
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 15 July 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1340430
This article is part of the Research TopicFuture Frontiers in the Management of Metastatic Colorectal CancerView all 8 articles
 Federico Pinto1
Federico Pinto1 Marco Di Pangrazio1
Marco Di Pangrazio1 Alessandro Martinino1
Alessandro Martinino1 Letizia Todeschini2
Letizia Todeschini2 Francesco Toti1
Francesco Toti1 Luca Cristin2
Luca Cristin2 Miriam Caimano3
Miriam Caimano3 Amelia Mattia3
Amelia Mattia3 Giuseppe Bianco3
Giuseppe Bianco3 Gabriele Spoletini3
Gabriele Spoletini3 Francesco Giovinazzo3,4,5,6*
Francesco Giovinazzo3,4,5,6*Introduction: This study comprehensively compared laparoscopic liver resection (LLR) to open liver resection (OLR) in treating colorectal cancer liver metastasis (CRLM).
Methods: A systematic review of relevant literature was conducted to assess a range of crucial surgical and oncological outcomes.
Results: Findings indicate that minimally invasive surgery (MIS) did not significantly prolong the duration of surgery compared to open liver resection and notably demonstrated lower blood transfusion rates and reduced intraoperative blood loss. While some studies favored MIS for its lower complication rates, others did not establish a statistically significant difference. One study identified a lower post-operative mortality rate in the MIS group. Furthermore, MIS consistently correlated with shorter hospital stays, indicative of expedited post-operative recovery. Concerning oncological outcomes, while certain meta-analyses reported a lower rate of cancer recurrence in the MIS group, others found no significant disparity. Overall survival and disease-free survival remained comparable between the MIS and open liver resection groups.
Conclusion: The analysis emphasizes the potential advantages of LLR in terms of surgical outcomes and aligns with existing literature findings in this field.
Systematic review registration: [website], identifier [registration number].
Colorectal cancer’s tendency to spread to the liver poses a substantial treatment challenge (1). Traditional open surgical approaches, while effective, entail considerable morbidity and protracted recovery periods. The foundation of minimally invasive resection lies in applying laparoscopic and robotic-assisted techniques. Laparoscopy, introduced in the 1980s, revolutionized surgery by enabling internal organ visualization and manipulation through small incisions. This technique mitigates tissue trauma, reducing pain and quicker postoperative recovery (2). Robotic-assisted surgery further enhances precision and dexterity through robotic arms operated by surgeons (3). Recent research underscores the efficacy and safety of minimally invasive liver resections for CRLM, demonstrating comparable oncological outcomes to traditional open surgeries, reduced blood loss, diminished post-operative complication rates, and shorter hospital stays (4, 5). The less invasive nature of these procedures augments patient satisfaction and cosmesis, thus improving overall quality of life during the recovery phase.
Nonetheless, refining patient selection criteria and optimizing techniques for complex cases remain ongoing challenges. Advancements in imaging technologies, intraoperative navigation systems, and instrumentation continually shape the minimally invasive liver surgery landscape. Ongoing research endeavors are dedicated to unraveling long-term oncological outcomes and refining the technical facets of these procedures. This umbrella review explores the safety and efficacy of laparoscopic liver resection (LLR) in contrast to open liver resection (OLR) for the treatment of colorectal liver metastases (CRLM).
The transformative period in hepatic surgery during the early 1990s witnessed the emergence of laparoscopic liver resection (LLR). Pioneering efforts by Reich et al. (6), Katkhouda et al. (7), and Gagner et al. (8) in 1991 and 1992 inaugurated this revolutionary approach, heralding a new era in surgical techniques. Building upon this foundation, subsequent years saw substantial advancements, including the groundbreaking left lateral sectionectomy (LLS) in 1996 and the progressive evolution toward hepatectomy by 1998 (9, 10). These sequential developments underscored the swift progression of LLR methodologies, adapting to the ever-evolving landscape of surgical innovation.
