buffer formalin to make a cell block. The smears were subjected to Pap staining; 4 μm sections were taken from the formalin-fixed and paraffin-embedded cell block and then stained for hemoglobin and eosin (H&E).
Immunocytochemistry (ICC) was conducted on 4 μm sections taken from the cell block using a Roche VENTANA ULTRA automated immunostaining machine following the steps of EnVision. The following antibodies were employed: Desmin, Myogenin, MyoD1, CD56, Syna, ALK-1, LCA, PCK, CD99, MPO, S100, HMB45, CD79a, CD20, CD3, CD30, TIA-1, CD117, Granzyme B, OCT3/4, CgA, and Ki-67. In situ hybridization (ISH) test was performed for EBER1/2 also using a 4μm section of the cell block.
A FOXO1 (FKHR) two-color break-apart probe (Abbott Molecular, Abbott Park, IL, USA) was used, which consisted of a 720-kb probe labeled with the Spectrumgreen™ fluorophore located proximal to the FOXO1 gene as well as a 650-kb probe labeled with the SpectrumOrange™ fluorophore located at 13q14 distal to the FOXO1 gene.
The smears and cell block sections were highly cellular. Many small blue-round cells with high N/C ratios were present, with some small lymphocytes in the background. Most abnormal cells were scatter-distributed, and some loosely packed clusters could also be found. The nuclei were irregular and eccentric, with coarse chromatin and small nucleoli, which showed marked heteromorphism. Apoptosis and mitosis were frequent. Some binucleate and multinucleate abnormal cells were observed (Figure 2).
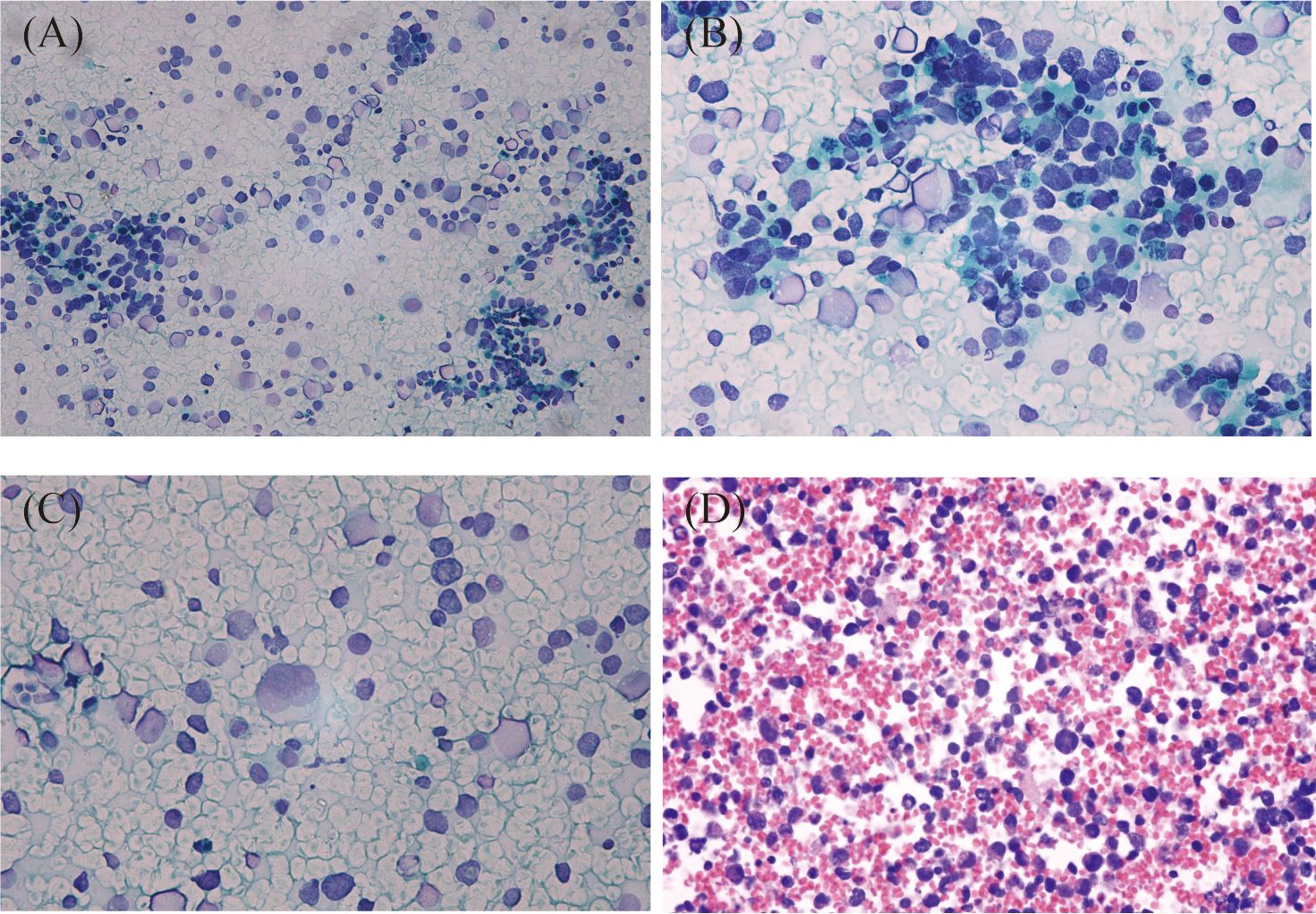
Figure 2 Cytologic features of metastatic alveolar rhabdomyosarcoma of the lymph nodes. Most tumor cells were scatter-distributed, and some loosely packed clusters could also be found in the background of lymphocytes (A), Papanicolaou staining, ×200). The nuclei were irregular and eccentric, with coarse chromatin and small nucleoli (B), Papanicolaou