- 1Clinical and Experimental Medicine PhD Program, Department of Biomedical, Metabolic, and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 2Department of Medical and Surgical Sciences for Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
- 3Institute of Pathologic Anatomy, Department of Medicine, University of Udine, Udine, Italy
- 4Department of Surgery, Medicine, Dentistry and Morphological Sciences with Interest in Transplant, Oncology and Regenerative Medicine, University of Modena and Reggio Emilia, Modena, Italy
- 5Dermatology Unit, Department of Surgery, Medicine, Dentistry and Morphological Sciences with Interest in Transplant, Oncology and Regenerative Medicine, University of Modena and Reggio Emilia, Modena, Italy
- 6Unit of Pathology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Casa Sollievo della Sofferenza, San Giovanni Rotondo, Foggia, Italy
- 7Section of Molecular Pathology, Department of Emergency and Organ Transplantation (DETO), University of Bari “Aldo Moro”, Bari, Italy
- 8Pathology Unit, University Hospital of Modena, Modena, Italy
- 9Dermatology Clinic, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Roma, Italy
Purpose: Even today, melanoma is a highly aggressive neoplasm with a high mortality rate. The nodular type is very aggressive and has cerebroid nests of melanocytes (CNMs) at the growth edge, morphologically similar to the poorly differentiated neoplastic epithelial cell clusters described in colorectal, breast, and endometrioid endometrial cancers.
Patients and methods: We selected 25 nodular melanomas (NMs) with known molecular profiles, of which the entire paraffin-embedded lesion was available. We counted CNMs under a microscopic at a magnification of 20x (i.e., a microscopic field with a major axis of 1 mm). Based on the number of CNMs in the area, melanomas were classified into three groups: G1 (CNMs ranging from 0 to 4), G2 (CNMs ranging from 5 to 9), and G3 (CNMs ≥ 10). The presence of CNMs and their counts were compared with molecular and histopathological data.
Results: Seventeen (NMs) were grouped as G1 (68%), 5 as G2 (20%), and 3 as G3 (12%) based on CNMs count. The presence of CNMs correlated with epithelioid cell morphology (p < 0.05), Clark IV and V levels (p < 0.05), vascular invasion (p < 0.05), and biological mutants (p < 0.05). Melanomas with ≥ 10 CNMs more frequently show ulceration (p < 0.02) and the BRAF V600E mutation (p < 0.02).
Conclusion: CNMs count has a predictive role regardless of tumor size; their association with the BRAF V600E mutation suggests their predictive significance in response to biologics. However, further investigations are needed to strengthen this hypothesis.
Introduction
Malignant melanomas are the most aggressive skin cancers, with increasing morbidity in recent years. They include heterogeneous neoplasms characterized by different dermatoscopic, histological, and molecular profiles (1–3). A particular type of melanoma is the nodular variant, characterized by deep growth, frequent metastasis, and a high rate of genetic aberrations (4–6). Histologically, nodular melanomas (NMs) are composed of large, atypical spindle or epithelioid melanocytes, pleomorphic, sometimes organized in aggregates morphologically attributable to cerebroid nests of melanocytes (CNMs). They are mainly described in the peripheral portion of the tumors, where they assume an infiltrative profile or within the tumor mass. CNMs are round or oval in shape and are composed of aggregates of at least 5 undifferentiated atypical melanocytes, primarily devoid of pigmentation (7, 8). However, no attention has been paid to the count of nests detected in the neoplasm. No specific correlation has been investigated between their maximum concentration and clinical-pathological features.
In nodular melanoma, CNMs could be compared to poorly differentiated clusters (PDCs) of tumor cells. PDCs have been identified at the growing edge of the tumor in some types of epithelial cancers, including colorectal, gastric, breast, and endometrioid endometrial cancers (9–12). By definition, they are composed of ≥ 5 undifferentiated cells and are counted in the microscopic field under a × 20 objective lens (i.e., a microscopic field of 1 mm) (9, 13). In colorectal cancer, their highest number observed identifies the grade of malignancy: < 5 clusters for grade 1 (G1), 5 to 9 clusters for grade 2 (G2), and 10 or more clusters for grade 3 (G3) (14). The high number of PDCs is strongly associated with lymphatic vascular invasion and lymph node metastases, irrespective of the TNM stage (14–16). The correlation between the number of PDCs and the depth of infiltration of the submucosa in colorectal and gastric tumors is relevant (10, 14, 17). The unfavorable prognostic significance in terms of survival, demonstrated in studies with large case series of colorectal and gastric cancers, encourages to consider PDCs as possible tools in assessing the risk of lymph node involvement and progressive disease, regardless of pTNM stages and other histological features (10, 18). KRAS, NRAS and BRAF mutant colorectal carcinomas tend to form a high number of PDCs suggesting that the state of genomic instability may be a condition favoring their formation (19).
The aim of this preliminary study is to investigate the CNMs in a series of 25 NMs and to describe their association with their biological and histological characteristics.
Materials and methods
Case selection and histological analysis
Twenty-five NMs were selected from the archive of the Unit of Pathology of Modena University from 2000 to 2020. Clinical information, including sex, age at diagnosis, tumor location and size, and biological profile, were collected. Three expert pathologists independently reviewed hematoxylin and eosin (H&E) stained slides representative of the melanomas (LRB, LB & PP). The following data were collected: tumor cell morphology (epithelioid or spindle), Breslow thickness, Clark’s level, mitosis x mm2, ulceration, lymphovascular invasion, and tumor-infiltrating lymphocytes (TILs).
