
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 April 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1334845
This article is part of the Research Topic Women in Genitourinary Oncology Vol III: 2023 View all 5 articles
 Maria T. Bourlon1,2*
Maria T. Bourlon1,2* Shaddai Urbina-Ramirez3
Shaddai Urbina-Ramirez3 Haydee C. Verduzco-Aguirre1
Haydee C. Verduzco-Aguirre1 Mauricio Mora-Pineda1
Mauricio Mora-Pineda1 Hugo E. Velazquez4
Hugo E. Velazquez4 Eucario Leon-Rodriguez1
Eucario Leon-Rodriguez1 Yemil Atisha-Fregoso5
Yemil Atisha-Fregoso5 María G. De Anda-Gonzalez3
María G. De Anda-Gonzalez3Introduction: Patients with adverse pathological features (APF) at radical prostatectomy (RP) for prostate cancer (PC) are candidates for adjuvant treatment. Clinicians lack reliable markers to predict these APF preoperatively. Protein tyrosine phosphatase 1B (PTP-1B) is involved in migration and invasion of PC, and its expression could predict presence of APF. Our aim was to compare PTP-1B expression in patients with and without APF, and to explore PTP-1B expression as an independent prognostic factor.
Methods: Tissue microarrays (TMAs) were constructed using RP archival specimens for immunohistochemical staining of PTP-1B; expression was reported with a standardized score (0-9). We compared median PTP-1B score between cases with and without APF. We constructed two logistic regression models, one to identify the independence of PTP-1B score from biologically associated variables (metformin use and type 2 diabetes mellitus [T2DM]) and the second to seek independence of known risk factors (Gleason score and prostate specific antigen [PSA]).
Results: A total of 73 specimens were suitable for TMA construction. Forty-four (60%) patients had APF. The median PTP-1B score was higher in those with APF: 8 (5-9) vs 5 (3-8) (p=0.026). In the logistic regression model including T2DM and metformin use, the PTP-1B score maintained statistical significance (OR 1.21, 95% CI 1.01-1.45, p=0.037). In the model including PSA and Gleason score; the PTP-1B score showed no independence (OR 1.68, 95% CI 0.97-1.41, p=0.11). The area under the curve to predict APF for the PTP-1B score was 0.65 (95% CI 0.52-0.78, p=0.03), for PSA+Gleason 0.71 (95% CI 0.59-0.82, p=0.03), and for PSA+Gleason+PTP-1B score 0.73 (95% CI 0.61-0.84, p=0.001).
Discussion: Patients with APF after RP have a higher expression of PTP-1B than those without APF, even after adjusting for T2DM and metformin exposure. PTP-1B has a good accuracy for predicting APF but does not add to known prognostic factors.
Prostate cancer is one of the most common cancers in men worldwide (1), with most cases being diagnosed at early stages. Localized prostate cancer represents a heterogeneous disease exhibiting a broad spectrum of presentation, ranging from asymptomatic disease detected by screening to aggressive tumors with poorer long-term outcomes. Consequently, localized prostate cancer is stratified at diagnosis based on the risk of progression into low-, intermediate- and high-risk disease. This categorization utilizes pretreatment prostate specific antigen (PSA) levels, the Gleason score from the initial biopsy, and the extent of the primary tumor as determined by digital rectal examination (2). Patients with high-risk disease usually require a multimodal approach, combining androgen deprivation therapy with radiotherapy, as prostatectomy alone can be insufficient in those with locally advanced disease. For low- or intermediate-risk patients who are candidates for definitive treatment, surgery is a therapeutic option with a curative intent (3).
Pathological staging may differ from the initial clinical stage, and the final prostatectomy specimen may reveal adverse pathological factors (APF) such as extraprostatic extension, seminal vesicle invasion, or positive surgical margins. Patients with APF are at an increased risk of recurrent disease (4–6), which is frequently managed with adjuvant or salvage radiotherapy (7), which carries an increased probability of significant gastrointestinal and genitourinary toxicity. Even among patients initially classified as low- or intermediate-risk, APFs may be present, suggesting that clinical risk factors alone may not adequately predict the necessity for additional treatment following prostatectomy.