The expansion of LLR procedures mirrored the historical evolution observed in open liver resections (OLR). In 2009 Nguyen et al. published the first international multicenter study supporting the idea that laparoscopic liver resection for colorectal cancer metastasis was safe, feasible, and comparable in terms of oncologic outcomes to open liver resection. The significance of LLR was underscored by two pivotal international consensus conferences held in 2008 in Louisville and 2014 in Morioka (11, 12). The first one focused on the viability of LLR, and the second conference centered around contrasting laparoscopic approaches with the then-standard open resection procedure, highlighting the evident relevance of a laparoscopic approach in the contemporary landscape of liver surgery. A third international conference took place in Seoul, Korea, in 2016. During this event, a panel of experts concentrated their efforts on formulating a statement concerning laparoscopic living donor hepatectomy (13). In 2017 the first European guidelines meeting took place in Southampton, where the primary objective was to present and validate clinical practice guidelines concerning laparoscopic liver surgery (14).
These consensus conferences provided a platform for leading experts to convene, discuss, and deliberate upon the state of LLR, sharing insights and perspectives that shaped its trajectory. Moreover, these conferences fostered a dynamic space for exchanging knowledge and best practices, facilitating the dissemination of advancements and fostering a global dialogue on LLR’s progress. One of the critical considerations that emerged on this transformative journey was the management of LLR-specific complications. This concern was effectively addressed through meticulous procedural implementation and the systematic evaluation of outcomes. As careful application and patient assessment became routine, these efforts alleviated anxieties and validated the advantages of LLR over its conventional counterpart, OLR.
In the umbrella review, a comprehensive search and analysis of various systematic reviews and meta-analyses concerning minimally invasive surgery (MIS) in liver resections for colorectal cancer (CRC) was conducted, as previously described (15). This study adhered to an already established research protocol (16). An AMSTAR 2 checklist is provided as Supplementary Material to assist in the evaluation and assessment of the systematic review presented herein (17).
The primary objective of this umbrella review is the assessment of postoperative mortality and overall/disease-free survival in the two analyzed groups. The secondary objectives encompass assessing parameters such as blood loss, blood transfusion, duration of surgery, complication rate, hospitalization time, surgical margins R0, and recurrence.
Utilizing the PICO criteria in framing a research question, the study aimed to investigate the following: “In patients undergoing surgical treatment for CRC liver metastasis (P), does laparoscopic surgery (I) compare to traditional open surgery (C), result in differences in postoperative mortality, overall/disease-free survival, blood loss, blood transfusion requirements, duration of surgery, complication rate, hospitalization time, surgical margins, and recurrence (O)?” (18).
The systematic review adhered to the guidelines outlined in the PRISMA statement for the conduct and reporting of data (19). The research encompassed an exhaustive computerized exploration of the PubMed and Cochrane Library databases. Employing an advanced search strategy, we employed terms such as “colorectal neoplasm” OR “colorectal” AND “liver metastases” AND “liver neoplasm” AND “therapeutics” OR “treatment” AND “meta-analysis” OR “systematic review”. Results were admitted from the time of inception up to and including June 7, 2023. Moreover, manual screenings of reference lists from pertinent articles were conducted, aiming to identify further relevant studies.
Articles were eligible for inclusion if they were systematic reviews or meta-analyses focusing on patients with CRC and liver metastasis. The selected articles were required to analyze laparoscopic liver resections versus open liver resections performed in individuals who were 18 years of age or older. We excluded all non-English language studies.
At least two reviewers independently gathered all data, resolving discrepancies through collaborative discussion and consensus. The diverse outcomes within different meta-analyses were independently extracted to ensure a meticulous and nuanced collection of data. Data collection encompassed the following information: authorship details, year of publication, the number of articles scrutinized, and the number of patients enrolled in each study. Additionally, the review calculated pooled outcome measures, presented values with 95% confidence intervals (95% CI), assessed statistical heterogeneity, and evaluated potential publication bias. Furthermore, a quality assessment of the included meta-analyses was conducted using the specific quality assessment tool developed by the Centre of Evidence-Based Medicine at the University of Oxford (Table 1).
An extensive search retrieved a total of 2203 records. During the initial screening phase, 216 articles were excluded due to duplication, and 1979 were excluded for not meeting the inclusion criteria. Consequently, only eleven articles remained eligible for a thorough full-text review (Figure 1).
Ultimately, our study comprised eleven included articles, all of which were meta-analyses (Table 2) (20–30). In Table 3, we present the analyzed outcomes comparing minimally invasive hepatectomy with open hepatectomy for CRLM.

Table 3 Results for different outcomes in patients undergoing laparoscopic versus open liver resection for CRLM.