Cerebroid nests of melanocytes
CNMs morphology and count were defined according to the definition of PDC proposed by Ueno et al. in colorectal cancer (13). Thus, we described CNMs as round or oval aggregates of undifferentiated epithelioid atypical melanocytes, primarily devoid of pigmentation, composed of at least 5 tumor cells, detected in hematoxylin and eosin (H&E) stained slides. We evaluated the entire surface of the tumors and its peripheral zone, identifying the highest CNMs concentration – hot spot under a microscopic field of an objective lens 20x (i.e., a microscopic field with a major axis of 1 mm). First CNMs evaluation was performed in a dichotomy system (present-absent) defining CNMs+ melanomas and CNMs- melanomas. Thus, according to a significant number of CNMs at 20x, we grouped melanomas into grade G1-CNMs+ melanoma (0 – 4 CNMs), grade G2-CNMs+ melanoma (5 – 9 CNMs), and grade G3-CNMs melanoma (≥ 10 CNMs). We distinguished central CNMs (cCNMs) from peripheral CNMs (pCNMs) concerning the primary neoplastic lesion.
Statistical analysis
Statistical analysis was performed with STATA software, version 14 (Stata Corp LP 4905 Lakeway Drive College Station, Texas 77845 USA). Qualitative data were expressed as frequency and percentage. The Chi-square test (Fisher’s exact test) examined the relationship among qualitative variables. A p-value < 0.05 was considered significant.
Results
The study included 25 NMs: 13 patients were males and 12 females, with a mean age of 67 years old (range 34 – 75 years). In detail, the mean diameter of the melanomas was 1.8 cm (range 0.9 – 2.3 cm), and tumor location included limbs (12 cases; 48%), chest-abdomen (8 cases; 32%), and other skin areas (5 cases; 20%). Breslow thickness was ≤ 1.0 mm in 13 cases (52%) (all pT1b) and > 1.0 mm in 12 (48%); Clark’s levels III, IV, and V were reached by 8 (32%), 15 (60%), and 2 (8%) tumors, respectively.
Epithelioid morphology of the tumor cells was observed in 16 cases (64%), and spindle cells in 9 of them (36%); 22 tumors (88%) showed > 5 mitosis x mm2; 6 masses (24%) were ulcerated. Lymph vascular invasion was observed in 12 cases (48%); TILs were present in 14 tumors (56%).
Eleven melanomas (44%) showed mutations. BRAF (V600E) mut was present in 6 cases (24%), NRAS (Q61R) mut in 4 cases (16%), and c-KIT (EXE11-G565E) mut in 1 case (4%).
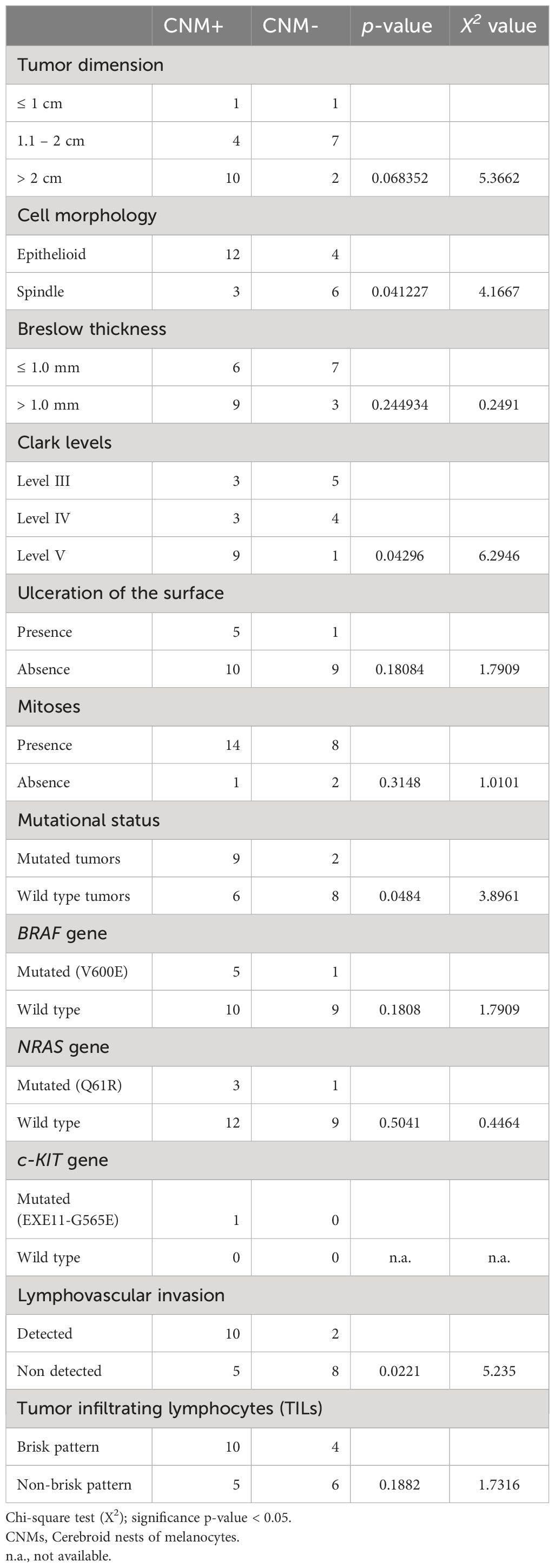
CNMs were observed in the periphery of 15 melanomas (60%) (Figure 1). Six of them (40%) were also detected within the tumor mass. The higher number of CNMs at 20x was seen mainly at the peripheral zone of the melanoma and, according to this, 17 melanomas (68%) were classified as G1 (0 – 4 CNMs), 5 (20%) as G2 (5 – 9 CNMs), and 3 (12%) as G3 (10 or more CNMs) (Figures 2A–C).