Deregulation of protein tyrosine phosphatases (PTPs) or protein tyrosine kinases (PTKs) leads to aberrant tyrosine phosphorylation, which has been implicated in the etiology of several diseases, including prostate cancer (8–10). The phosphatase PTP-1B, encoded by the PTPN1 gene, plays a role in metabolic signaling pathways, particularly through regulation of the insulin receptor and leptin pathways (11). PTP-1B-deficient mice show increased insulin sensitivity and resistance to obesity (12). This phosphatase also interacts with other receptor protein tyrosine kinases such as PDGFR and EGFR, both in vitro and in vivo (13), and plays a role in the activation of the tyrosine kinases c-Src, which are implicated in the development of some types of cancer (14). PTP-1B can also promote apoptosis through down-regulation of pro-survival signaling, and promotion of caspase activity (15).
Despite exhibiting both tumor suppressing and tumor promoting effects, PTPN1 appears to act predominantly as an oncogene. For instance, silencing PTP-1B in breast cancer cell lines is associated with decreased cell proliferation through the negative regulation of HER2 expression (16). However, clinical evidence remains conflicting: expression of PTP-1B has been identified as a favorable prognostic factor for survival in breast cancer (17); overexpression of PTP-1B has also been proposed as a marker for response to chemotherapy in high-grade serous carcinoma (18). Conversely PTP-1B amplification is associated with poorer survival in gastric and colorectal cancer (19, 20).
PTPN1, encoding PTP-1B, has been found to be co-amplified with the androgen receptor (AR) (21). PTP-1B expression can be induced through stimulation of the AR and is associated with nuclear localization of the AR and a higher Ki67 in primary prostate tumors (22). Depletion of PTP-1B delays androgen-dependent tumor growth and alters in vitro migration and invasion. PTP-1B also contributes to the neuroendocrine differentiation of prostate cancer (23), associated with a worse prognosis.
Given that the role of PTP-1B in prostate cancer has mostly been explored in preclinical settings, this study aimed to elucidate how PTP-1B expression correlates with adverse prognostic factors in localized disease. The primary objective of this study was to compare the expression of PTP-1B, quantified by immunohistochemistry, in patients with and without adverse pathological factors in prostatectomy specimens. Secondary objectives were to establish a correlation between PTP-1B expression and pre-prostatectomy PSA values, and PTP-1B expression and Gleason score; to estimate overall and cancer-specific survival according to PTP-1B expression; and to evaluate PTP-1B as an independent prognostic factor.
After institutional review board approval, we identified all cases of prostate cancer with a history of radical prostatectomy at our center from January 1990 to December 2015. We included cases with complete clinical and pathological data. We excluded cases that received neoadjuvant therapy, presented evidence of metastatic disease prior to prostatectomy, or lacked available tissue in the pathology archives. Additionally, cases with insufficient tissue for the planned analyses were eliminated.
We collected the following clinical information from both physical and electronic medical records: patient age at the time of surgery, pre-surgical PSA, prior diagnosis and treatment of type 2 diabetes mellitus (T2DM), pathological staging according to the AJCC/TNM version 7.0, Gleason score, and presence of adverse pathological factors as defined by the International Society of Urological Pathology (ISUP). These factors include extraprostatic extension, seminal vesicle invasion, or any positive surgical margins other than apical. Overall survival was calculated from the date of prostatectomy until death from any cause. Cancer-specific survival was calculated from the date of prostatectomy until death from any prostate cancer-related cause.
Upon identification of the prostatectomy specimens, we used hematoxylin and eosin-stained slides to identify representative areas of each specimen. These areas were characterized by the highest Gleason score, and lacked necrosis, inflammation, fibrosis or desmoplasia. Corresponding areas in the paraffin blocks were manually harvested by tru-cut biopsy and placed into a tissue microarray (TMA) base. After constructing the TMA, we reassessed the samples to ensure every sample contained the selected tumor and Gleason score, and a minimum of 30 tumor cells. We ensured every constructed TMA had at least two cases with normal prostate tissue, benign hyperplasia, or intraepithelial prostatic neoplasm to serve as internal controls.