The analysis of blood loss consistently favored the minimally invasive group across all meta-analyses from non-randomized studies (20–22, 24, 26–29). In the assessment of this parameter within randomized controlled trials (RCTs), Ozair et al. (29) observed a lower estimated blood loss (EBL) in the MIS group. Although not statistically significant, this information aligns with the findings of observational studies, consistently reporting significantly reduced EBL with minimally invasive hepatectomy. This unanimity in findings underscores that laparoscopic/MIS techniques significantly reduce intraoperative blood loss, which can be crucial in minimizing the risk of complications and ensuring patient safety.
When assessing the need for blood transfusion, eight meta-analyses from non-randomized studies (20–22, 24, 26–29) reported a lower rate in the MIS group compared to the open liver resection group. The finding suggests that patients undergoing MIS are less likely to require blood transfusions, signifying a potential advantage in blood preservation. When evaluating this parameter within randomized controlled trials (RCTs), a lower, although not statistically significant, need for transfusion was reported with minimally invasive hepatectomy (29).
The analysis of duration of surgery across seven meta-analyses revealed no significant difference between the MIS group and the open liver resection group (20–22, 24, 26–28), suggesting that, in most cases, MIS does not significantly extend the duration of the procedure.
Seven meta-analyses of non-RCTs (20–22, 24, 27–29) indicated a lower rate of perioperative complications in the MIS group, emphasizing the potential benefit of MIS in reducing post-operative complications. However, one included study (26) found no significant difference between the two groups, suggesting that the effectiveness of MIS in reducing complications may depend on specific patient characteristics or procedural factors, such as patient fitness, the presence of comorbidities, or the surgeon’s experience and used technique. Evidence from RCTs revealed a lower risk of complications with minimally invasive liver resections (29).
The analysis of hospitalization time revealed that seven meta-analyses from non-RCTs (20–22, 26–29) detected a shorter hospital stay for patients in the MIS group. Correspondingly, data from RCTs align with these findings (29), supporting the notion that MIS promotes faster post-operative recovery and reduces hospitalization duration. However, one meta-analysis (24) found no significant difference between the two groups, indicating that other factors may influence the length of hospitalization.
Among the included meta-analyses, five considered post-operative mortality as an operative outcome. All five of the cited studies (20–22, 24, 27) reported no significant difference in mortality rates between the MIS and open liver resection groups.
Among the meta-analyses that evaluated this oncologic outcome, three reported higher rates of surgical margins R0 in the MIS group (21, 22, 28). However, one study (29) reported nearly identical rates of R0 resection between the two groups. Another study (20) indicated a slightly higher rate of R0 margins in the open liver resection (OLR) group, highlighting potential variability in outcomes. One meta-analysis (24) found a lower incidence of R1 resection in the LLR group, however, Luo at al (27). did not find any significant difference in terms of increased R1 positive margins between the two groups. Data from RCTs (29) did not detect any significant difference between the MIS and OLR groups.
Regarding cancer recurrence, three meta-analyses were included in the analysis. While two of these meta-analyses (20, 21) reported a lower recurrence rate in the MIS group, the statistical significance was not reached in the latter. These findings imply a potential advantage of MIS in controlling cancer recurrence. However, a third meta-analysis (22) did not find a statistically significant difference between the two groups, indicating the need for additional research to comprehensively assess the impact of MIS on recurrence rates.
Data from eight meta-analyses (20–22, 24, 26–29), presented no significant difference was observed between the MIS and open liver resection groups regarding overall survival and disease-free survival. Notably, Syn et al. (30), in their meta-analysis of Individual Patient Data From Randomized Trials and Propensity-score Matched Studies, reported a consistent survival advantage favoring laparoscopic over open hepatectomy for colorectal liver metastases (CLM).
- (In Table 4, we assessed outcomes pertaining to minimally invasive versus open hepatectomy for CRLM, specifically when performed simultaneously with the resection of the primary tumor).