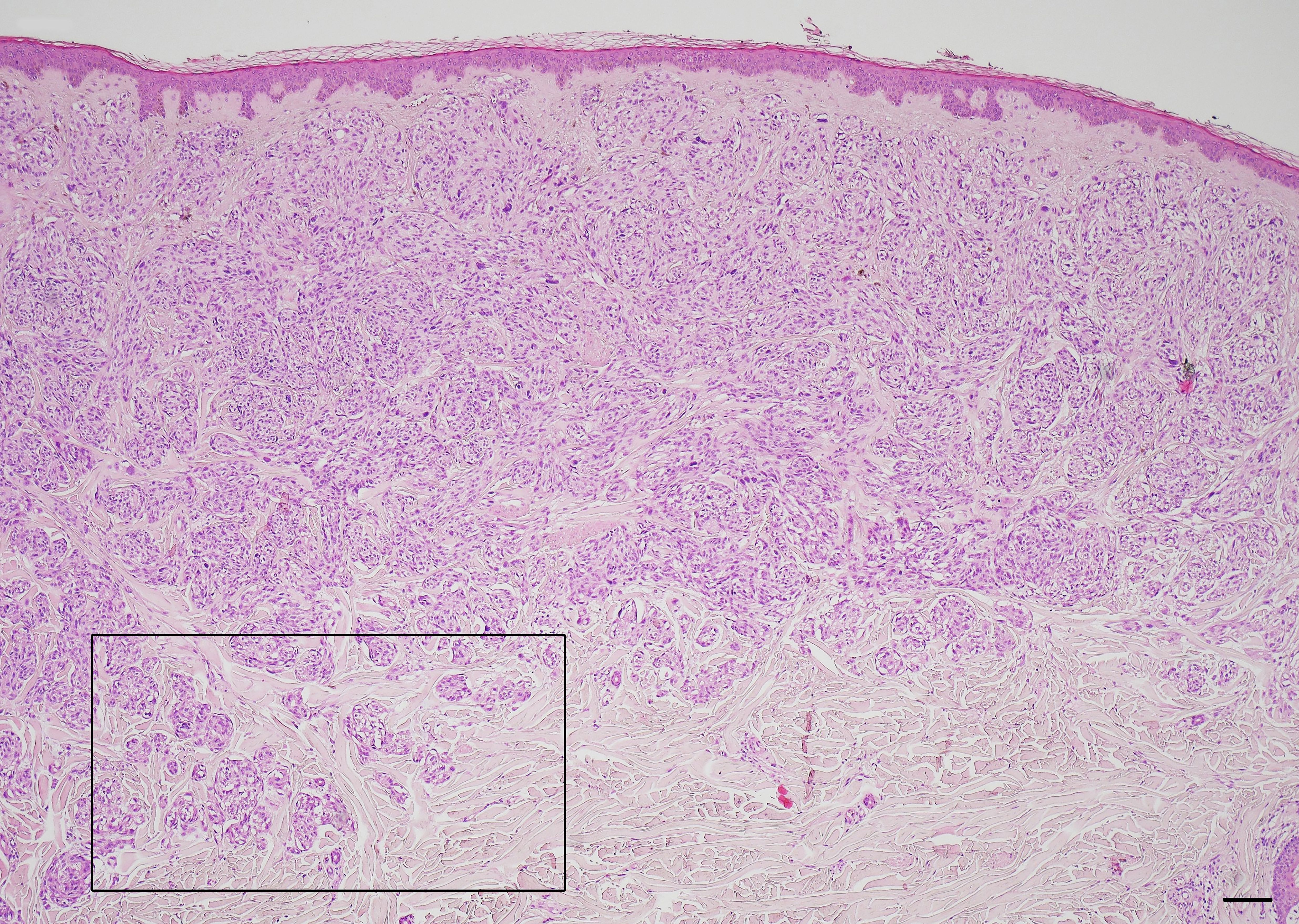
Figure 1 Cerebroid nests of melanocytes at the periphery – growth edge – of nodular melanoma. Hematoxylin and eosin stained slide. Magnification 4x, scale bars 200 µm.