staining, ×400). Some binucleated and multinucleated cells were present (C), Papanicolaou staining, ×400). Scattered small blue-round cells with rhabdoid features were seen on the cell block section. The nuclei were deeply stained, and tumor giant cells were observed in this figure (D), HE staining, ×400).
Based on the cytological morphology and imageological findings, lymphohematopoietic tissue tumors were first considered, and relevant immunophenotyping tests, in situ hybridization tests, and gene rearrangement tests were performed. However, ICC showed LCA, CD3, CD20, CD79a, CD30, TIA-1, Granzyme B, and MPO were all negative, while only CD56, ALK, and Ki-67 (80%) were positive. ISH test showed EBER1/2 (−). Gene rearrangement tests for IgH and TCRG were all negative. According to the above test results, lymphohematopoietic tissue tumors were basically ruled out, and then metastatic tumors of the lymph node were considered. The second round immunophenotyping test showed PCK, CD99, OCT3/4, CD117, S100, and HMB45 were all negative while only Syna was positive, which can rule out metastatic carcinoma, germ cell tumor, melanoma, and primitive neuroectodermal tumor. Combined with the patient’s age, cytological morphology, and the results of the first two rounds of immunophenotyping tests (CD56+, Syna+, and ALK+), metastatic RMS was considered, and the third round of immunophenotyping test was carried out, which showed Desmin, Myogenin, and MyoD1 were all positive (Figure 3). So, the diagnosis of metastatic RMS was made. To further determine the subtype of RMS, the Fluorescence in situ hybridization (FISH) test for FOXO1 (FKHR) gene rearrangement was performed, and the FOXO1 gene translocation was detected (Figure 4). At last, metastatic ARMS of the left paravascular iliac lymph node was diagnosed by FNAC, and the pelvic lesion was considered ARMS by the clinicians.