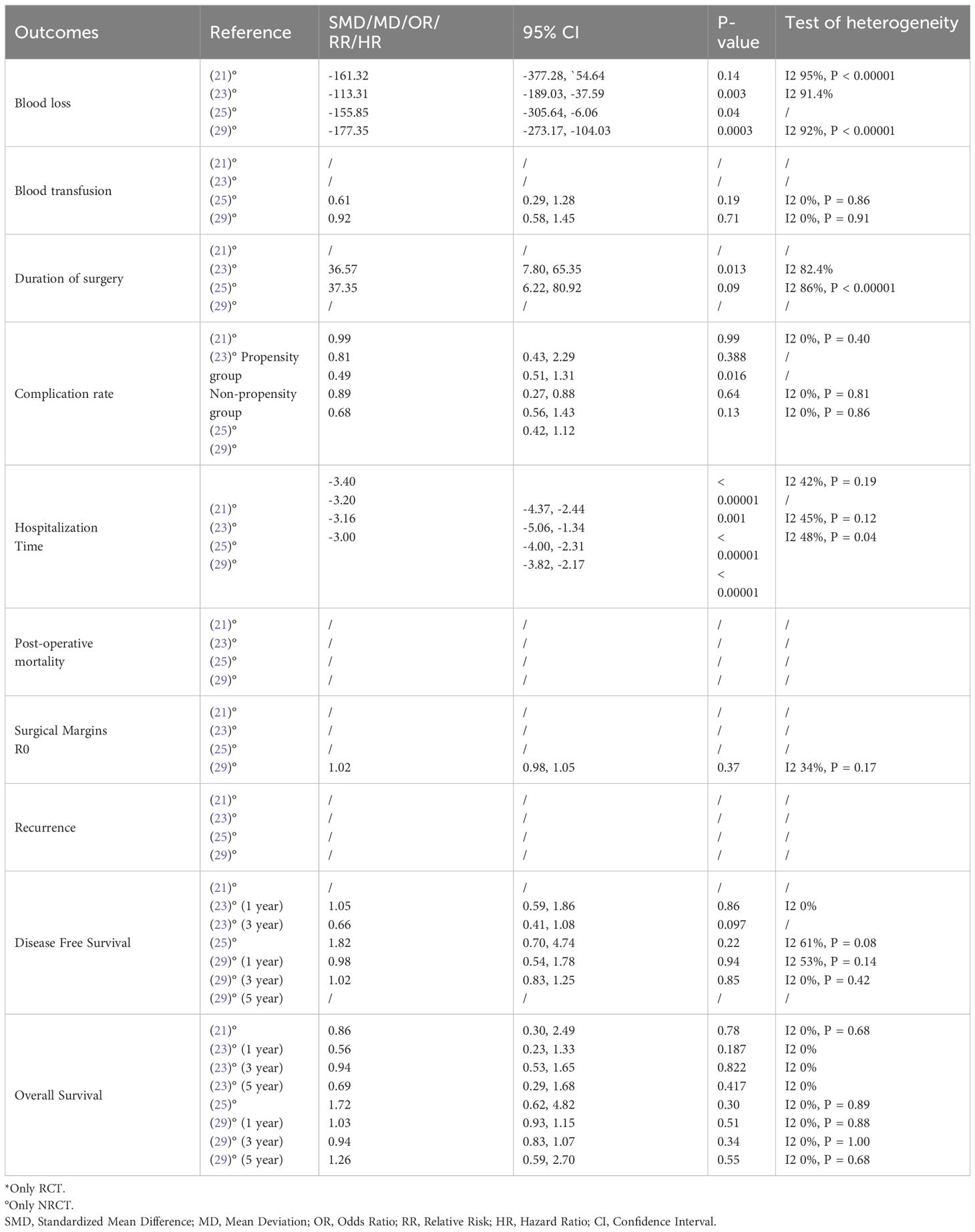
Table 4 Results for different outcomes in patients undergoing laparoscopic versus open simultaneous resection of CRLM and primary CRC.
- (In Table 5, we present a citation matrix that details the primary studies and meta-analyses).
Laparoscopy for liver resections has come long since it was first introduced in the 1990s (31). Nowadays, it is considered a practical option for various liver surgeries, even for cases involving colorectal cancer that has spread to the liver (CRLM). This approach has gained support from studies like case series, meta-analyses, and comparisons with traditional open surgery (32).
There is a solid consensus in the medical community that laparoscopic hepatic resection is safe, feasible, and offers advantages compared to open procedures. However, using laparoscopic techniques for liver surgery is quite complicated. Surgeons need extensive training to master the skills required. The liver’s complex anatomy demands a deep understanding of its structure and the use of tools like intraoperative ultrasound to enable enhanced identification and characterization of tumors, directing intraoperative procedures (33, 34). Moreover, applying laparoscopic techniques becomes even more intricate in oncologic surgery. Adherence to radical resection criteria is paramount, necessitating a meticulous and nuanced approach. The surgeon must balance the intricacies of minimally invasive surgery (MIS) with the imperative to achieve the necessary oncological outcomes while preserving the patient’s overall well-being.