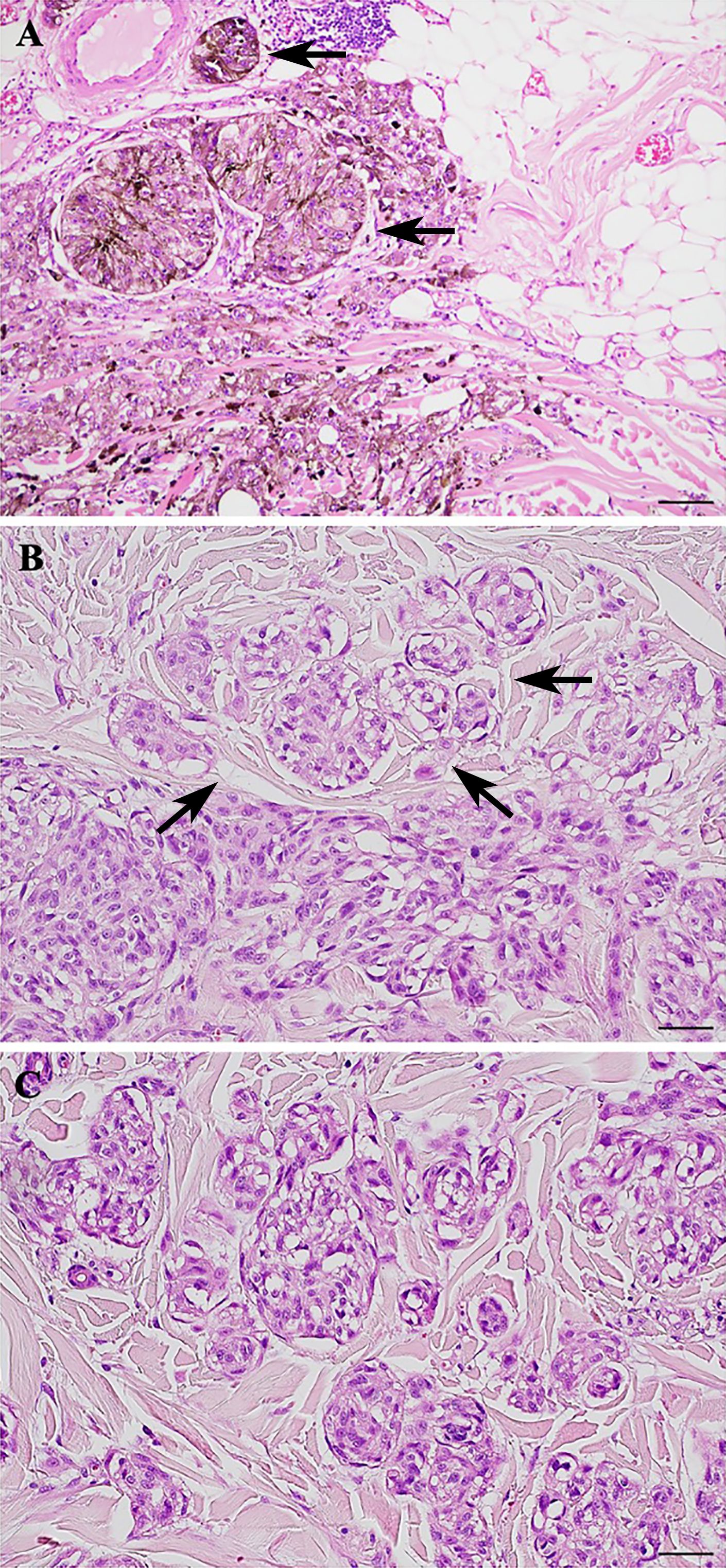
Figure 2 Cerebroid nests of melanocytes (CNMs) grading in nodular melanoma. (A) CNMs G1 (from 0 to 4 buds). (B) CNMs G2 (from 5 to 9 buds). (C) CNMs G3 (≥ 10 buds). Hematoxylin and eosin stained slide. Magnification 20x, scale bars 100 µm.
Histological and molecular features of the CNM+ and CNM- melanomas are listed in Table 1. In detail, CNM+ tumors were mainly epithelioid in morphology (p = 0.041), showed more depth invasion (p = 0.042), showed more frequently lymph vascular invasion (p = 0.022) and a mutated status (p = 0.048); CNM+ melanomas were larger in diameter, although no statistical significance was reached (p = 0.068).
Histological and molecular features are listed in Table 2, G1-CNM+, G2-CNM+, and G3-CNM+ melanomas, respectively. High-grade CNM+ melanomas (G3-CNM+) were more frequently ulcerated (p = 0.025), and BRAF (V600E) mutated (p = 0.028). Although statistical significance was not reached, they exhibited predominantly epithelioid cell morphology, and were lymph vascular invasive.
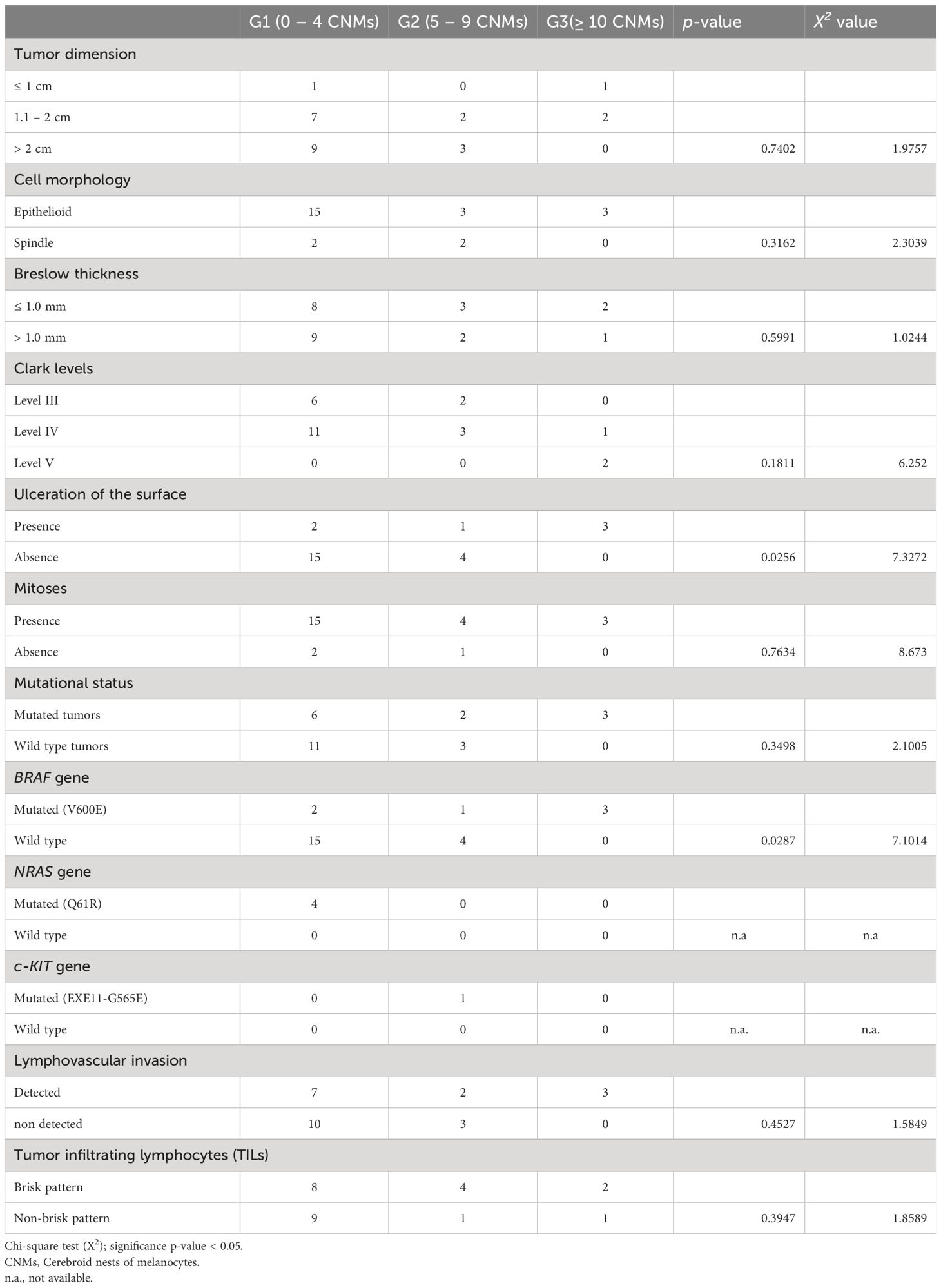
Table 2 Correlation between cerebroid nests of melanocytes grading and clinico-pathological features.
Discussion
NM represents a clinically aggressive histologic variant characterized by atypical pleomorphic melanocytic cells with large cytoplasm, spindle or epithelioid shape, and prominent nucleoli (3, 20, 21). In many tumors in the periphery, CNMs can be detected, which detach from the main mass, taking on an infiltrative appearance. CNMs are readily detectable in slides stained with H&E and show a morphology similar to the aggregates of PDCs observed in colorectal carcinoma (9). Although CNMs are described in NMs, no studies currently consider their histopathologic or prognostic significance. Concerning this, we examined the histological slides of a selected group of NMs and demonstrated the presence of CNMs at the periphery of 15 (60%) of them and a number ≥ 10 nests at 20x field in 3 cases (12%). We showed that CNMs were significantly associated with lymphatic vascular invasion, and higher Clark level, suggesting a possible role in tumor growth. This would be responsible for vascular invasion and, thus, metastasis, typical of their more aggressive clinical behavior (20, 22–28). Therefore, CNMs represent the active growth front of these melanomas, typically