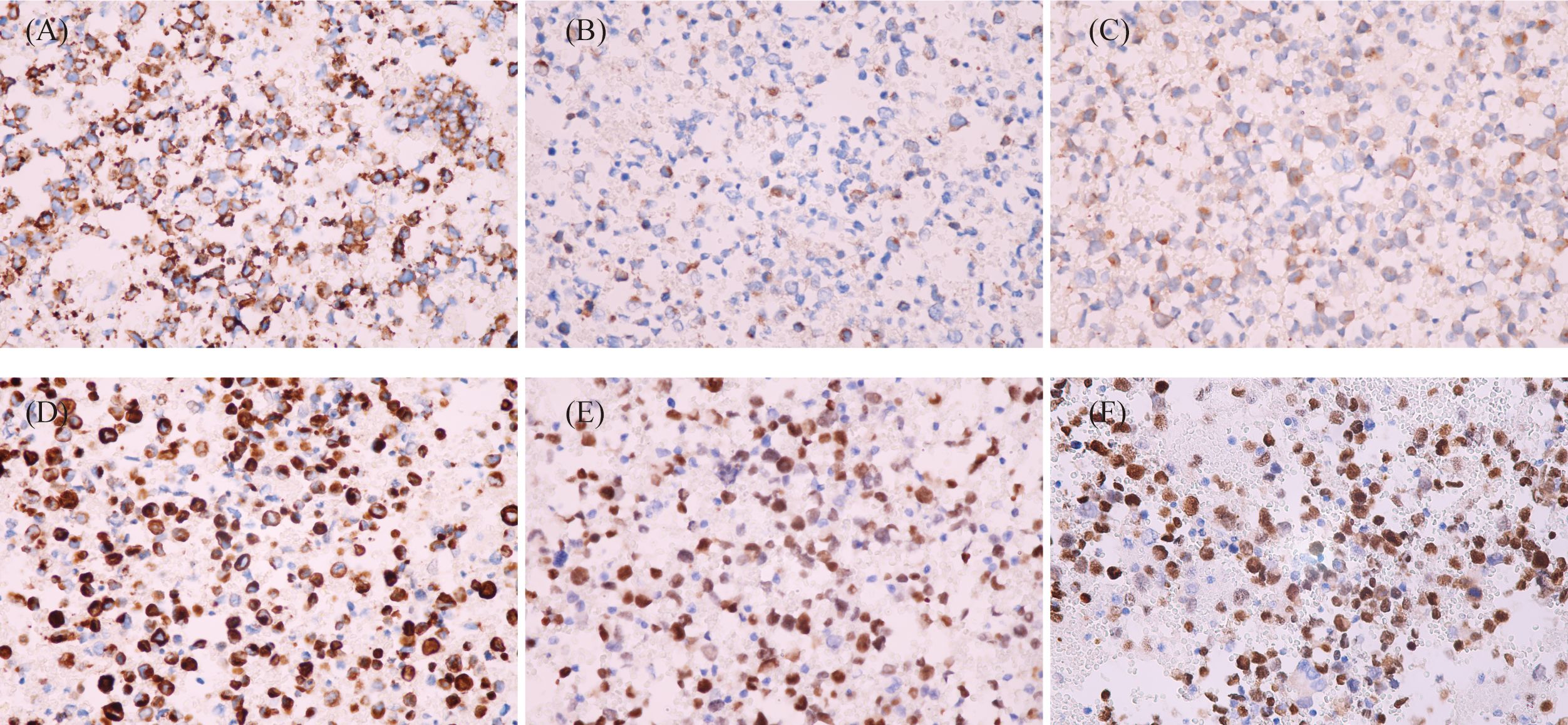
Figure 3 Immunocytochemistry of cell block sections (EnVision, ×400). (A) Tumor cells showed CD56-positive stains. (B) Tumor cells showed Syna-positive stains. (C) Tumor cells showed ALK-positive stains. (D) Tumor cells showed Des-positive stains. (E) Tumor cells showed Myogenin-positive stains. (F) Tumor cells showed MyoD1-positive stains.
However, the surgical treatment and core needle aspiration biopsy were not put into practice because of the wide extent of the pelvic lesion and the poor general condition of this patient. The patient chose to receive chemotherapy and symptomatic supportive treatment. After two courses of chemotherapy, the patient was discharged. Follow-up was conducted 6 months later, and we learned the patient turned worse and only received symptomatic supportive treatment such as pain relief and nutrition at home. After 3 months, the patient was lost to follow-up.
RMS is one of the most common sarcomas with lymph node metastasis, while other types of sarcomas usually spread via blood vessels (12, 13). It can be categorized into four subtypes: embryonic, alveolar, polymorphic, and sclerosing RMS. In terms of cytological morphology, most RMS share certain features, such as high cellularity with predominantly small, round, single blue cells with occasional loosely packed clusters (14, 15).