The results of this review’s comprehensive analysis shed light on the comparative outcomes of MIS, particularly laparoscopic liver resection (LLR) versus open liver resection (OLR) in the context of colorectal cancer liver metastasis (CRLM). Findings provide valuable insights into the advantages and limitations of these surgical approaches, contributing to the ongoing dialogue surrounding the optimal treatment strategy for this challenging condition.
A pivotal investigation in this domain is the OSLO-COMET Randomized Controlled Trial (RCT) (35), which, notably, was not incorporated into the included meta-analysis. Nevertheless, it is worth highlighting that our findings exhibit striking congruence with the OSLO-COMET study, particularly in the context of reduced postoperative complications observed in the LLR group when compared to OLR. In addition to the OSLO-COMET trial, another RCT, conducted by the same research group 3-years later, reported comparable survival outcomes between the LLR and OLR groups (36). Importantly, this review yields findings that are consonant with this data, further reinforcing the assertion that there may be no substantial survival advantage associated with either surgical approach.
Another noteworthy randomized controlled trial to discuss is the LapOpHuva, which reported no significant differences in short-term outcomes, including surgical duration, blood loss, transfusion requirements, or mortality. Moreover, it demonstrated similar oncological outcomes to OLR (37). These results are consistent with the findings of this umbrella review, further corroborating the notion that LLR can yield comparable outcomes to OLR across various dimensions of surgical and oncological evaluation.
A key observation from the analysis is that MIS does not significantly prolong the duration of surgery in most cases compared to OLR. The result dispels concerns about excessively prolonged surgeries associated with laparoscopic liver resections and highlights that careful patient selection and surgical planning are pivotal factors in optimizing operative durations. Furthermore, LLR is associated with a lower rate of blood transfusion and significantly reduced intraoperative blood loss. These outcomes underscore the potential advantages of MIS in terms of minimizing the need for blood products and preserving hemostasis. The benefits of reduced blood loss extend beyond transfusion-related concerns, as they may also contribute to decreased post-operative complications and expedited recovery. While the majority of meta-analyses, incorporating data from both non-randomized controlled trials (non-RCTs) and RCTs, indicated a favorable trend toward lower complication rates with minimally invasive surgery (MIS), it is noteworthy that a singular study did not detect a significant difference between MIS and open liver resection. These findings underscore the intricacies involved in evaluating complication rates, emphasizing the impact of different factors such as patient comorbidities and the specific surgical techniques employed.
Nonetheless, the potential reduction in perioperative complications associated with MIS remains a compelling aspect, potentially improving the overall safety profile of these procedures. The analysis consistently showed that MIS is associated with a shorter hospitalization time. The finding aligns with the concept of minimally invasive surgery promoting faster post-operative recovery and shorter lengths of stay, which can lead to substantial cost savings and improved patient satisfaction.
The analysis of surgical margins (R0) in the context of liver resections for colorectal cancer metastasis presents a complex and multifaceted picture. While some meta-analyses suggest a potential advantage in achieving R0 resections with LLR, variations in outcomes, as highlighted by individual studies and RCTs, underscore the need for cautious interpretation. The choice between LLR and open techniques should be tailored to the specific characteristics of the tumor and the nuances of the anatomical context, recognizing the intricacies involved in achieving optimal oncologic outcomes.
Concerning cancer recurrence, although two meta-analyses reported a reduced recurrence rate in the MIS group, it is crucial to note that statistical significance was not observed in one of these studies. This outcome underscores the importance of ongoing research to delineate the impact of MIS on recurrence rates and to elucidate the patient subgroups that may benefit most from this approach.
The disparity in findings regarding survival outcomes is likely influenced by variations in study populations, methodologies, and the inclusion of different types of studies. This highlights the intricacies involved in comparing outcomes in surgical interventions and underscores the importance of considering diverse factors when interpreting results from meta-analyses. The meta-analysis conducted by Syn et al. (30), which integrates individual patient data and propensity-score matched studies, offers a more detailed and patient-specific perspective. This approach has the potential to capture nuanced differences that broader analyses may overlook. The identification of potentially improved survival among patients undergoing laparoscopic liver resections introduces a new perspective that warrants further investigation.