observed in epithelial malignancies. In support of this, it is interesting to report that CNMs were significantly associated with the epithelioid phenotype of the melanoma from which they originated. As shown in Figure 3, where we compare PDCs and CNMs, we hypothesize that NMs with epithelioid morphology have an aggressive growth attitude, probably comparable to poorly differentiated epithelial neoplasms with active neoplasm “buds” (17). Indeed, in our experience reported here, CNM+ melanomas reach Clark’s IV and V levels of infiltration and are clinically characterized by recurrence and metastasis (p = 0.011; Table 2). This aspect of invasiveness has also been similarly demonstrated in colorectal cancers with a high number of PDCs in tumors deeply infiltrating the intestinal wall (pT3 and pT4 tumors) (15, 16, 29).
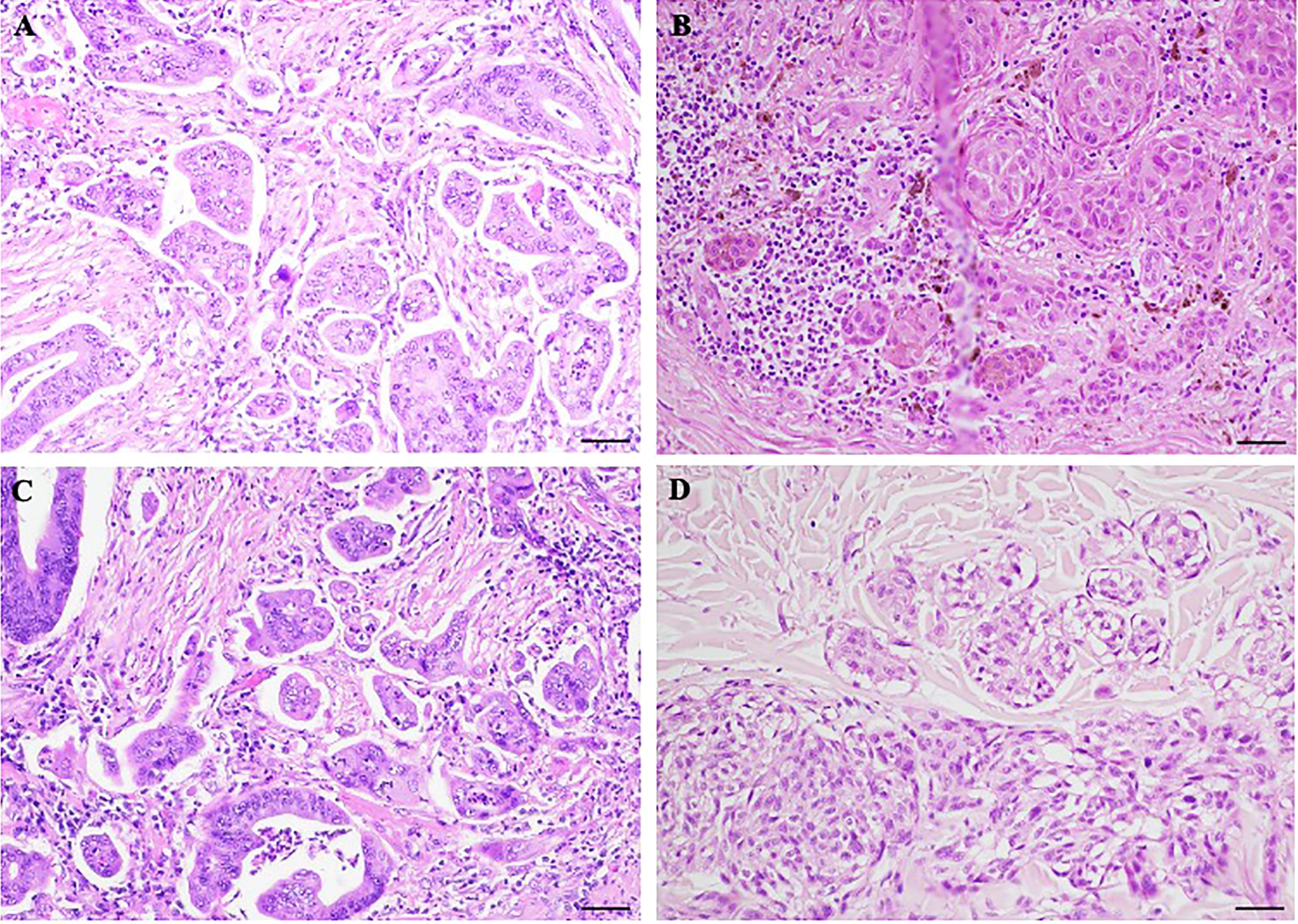
Figure 3 Morphological comparison between poorly differentiated clusters (PDCs) and cerebroid nests of melanocytes (CNMs). (A–C) PDCs in colorectal cancer. (B–D) CNMs in nodular melanoma. Hematoxylin and eosin stained slide. Magnification 20x, scale bars 100 µm.
CNMs are more abundant at the periphery of melanomas than those detectable within masses, which are rarer to identify. As surmised for colorectal PDCs (30, 31), this could indicate that their origin derives from the epithelio-stroma interaction, which belongs to the mechanisms that drive cell growth and proliferation (30). This aspect is typically observed in epithelial tumors (32) and therefore now acceptable as a possible explanation to the formation of PDCs (14). It must, however, be transferred with caution in the context of NMs with CNMs since the origin is neuroectodermal and for that reason melanoma cells may not be subject to epithelial-mesenchymal transition (EMT)-mediated effects (33). On the other hand, it is also known that in the dermal invasive phase, tumor melanocytes acquire molecular changes in cell-cell adhesion proteins, including the downregulation of the junctional protein E-cadherin (33–35) and that this process becomes particularly evident during the transition from radial to vertical growth phase (36). On the other hand, it should be mentioned that nonepithelial tumors, including melanoma, can acquire mesenchymal-like properties (37). However, it is still not entirely clear how transcription factors that induce EMT drive the growth and progression of nonepithelial tumors, such as melanoma (37, 38).