ERMS consists of stellate, small blue-round or short spindle-shaped mesenchymal cells that are morphologically diverse and show a mixed cellular pattern. Tumor cells in ARMS are larger and have more cytoplasm than those from the ERMS; binucleate or multinucleate cells are more commonly seen; and alveolar structures may be present. Tumor cells in PRMS are highly variable in size and morphology, exhibiting marked pleomorphism and larger rhabdomyoblastic cells. In SRMS, a dense sclerotic background is typically seen. However, a reliable subclassification of RMS cannot be made only by FNA smears due to the lack of histological structure. Moreover, the ARMS also needs to be differentiated from other small blue-round cell tumors, including lymphoma, Ewing/PNET, neuroblastoma, and desmoplastic small round tumors (5). The rhabdoid feature can provide a hint and help with the differential diagnosis, which is more obvious on the cell block sections. However, it is not easy to be observed on Pap staining and may lead to misdiagnosis. So, a cell block should be made, and an ICC test should be used for the differential diagnosis.
Immunohistochemical detection of skeletal muscle phenotype-specific markers, including Desmin, Myogenin, and MyoD1, can accurately lead to a diagnosis of RMS. However, the expression of Desmin, Myogenin, and MyoD1 differs across the subtypes. It has been shown that ERMS showed patchy staining of Myogenin, whereas ARMS showed diffuse staining, which may help differentiate ERMS and ARMS (16). As the two rare subtypes of RMS, PRMS only focally expresses Myogenin and MyoD1 (17), SRMS often diffusely expresses MyoD1, and focally expresses Myogenin, while both of them predominantly express Desmin (18). It is worth noting that some cases of ARMS can also express Syn, CD56, AE1/AE3, and ALK, which makes it necessary to distinguish ARMS from small cell carcinomas and neuroendocrine carcinomas (19, 20). The tumor cells in this case diffusely expressed Desmin, Myogenin, and MyoD1, which can lead to the diagnosis of ARMS. Furthermore, the positive stains of Syna, CD56, and ALK were also important clues to the diagnosis of ARMS.
The genetic characteristics can also help identify the subtypes of RMS (21). It has already been discovered that tumor cells of ARMS usually contain t(2;13) or t(1;13) translocations producing PAX3-FOXO1 or PAX7-FOXO1 fusion genes, which are absent in other subtypes of RMS. In this case, though the tests for these two fusion genes were not performed, the break-apart of the FOXO1 gene at locus 13q14 was detected by FISH, which further strengthens the possibility of an ARMS diagnosis.
FNAC is used to examine neoplastic and non-neoplastic lesions at different sites. Because of the tiny caliber of the puncture needle, fine needle aspiration minimizes the risks of blooding and tumor dissemination. The real-time guidance of the ultrasound allows the diseased tissue to be accessed more accurately. Many soft tissue tumors have distinctive clinical characteristics, such as specific age ranges and predilection body sites, which can be helpful in making a diagnosis. However, it still faces great challenges when only cytological materials are available and a definite diagnosis must be made (22). With the advancement of modern pathology, some cases that are difficult to diagnose morphologically can be determined in combination with immunophenotyping and genetic features. It is not uncommon in clinical practice to make a diagnosis of RMS by FNAC (23, 24), but only a few studies have discussed the cytologic diagnosis of metastatic ARMS in lymph nodes. Those three cases had been reported arising from the nasal cavity, paranasal sinus, or nasal vestibule with metastasis to cervical lymph nodes. However, none of these cases was proved as ARMS just by using cytological materials because histological biopsies were available (25–27).
In this case, the surgical excisional biopsy and core needle aspiration biopsy could not be performed due to the poor general condition of the patient. When a histological biopsy is unavailable, FNA is the only method to get the diseased tissue, and the cytological diagnosis is very essential in instructing the choice of subsequent treatment and prognostic judgment. In such a situation, the cytological diagnosis should be made with great caution. Communication between the cytopathologist, radiologist, and clinician is important to ensure an optimal interpretation and avoid diagnostic pitfalls. A correct diagnosis should be made based on the clinical history, imaging features, cytomorphology, immunophenotypes, and molecular detection. Even so, many soft tissue tumors still cannot be diagnosed by FNAC without histological confirmation. However, this case had typical cytological features, including small, blue-round tumor cells, rhabdomyoblasts, and binucleate or multinucleate abnormal cells. Immunophenotyping tests showed positivity of tumor cells for Desmin, Myogenin, MyoD1, Syna, CD56, and ALK. Moreover, the break-apart of the FOXO1 gene at locus 13q14 was detected by FISH. So, a definite diagnosis of ARMS can be made by FNAC.