For immunohistochemistry, TMA paraffin blocks were sectioned into 4 μm slides. The last slide of each series was stained with hematoxylin and eosin to ensure the presence of sufficient neoplastic tissue in the TMA blocks. The remaining slides were processed sequentially in xylene and ethanol washes before being submerged in water. Afterwards, citrate buffer was used for heat-induced antigen retrieval, and slides were then washed and rinsed. The mouse monoclonal antibody against PTPN1 (PTP-1B), clone OTI2G3, (formerly known 2G3 [TA503188], OriGene Technologies, Rockville, MD), was applied. The slides were incubated with the primary antibody for 45 minutes at room temperature, then for 10 minutes with a biotin solution, and 10 minutes with an HRP solution. Slides were developed with diaminobenzidine and counterstained with hematoxylin.
Antibody standardization was achieved by comparison with normal breast and prostatic tissue processed with the same immunohistochemistry technique described above. Each sample was tested with antibody dilutions of 1:150, 1:100 and 1:50. The staining with the best quality was selected for the dilution. These were used as external controls for the TMAs.
To evaluate the antibody in the selected prostate cancer samples, initial examination was conducted at 4x under light microscopy (Nikon Eclipse 80) to ensure antibody expression was visible in a panoramic view. Then, slides were reviewed at 10x and 40x for every TMA cylinder. Antibody expression was evaluated qualitatively as follows: 0, no antibody staining in neoplastic tissue; 1+, weak cytoplasmic staining in neoplastic tissue; 2+ moderate cytoplasmic staining in neoplastic tissue; 3+, strong cytoplasmic staining in neoplastic tissue; NE, not evaluable due to unspecific staining, background staining, no delimitation of malignant cells, or staining of lymphocytes or fibroblasts (Figure 1). A second, semi-quantitative assessment categorized staining as: NE; not evaluable, 0, no antibody staining in neoplastic tissue; 1+, 1-50% of staining in neoplastic cells; 2+, 51-75% staining of neoplastic cells; 3+, 76-100% staining of neoplastic cells. An expert pathologist in molecular biology, blinded to clinical data, reviewed the cases. An intensity scale was then created based on the primary and secondary readings, as described by Lessard et al. (22) (Table 1).
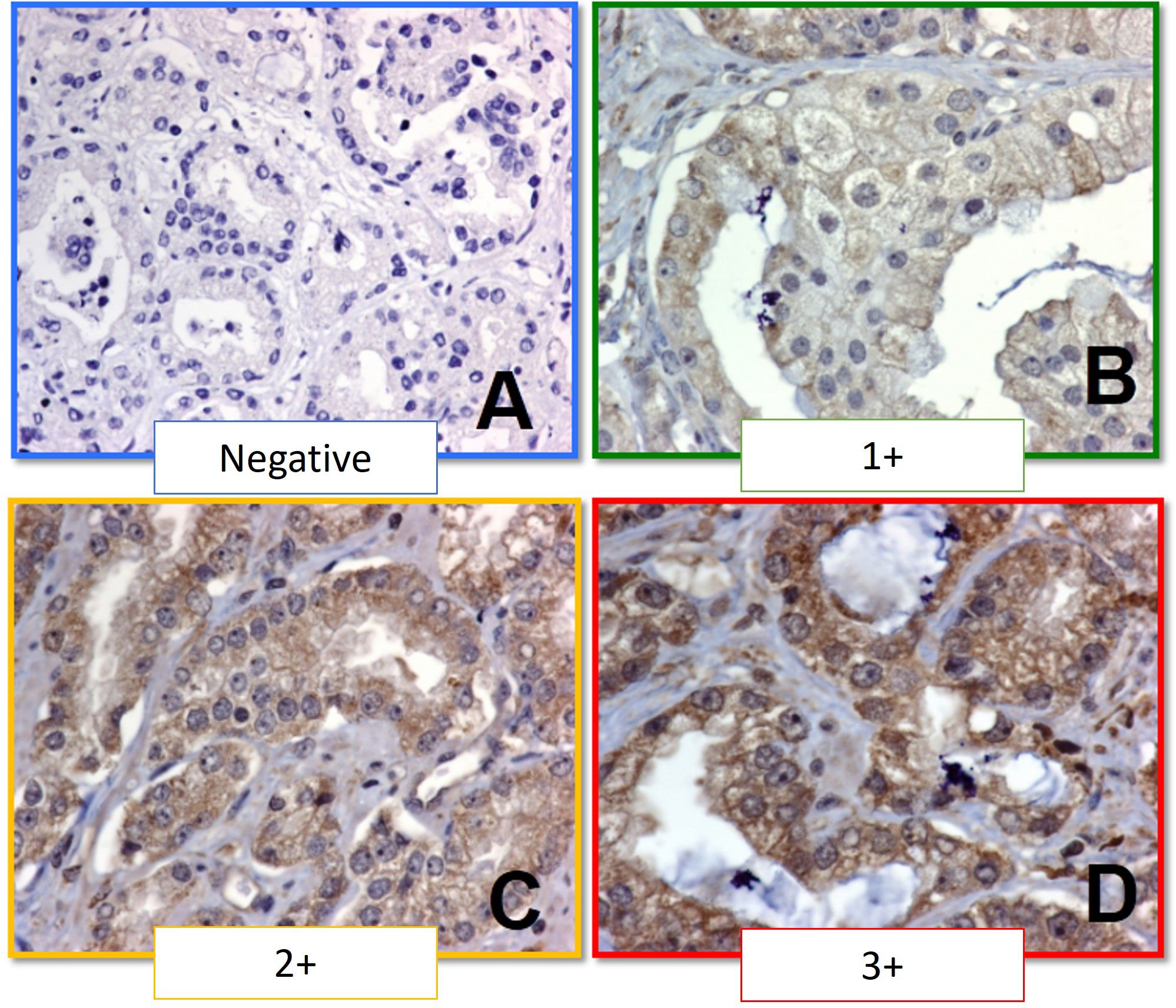
Figure 1 Immunohistochemistry staining scale for PTP-1B. (A) Negative (0+). (B) Weak staining (1+). (C) Moderate staining (2+). (D) Strong staining (3+).