Presently, MIS is embarking on a new era with the integration of robotic technology into clinical practice. Although it initially made strides in urologic procedures, robotic applications have now branched out into various surgical domains. Among these, it has notably risen to prominence and seen extensive use in the field of general surgery. The hallmark features of robotic surgery include high-definition 3D magnified vision, endo-wristed movements, precision, and surgical finesse. These characteristics have effectively surmounted some of the technical constraints associated with laparoscopic surgery. As a result, they have garnered significant recognition, firmly establishing robot-assisted liver surgery as a universally accepted approach for the management of a wide range of hepatic conditions. In 2010, Giulianotti et al. published a pioneering series comprising a total of 70 cases of robotic hepatectomies. This initial experience provided compelling evidence of the safety of the robotic approach in liver resections, as demonstrated by low rates of conversion, minimal bleeding, and postoperative complications (38). In 2018, a significant milestone was achieved when the Asian group led by Rong Liu recorded the first consensus regarding robotic hepatectomies (39). Their findings yielded strong recommendations for the safety and efficacy of robotic procedures when compared to both open (2C) and laparoscopic (2D) approaches. Furthermore, the comparison with open hepatectomies (OD) for malignancies garnered a 2D recommendation. Notably, even the indication for living-donor robotic hepatectomy received a 2D recommendation, underscoring the growing acceptance and endorsement of this advanced surgical modality.
Today the majority of the studies found in the literature consider robotic liver surgery a safe approach and effective approach to liver malignancies as for the laparoscopic approach (40–43). There is wide acceptance among surgeons of the use of robotic surgery in complex cases like in cirrhotic patients or delicate procedures requiring, for example, micro-suturing, vascular resections (44), or bilio-enteric anastomosis (43, 45). However, it’s important to note that standardization of many of the techniques within this approach has not yet been fully realized and no research has provided conclusive guidelines for when to recommend or discourage robotic surgery due to the absence of randomized control trials (46).
The study may face limitations regarding the availability and quality of the primary research studies included in the umbrella review. Heterogeneity among the included studies could affect the overall conclusions. However, rigorous inclusion criteria were applied to ensure the reliability of the selected studies.
In conclusion, this analysis indicates that laparoscopic liver resections exhibit notable advantages over open liver resections. The observed reductions in blood loss, decreased transfusion requirements, and shorter hospitalization times suggest that adopting laparoscopic approaches could contribute to more efficient and patient-friendly postoperative experiences. Moreover, the lower complication rates associated with laparoscopy indicate a potential enhancement in the overall safety profile of these procedures. These practical implications are particularly relevant in the context of personalized treatment strategies, where consideration of patient-specific factors and tumor characteristics plays a crucial role in decision-making. To improve our understanding of laparoscopic liver resections’ oncological efficacy and long-term impact, there is a compelling need for additional high-quality randomized controlled trials (RCTs) and multicentric observational studies. These studies will not only contribute crucial insights into the intervention’s effectiveness but also address the complexities inherent in comparing outcomes across diverse patient populations.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
FP: Methodology, Writing – original draft. MDP: Writing – original draft. AMar: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. LT: Writing – original draft. FT: Writing – original draft. LC: Writing – original draft. MC: Methodology, Writing – original draft. AMat: Writing – original draft. GB: Writing – review & editing. GS: Writing – review & editing. SA: Supervision, Writing – review & editing. FG: Conceptualization, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We gratefully thank Prof. Salvatore Agnes for his supervisory role in the study and express our sincere appreciation for his guidance and support throughout the research process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1340430/full#supplementary-material
1. Taillieu E, De Meyere C, Nuytens F, Verslype C, D’Hondt M. Laparoscopic liver resection for colorectal liver metastases — short- and long-term outcomes: A systematic review. World J Gastrointest Oncol. (2021) 13:732–57. doi: 10.4251/wjgo.v13.i7.732
2. Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. (2008) 22:38–44. doi: 10.1007/s00464-007-9527-y
3. Köckerling F. Robotic vs. Standard laparoscopic technique – what is better? Front Surg. (2014) 1:15. doi: 10.3389/fsurg.2014.00015
4. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection—2,804 patients. Ann Surg. (2009) 250:831. doi: 10.1097/SLA.0b013e3181b0c4df
5. Lo WM, Tohme ST, Geller DA. Recent advances in minimally invasive liver resection for colorectal cancer liver metastases—A review. Cancers. (2022) 15:142. doi: 10.3390/cancers15010142
6. Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. (1991) 78:956–8.