In recent years, it has been identified that genetic factors, including activating mutations in the BRAF and NRAS oncogenes, contribute to melanoma initiation, promoting its growth and metastasis, as well as the transition of melanoma cells into different epithelial and mesenchymal states (39). Our study reported that BRAF (V600E) mutated NMs have a higher number of CNMs at the periphery of the mass, suggesting that the accumulation of genetic mutations in tumor cells could promote the creation of clones of cells that can aggregate and migrate by invading the dermis. Studies on PDCs and colorectal cancer report that a high number of PDCs in colon cancer are associated with the V600E mutation in the BRAF gene, although without statistical significance due to the limited number of cases collected (14, 19). BRAF is a Ras-activated serine/threonine protein kinase that participates in the MAP kinase/ERK signaling pathway (40), mutated in approximately 50% of melanomas [Catalogue of Somatic Mutations in Cancer (COSMIC) at http://www.sanger.ac.uk/cosmic] (41). BRAF (V600E) mut has been implicated in melanoma progression, senescence evasion, apoptosis, uncontrolled replication potential, and angiogenesis, resulting in tissue invasion and metastasis (42). The interaction between melanocytic tumor cells and the surrounding microenvironment, consisting of different extracellular matrix components and growth factors, and the induction of EMT in melanoma are influenced by common mutations and/or deregulated expression of BRAF, NRAS, and PTEN, which appear to act synergistically with each other and with different microenvironmental factors (43). The microenvironment can integrate aberrant genetic changes such as mutations in BRAF to promote melanomagenesis and support an invasive cell phenotype. Therefore, BRAF mutation could promote the genesis of tumor melanocyte clones that would take on the biomolecular characteristics required to generate CNMs (40, 44).
Our study has some limitations: first, we have a “small” series of NMs, and second, molecular mutations were detected in only 11 out of 25 cases, but this is due to the difficulty in enrolling cases with CNMs as well as the fact that this is a pilot study, so the results should be considered preliminary.