In conclusion, though sarcomas generally develop bloodstream metastases, RMS is relatively prone to lymph node metastasis. If small blue-round cells are found when performing FNAC on suspicious lymph nodes of children or young adults, lymph node metastasis of RMS should be considered in addition to other malignant tumors in this age group. When histological biopsy is unavailable, FNAC can play an essential role in making the diagnosis. The clinical history, imaging features, cytomorphology, immunophenotyping, and molecular testing should be combined together to make a correct diagnosis, which can guide the following treatment and prognostic judgment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XH: Writing – original draft, Writing – review & editing. CH: Writing – original draft, Writing – review & editing. QL: Writing – original draft. BW: Writing – original draft. CY: Writing – original draft. XS: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU 3rd, et al. Soft tissue sarcoma. J Natl Compr Canc Netw. (2010) 8:630–74. doi: 10.6004/jnccn.2010.0049
2. Loeb DM, Thornton K, Shokek O. Pediatric soft tissue sarcomas. Surg Clin North Am. (2008) 88:615–27, vii. doi: 10.1016/j.suc.2008.03.008
3. Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. (2009) 27:3391–7. doi: 10.1200/JCO.2008.19.7483
4. Skapek SX, Ferrari A, Gupta AA, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. (2019) 5:1. doi: 10.1038/s41572-018-0051-2
5. Domanski HA. The small round cell sarcomas complexities and desmoplastic presentation. Acta Cytol. (2022) 66:279–94. doi: 10.1159/000524260
6. Xu H, Fan F, Gong Y, Jing X, Lin X, Wang H, et al. Diagnostic challenges in fine-needle aspiration cytology of mediastinal tumors and lesions. Arch Pathol Lab Med. (2022) 146:960–74. doi: 10.5858/arpa.2021-0108-RA
7. Goyal A. Role of fine needle aspiration cytology in the diagnosis of gynecologic tumors. Acta Cytol. (2023) 67:195–212. doi: 10.1159/000529364
8. Rakheja G, Handa U, Punia RS, Attri AK. Fine-needle aspiration cytology in soft tissue tumors-5-year institutional experience. Diagn Cytopathol. (2022) 50:463–70. doi: 10.1002/dc.25017
9. Sheppard SC, Borner U, Wartenberg M, Giger R, Nisa L. Diagnostic use of fine-needle aspiration cytology and core-needle biopsy in head and neck sarcomas. Head Neck. (2021) 43:1939–48. doi: 10.1002/hed.26670
10. Buzatto IPC, Dos Reis FJC, de Andrade JM, Rodrigues T, Borba JMC, Netto AH, et al. Axillary ultrasound and fine-needle aspiration cytology to predict clinically relevant nodal burden in breast cancer patients. World J Surg Oncol. (2021) 19:292. doi: 10.1186/s12957-021-02391-3
11. Choy KCC, Bundele MM, Fu EW, Li H, Gan JYJ, Rao NCL, et al. Risk stratification of parotid neoplasms based on intraoperative frozen section and preoperative fine needle aspiration cytology. Eur Arch Otorhinolaryngol. (2022) 279:2117–31. doi: 10.1007/s00405-021-07015-w
12. Gandhi J, Mehta S, Patel T, Gami A, Shah M, Jetly D. Metastasis of soft tissue sarcomas in lymph node: A cytomorphological study. Diagn Cytopathol. (2017) 45:784–8. doi: 10.1002/dc.23754
13. Lee RE, Valaitis J, Kalis O, Sophian A, Schultz E. Lymph node examination by fine needle aspiration in patients with known or suspected Malignancy. Acta Cytol. (1987) 31:563–72.