7. Katkhouda N, Fabiani P, Benizri E, Mouiel J. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. (1992) 79:560–1. doi: 10.1002/bjs.1800790628
8. Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. (1992) 6:97–8.
9. Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy—technical aspects. Surg Endosc. (1996) 10:758–61. doi: 10.1007/BF00193052
10. Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: Technique and results of a clinical series. Surgery. (1996) 120:468–75. doi: 10.1016/S0039-6060(96)80065-1
11. Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the louisville statement, 2008. Ann Surg. (2009) 250:825. doi: 10.1097/SLA.0b013e3181b3b2d8
12. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. (2015) 261:619–29.
13. Cho JY, Han HS, Kaneko H, Wakabayashi G, Okajima H, Uemoto S, et al. Survey results of the expert meeting on laparoscopic living donor hepatectomy and literature review. Dig Surg. (2017) 35:289–93. doi: 10.1159/000479243
14. Abu Hilal M, Al E, Clavien PA. The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. (2018) 268:11–8. doi: 10.1097SLA.0000000000002524
15. Martinino A, Pereira JPS, Spoletini G, Treglia G, Agnes S, Giovinazzo F. The use of the T-tube in biliary tract reconstruction during orthotopic liver transplantation: An umbrella review. Transplant Rev Orlando Fla. (2022) 36:100711. doi: 10.1016/j.trre.2022.100711
16. Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. BMJ Ment Health. (2018) 21:95–100. doi: 10.1136/ebmental-2018-300014
17. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
18. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc JMLA. (2018) 106:420–31. doi: 10.5195/jmla.2018.345
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
20. qiang TZ, fang S, yong LZ, chao W, xin WL, He J. Meta-analysis of laparoscopic versus open liver resection for colorectal liver metastases. Oncotarget. (2016) 7:84544–55. doi: 10.18632/oncotarget.v7i51
21. Wei M, He Y, Wang J, Chen N, Zhou Z, Wang Z. Laparoscopic versus Open Hepatectomy with or without Synchronous Colectomy for Colorectal Liver Metastasis: A Meta-Analysis. PloS One. (2014) 9:e87461. doi: 10.1371/journal.pone.0087461
22. Zhou Y, Xiao Y, Wu L, Li B, Li H. Laparoscopic liver resection as a safe and efficacious alternative to open resection for colorectal liver metastasis: a meta-analysis. BMC Surg. (2013) 13:44. doi: 10.1186/1471-2482-13-44
23. Pan L, Tong C, Fu S, Fang J, Gu Q, Wang S, et al. Laparoscopic procedure is associated with lower morbidity for simultaneous resection of colorectal cancer and liver metastases: an updated meta-analysis. World J Surg Oncol. (2020) 18:251. doi: 10.1186/s12957-020-02018-z
24. Luo LX, Yu ZY, Bai YN. Laparoscopic hepatectomy for liver metastases from colorectal cancer: A meta-analysis. J Laparoendosc Adv Surg Tech. (2014) 24:213–22. doi: 10.1089/lap.2013.0399
25. Guo Y, Gao Y, Chen G, Li C, Dong G. Minimally invasive versus open simultaneous resections of colorectal cancer and synchronous liver metastases: A meta-analysis. Am Surg. (2018) 84:192–200. doi: 10.1177/000313481808400224
26. Ye SP, Qiu H, Liao SJ, Ai JH, Shi J. Mini-invasive vs open resection of colorectal cancer and liver metastases: A meta-analysis. World J Gastroenterol. (2019) 25:2819–32. doi: 10.3748/wjg.v25.i22.2819
27. Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: A metaanalysis of 610 patients. Surgery. (2015) 157:211–22. doi: 10.1016/j.surg.2014.08.036
28. Kelly ME, Fahy M, Bolger JC, Boland PA, Neary C, McEntee GP, et al. Open versus laparoscopic liver resection of colorectal metastases: a meta-analysis of matched patient populations. Ir J Med Sci. (2022) 191:1531–8. doi: 10.1007/s11845-021-02780-3
29. Ozair A, Collings A, Adams AM, Dirks R, Kushner BS, Sucandy I, et al. Minimally invasive versus open hepatectomy for the resection of colorectal liver metastases: a systematic review and meta-analysis. Surg Endosc. (2022) 36:7915–37. doi: 10.1007/s00464-022-09612-0
30. Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, et al. Survival advantage of laparoscopic versus open resection for colorectal liver metastases: A meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg. (2020) 272:253–65. doi: 10.1097/SLA.0000000000003672
31. Morise Z, Wakabayashi G. First quarter century of laparoscopic liver resection. World J Gastroenterol. (2017) 23:3581–8. doi: 10.3748/wjg.v23.i20.3581
32. Coelho FF, Kruger JAP, Fonseca GM, Araújo RLC, Jeismann VB, Perini MV, et al. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg. (2016) 8:5–26. doi: 10.4240/wjgs.v8.i1.5
33. Nanashima A, Tobinaga S, Abo T, Kunizaki M, Takeshita H, Hidaka S, et al. Usefulness of sonazoid–ultrasonography during hepatectomy in patients with liver tumors: A preliminary study. J Surg Oncol. (2011) 103:152–7. doi: 10.1002/jso.21782
34. Zacherl J, Scheuba C, Imhof M, Zacherl M, Längle F, Pokieser P, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. (2002) 26:550–4. doi: 10.1007/s00268-001-0266-2
35. Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. (2018) 267:199. doi: 10.1097/SLA.0000000000002353
36. Aghayan DL, Kazaryan AM, Dagenborg VJ, Røsok BI, Fagerland MW, Waaler Bjørnelv GM, et al. Long-term oncologic outcomes after laparoscopic versus open resection for colorectal liver metastases. Ann Intern Med. (2021) 174:175–82. doi: 10.7326/M20-4011
37. Robles-Campos R, Lopez-Lopez V, Brusadin R, Lopez-Conesa A, Gil-Vazquez PJ, Navarro-Barrios Á, et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc. (2019) 33:3926–36. doi: 10.1007/s00464-019-06679-0
38. Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, et al. Robotic liver surgery: Results for 70 resections. Surgery. (2011) 149:29–39. doi: 10.1016/j.surg.2010.04.002
39. Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. (2019) 25:1432–44. doi: 10.3748/wjg.v25.i12.1432
40. Fahrner R, Rauchfuß F, Bauschke A, Kissler H, Settmacher U, Zanow J. Robotic hepatic surgery in Malignancy: review of the current literature. J Robot Surg. (2019) 13:533–8. doi: 10.1007/s11701-019-00939-w
41. Tsilimigras DI, Moris D, Vagios S, Merath K, Pawlik TM. Safety and oncologic outcomes of robotic liver resections: A systematic review. J Surg Oncol. (2018) 117:1517–30. doi: 10.1002/jso.25018
42. Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc. (2016) 30:862–75. doi: 10.1007/s00464-015-4306-7
43. Ocuin LM, Tsung A. Robotic liver resection for Malignancy: Current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol. (2015) 112:295–301. doi: 10.1002/jso.23901
44. Magistri P, Pang NQ, Guidetti C, Caracciolo D, Odorizzi R, Catellani B, et al. Robotic approach for perihilar cholangiocarcinoma: from Bismuth 1 to vascular resection. Eur J Surg Oncol. (2023) 49:107002. doi: 10.1016/j.ejso.2023.107002
45. Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D. Review of emerging surgical robotic technology. Surg Endosc. (2018) 32:1636–55. doi: 10.1007/s00464-018-6079-2
Keywords: minimally invasive surgery, laparoscopic liver resection, open liver resection, outcomes, colorectal liver metastasis
Citation: Pinto F, Pangrazio MD, Martinino A, Todeschini L, Toti F, Cristin L, Caimano M, Mattia A, Bianco G, Spoletini G and Giovinazzo F (2024) Laparoscopic versus open liver resection for colorectal liver metastasis: an umbrella review. Front. Oncol. 14:1340430. doi: 10.3389/fonc.2024.1340430
Received: 18 November 2023; Accepted: 19 January 2024;
Published: 15 July 2024.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Krzysztof Zieniewicz, Medical University of Warsaw, PolandCopyright © 2024 Pinto, Pangrazio, Martinino, Todeschini, Toti, Cristin, Caimano, Mattia, Bianco, Spoletini and Giovinazzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Giovinazzo, Z2lvdmluYXp6b19mcmFuY2VzY29AbGl2ZS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.