In conclusion, CNMs represent a promising additional unfavorable histologic feature of NMs with epithelioid morphology. Because CNMs+ melanomas appear to be associated with the BRAF V600E mutation, the presence of CNMs could have cancer predictive significance for identifying a subgroup of patients who might benefit from specific biologic drugs. However, further studies are needed to confirm this hypothesis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethical Review Committee of the Modena University Hospital (protocol number CE 289\13) and conducted according to the Helsinki Declaration. Written informed consent was obtained from each patient enrolled. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SC: Writing – original draft. AM: Writing – original draft, Writing – review & editing. LB: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. MM: Visualization, Writing – review & editing. FF: Visualization, Writing – review & editing. PP: Visualization, Writing – review & editing. VA: Visualization, Writing – review & editing. GC: Visualization, Writing – review & editing. TS: Writing – review & editing. GP: Visualization, Writing – review & editing. LRB: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferrara G, Argenziano G. The WHO 2018 classification of cutaneous melanocytic neoplasms: suggestions from routine practice. Front Oncol. (2021) 11:675296. doi: 10.3389/fonc.2021.675296
2. Raimondi S, Suppa M, Gandini S. Melanoma epidemiology and sun exposure. Acta Derm Venereol. (2020) 100:adv00136. doi: 10.2340/00015555-3491
3. Beretti F, Bertoni L, Farnetani F, Pellegrini C, Gorelli G, Cesinaro AM, et al. Melanoma types by in vivo reflectance confocal microscopy correlated with protein and molecular genetic alterations: A pilot study. Exp Dermatol. (2019) 28:254–60. doi: 10.1111/exd.13877
4. Yaman B, Akalin T, Kandiloglu G. Clinicopathological characteristics and mutation profiling in primary cutaneous melanoma. Am J Dermatopathol. (2015) 37:389–97. doi: 10.1097/DAD.0000000000000241
5. Urvanegia AC, Tavoloni Braga JC, Shitara D, Fregnani JH, Neves JI, Pinto CA, et al. Reflectance confocal microscopy features of BRAF V600E mutated thin melanomas detected by immunohistochemistry. PloS One. (2017) 12:e0179745. doi: 10.1371/journal.pone.0179745
6. Newton-Bishop J, Bishop DT, Harland M. Melanoma genomics. Acta Derm Venereol. (2020) 100:adv00138. doi: 10.2340/00015555-3493
7. Segura S, Pellacani G, Puig S, Longo C, Bassoli S, Guitera P, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. (2008) 144:1311–20. doi: 10.1001/archderm.144.10.1311
8. Corneli P, Zalaudek I, Magaton Rizzi G, di Meo N. Improving the early diagnosis of early nodular melanoma: can we do better? Expert Rev Anticancer Ther. (2018) 18:1007–12. doi: 10.1080/14737140.2018.1507822
9. Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. (2012) 36:193–201. doi: 10.1097/PAS.0b013e318235edee
10. Sorrentino L, De Ruvo N, Serra F, Salati M, Ricciardolo AA, Bonetti LR, et al. Role of poorly differentiated cluster in gastric cancer: is it a new prognosis factor? Scand J Gastroenterol. (2022) 57:44–9. doi: 10.1080/00365521.2021.1974932
11. Sun Y, Liang F, Cao W, Wang K, He J, Wang H, et al. Prognostic value of poorly differentiated clusters in invasive breast cancer. World J Surg Oncol. (2014) 12:310. doi: 10.1186/1477-7819-12-310
12. Yamamoto M, Kaizaki Y, Kogami A, Hara T, Sakai Y, Tsuchida T. Prognostic significance of tumor budding, poorly differentiated cluster, and desmoplastic reaction in endometrioid endometrial carcinomas. J Obstet Gynaecol Res. (2021) 47:3958–67. doi: 10.1111/jog.14997
13. Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Tanaka M, Miyake O, et al. Site-specific tumor grading system in colorectal cancer: multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. (2014) 38:197–204. doi: 10.1097/PAS.0000000000000113
14. Reggiani Bonetti L, Barresi V, Bettelli S, Domati F, Palmiere C. Poorly differentiated clusters (PDC) in colorectal cancer: what is and ought to be known. Diagn Pathol. (2016) 11:31. doi: 10.1186/s13000-016-0481-7
15. Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Histological grading in colorectal cancer: new insights and perspectives. Histol Histopathol. (2015) 30:1059–67. doi: 10.14670/HH-11-633
16. Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Poorly differentiated clusters: clinical impact in colorectal cancer. Clin Colorectal Cancer. (2017) 16:9–15. doi: 10.1016/j.clcc.2016.06.002
17. Barresi V, Branca G, Ieni A, Reggiani Bonetti L, Baron L, Mondello S, et al. Poorly differentiated clusters (PDCs) as a novel histological predictor of nodal metastases in pT1 colorectal cancer. Virchows Arch. (2014) 464:655–62. doi: 10.1007/s00428-014-1580-z
18. Shivji S, Conner JR, Barresi V, Kirsch R. Poorly differentiated clusters in colorectal cancer: a current review and implications for future practice. Histopathology. (2020) 77:351–68. doi: 10.1111/his.14128