14. Atahan S, Aksu O, Ekinci C. Cytologic diagnosis and subtyping of rhabdomyosarcoma. Cytopathology. (1998) 9:389–97. doi: 10.1046/j.1365-2303.1998.00131.x
15. de Almeida M, Stastny JF, Wakely PE Jr., Frable WJ. Fine-needle aspiration biopsy of childhood rhabdomyosarcoma: reevaluation of the cytologic criteria for diagnosis. Diagn Cytopathol. (1994) 11:231–6. doi: 10.1002/dc.2840110308
16. Dias P, Chen B, Dilday B, Palmer H, Hosoi H, Singh S, et al. Strong immunostaining for myogenin in rhabdomyosarcoma is significantly associated with tumors of the alveolar subclass. Am J Pathol. (2000) 156:399–408. doi: 10.1016/S0002-9440(10)64743-8
17. Agaram NP. Evolving classification of rhabdomyosarcoma. Histopathology. (2022) 80:98–108. doi: 10.1111/his.14449
18. Yasui N, Yoshida A, Kawamoto H, Yonemori K, Hosono A, Kawai A. Clinicopathologic analysis of spindle cell/sclerosing rhabdomyosarcoma. Pediatr Blood Cancer. (2015) 62:1011–6. doi: 10.1002/pbc.25367
19. Rekhi B, Gupta C, Chinnaswamy G, Qureshi S, Vora T, Khanna N, et al. Clinicopathologic features of 300 rhabdomyosarcomas with emphasis upon differential expression of skeletal muscle specific markers in the various subtypes: A single institutional experience. Ann Diagn Pathol. (2018) 36:50–60. doi: 10.1016/j.anndiagpath.2018.07.002
20. Pillay K, Govender D, Chetty R. ALK protein expression in rhabdomyosarcomas. Histopathology. (2002) 41:461–7. doi: 10.1046/j.1365-2559.2002.01534.x
21. Anderson J, Gordon A, Pritchard-Jones K, Shipley J. Genes, chromosomes, and rhabdomyosarcoma. Genes Chromosomes Cancer. (1999) 26:275–85. doi: 10.1002/(SICI)1098-2264(199912)26:4<275::AID-GCC1>3.3.CO;2-V
22. Kilpatrick SE, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. (2001) 115:59–68. doi: 10.1309/YN14-K8U4-5FLJ-DGJE
23. Silas OA, Ige OO, Adoga AA, Nimkur LT, Ajetunmobi OI. Role of fine needle aspiration cytology (FNAC) as a diagnostic tool in paediatric head and neck lymphodenopathy. J Otol Rhinol. (2015) 4. doi: 10.4172/2324-8785.1000211
24. Mukundapai M, Agrawal M, Nargund A, Patil Okaly GV, Kavitha BL, Padma M, et al. Diagnosis of solid tumors in infants by fine-needle aspiration cytology: 5 years retrospective study from a tertiary care oncology center in South India. Diagn Cytopathol. (2021) 49:743–52. doi: 10.1002/dc.24735
25. Chen KT. Rhabdomyosarcoma in an adult presenting with nodal metastasis: a pitfall in fine-needle aspiration cytology of lymph nodes. Diagn Cytopathol. (2005) 32:303–6. doi: 10.1002/(ISSN)1097-0339
26. Zhao Z, Takano A, Lim AST, Lim TH, Selvarajan S. Metastatic alveolar rhabdomyosarcoma on the fine-needle aspiration cytology of cervical lymph node in an elderly patient, with FISH confirmation: A case report. Malays J Pathol. (2020) 42:115–9.
Keywords: fine needle aspiration cytology, alveolar rhabdomyosarcoma, lymph node metastasis, FNA (fine needle aspiration), ARMS
Citation: Hu X, Huang C, Li Q, Wu B, Yue C and Su X (2024) Case report: Lymph node metastasis of pelvic alveolar rhabdomyosarcoma diagnosed by fine needle aspiration cytology. Front. Oncol. 14:1340865. doi: 10.3389/fonc.2024.1340865
Received: 20 November 2023; Accepted: 29 April 2024;
Published: 17 May 2024.
Edited by:
Christos Kosmas, Metaxa Hospital, GreeceReviewed by:
Francesco Fabozzi, Bambino Gesù Pediatric Hospital (IRCCS), ItalyCopyright © 2024 Hu, Huang, Li, Wu, Yue and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueying Su, eHVleWluZy5zdUBpY2xvdWQuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.