19. Barresi V, Bonetti LR, Bettelli S. KRAS. NRAS, BRAF mutations and high counts of poorly differentiated clusters of neoplastic cells in colorectal cancer: observational analysis of 175 cases. Pathology. (2015) 47:551–6. doi: 10.1097/PAT.0000000000000300
20. Susok L, Stucker M, Bechara FG, Stockfleth E, Gambichler T. Multivariate analysis of prognostic factors in patients with nodular melanoma. J Cancer Res Clin Oncol. (2021) 147:2759–64. doi: 10.1007/s00432-021-03562-1
21. Lattanzi M, Lee Y, Simpson D, Moran U, Darvishian F, Kim RH, et al. Primary melanoma histologic subtype: impact on survival and response to therapy. J Natl Cancer Inst. (2019) 111:180–8. doi: 10.1093/jnci/djy086
22. Ueno H, Shinto E, Kajiwara Y, Fukazawa S, Shimazaki H, Yamamoto J, et al. Prognostic impact of histological categorisation of epithelial-mesenchymal transition in colorectal cancer. Br J Cancer. (2014) 111:2082–90. doi: 10.1038/bjc.2014.509
23. Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. (2012) 10:1403–18. doi: 10.1158/1541-7786.MCR-12-0307
24. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
25. Prall F, Ostwald C. High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol. (2007) 38:1696–702. doi: 10.1016/j.humpath.2007.04.002
26. Prall F. Tumour budding in colorectal carcinoma. Histopathology. (2007) 50:151–62. doi: 10.1111/j.1365-2559.2006.02551.x
27. Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of Malignant tumour progression. Nat Rev Cancer. (2005) 5:744–9. doi: 10.1038/nrc1694
28. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. (2003) 3:362–74. doi: 10.1038/nrc1075
29. Archilla I, Diaz-Mercedes S, Aguirre JJ, Tarragona J, MaChado I, Rodrigo MT, et al. Lymph node tumor burden correlates with tumor budding and poorly differentiated clusters: A new prognostic factor in colorectal carcinoma? Clin Transl Gastroenterol. (2021) 12:e00303. doi: 10.14309/ctg.0000000000000303
30. Bertoni L, Barresi V, Bonetti LR, Caramaschi S, Mangogna A, Lionti S, et al. Poorly differentiated clusters (PDC) in colorectal cancer: Does their localization in tumor matter? Ann Diagn Pathol. (2019) 41:106–11. doi: 10.1016/j.anndiagpath.2019.06.008
31. Caramaschi S, Mangogna A, Salviato T, Ammendola S, Barresi V, Manco G, et al. Cytoproliferative activity in colorectal poorly differentiated clusters: Biological significance in tumor setting. Ann Diagn Pathol. (2021) 53:151772. doi: 10.1016/j.anndiagpath.2021.151772
32. Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. (2003) 15:740–6. doi: 10.1016/j.ceb.2003.10.006
33. Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PloS One. (2012) 7:e40378. doi: 10.1371/journal.pone.0040378
35. Pla P, Moore R, Morali OG, Grille S, Martinozzi S, Delmas V, et al. Cadherins in neural crest cell development and transformation. J Cell Physiol. (2001) 189:121–32. doi: 10.1002/jcp.10008
36. Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. (2000) 156:1515–25. doi: 10.1016/S0002-9440(10)65023-7
37. Pedri D, Karras P, Landeloos E, Marine JC, Rambow F. Epithelial-to-mesenchymal-like transition events in melanoma. FEBS J. (2022) 289:1352–68. doi: 10.1111/febs.16021
38. Vandamme N, Berx G. Melanoma cells revive an embryonic transcriptional network to dictate phenotypic heterogeneity. Front Oncol. (2014) 4:352. doi: 10.3389/fonc.2014.00352
39. Bauer R, Hein R, Bosserhoff AK. A secreted form of P-cadherin is expressed in Malignant melanoma. Exp Cell Res. (2005) 305:418–26. doi: 10.1016/j.yexcr.2005.01.024
40. Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. (2012) 10:85. doi: 10.1186/1479-5876-10-85
41. (WSI) WSI. COSMIC (2023). Available online at: https://cancer.sanger.ac.uk/cosmic.
42. Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. (2011) 30:3477–88. doi: 10.1038/onc.2011.160
43. Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett. (2017) 391:125–40. doi: 10.1016/j.canlet.2017.01.029
Keywords: nodular melanoma, cerebroid nests, melanocytes, BRAF, prognosis
Citation: Caramaschi S, Mangogna A, Bertoni L, Manfredini M, Farnetani F, Parente P, Attino V, Cazzato G, Salviato T, Pellacani G and Reggiani Bonetti L (2024) High charge of cerebroid nests in nodular melanomas predicts tumor aggressiveness and high mutational tumoral burden: a pilot study. Front. Oncol. 14:1336895. doi: 10.3389/fonc.2024.1336895
Received: 11 November 2023; Accepted: 02 July 2024;
Published: 19 July 2024.
Edited by:
Suzie Chen, Rutgers, The State University of New Jersey, United StatesReviewed by:
Mithalesh Kumar Singh, University of Texas Southwestern Medical Center, United StatesLee Wheless, Vanderbilt Ingram Cancer Center, United States
Copyright © 2024 Caramaschi, Mangogna, Bertoni, Manfredini, Farnetani, Parente, Attino, Cazzato, Salviato, Pellacani and Reggiani Bonetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Reggiani Bonetti, bHVjYS5yZWdnaWFuaWJvbmV0dGlAdW5pbW9yZS5pdA==
 Stefania Caramaschi
Stefania Caramaschi Alessandro Mangogna
Alessandro Mangogna Laura Bertoni
Laura Bertoni Marco Manfredini
Marco Manfredini Francesca Farnetani5
Francesca Farnetani5 Gerardo Cazzato
Gerardo Cazzato Luca Reggiani Bonetti
Luca Reggiani Bonetti