The primary aim of the study utilized the Wilcoxon Mann-Whitney test to compare median scores of PTP-1B between groups with and without APF, considering a p-value ≤ 0.05 as statistically significant. Spearman’s test was used for correlation analyses. Logistic regression analysis was employed to examine the association of APF with biologically related variables (metformin use T2DM) and previously identified risk factors (Gleason score and PSA levels). The sensitivity and specificity of the PTP-1B IHC scale to classify prognostic outcomes was determined using 2x2 tables. Receiver operating characteristic (ROC) curves were constructed to identify the optimal cutoff point for the scale. The Kaplan-Meier method was used to estimate overall and cancer-specific survival, with comparisons made using Cox regression analysis.
We identified 502 cases of prostate cancer with a history of radical prostatectomy diagnosed between January 1990 and December 2015 at our center. Of these, 190 pathology specimens were available for review. An expert molecular pathologist eliminated 117 cases due to insufficient material, resulting in 73 cases being included for the construction of TMAs and subsequent PTP-1B IHC analysis.
For the 73 cases included, the median age was 62 years (range 44-78 years). The mean BMI was 27.2 kg/m2, with 68.85% of patients classified as overweight or obese. Prior to prostate cancer diagnosis, 20.3% had been diagnosed with T2DM, 21.9% had prior use of metformin (either for T2DM or metabolic syndrome), and 10.95% were on other oral T2DM medications. Regarding clinical characteristics, 38.4% of patients were diagnosed via PSA screening. Most cases (53.4%) had a low Gleason score (6), with approximately one-third of the cases falling into each of the low-, intermediate- and high-risk categories. Population characteristics are further described in Table 2.
Prostatectomy specimen characteristics are described in Table 3. Most patients had tumor sizes occupying less than 50% of the specimen. Gleason scores were 6 in 24.7% of cases, 7 in 40.3% and ≥8 in the remainder. Among these cases, 60.3% presented with at least one adverse pathological factor (APF), such as extraprostatic extension, seminal vesicle invasion, or positive surgical margins. Five cases (6.8%) had pelvic nodal disease.
The median PTP-1B expression score for the entire cohort was 6 (interquartile range [IQR] 4-9) (Figure 2). Patients without APFs had a median score of 5 (IQR 3-8), while those with APFs exhibited a significantly higher median score of 8 (IQR 5-9) (p=0.026) (Figure 3). A weak correlation was found between PTP-1B expression and both the prostatectomy Gleason score (r=0.24, p=0.042) and the pre-prostatectomy PSA value (r=0.243, p=0.046).
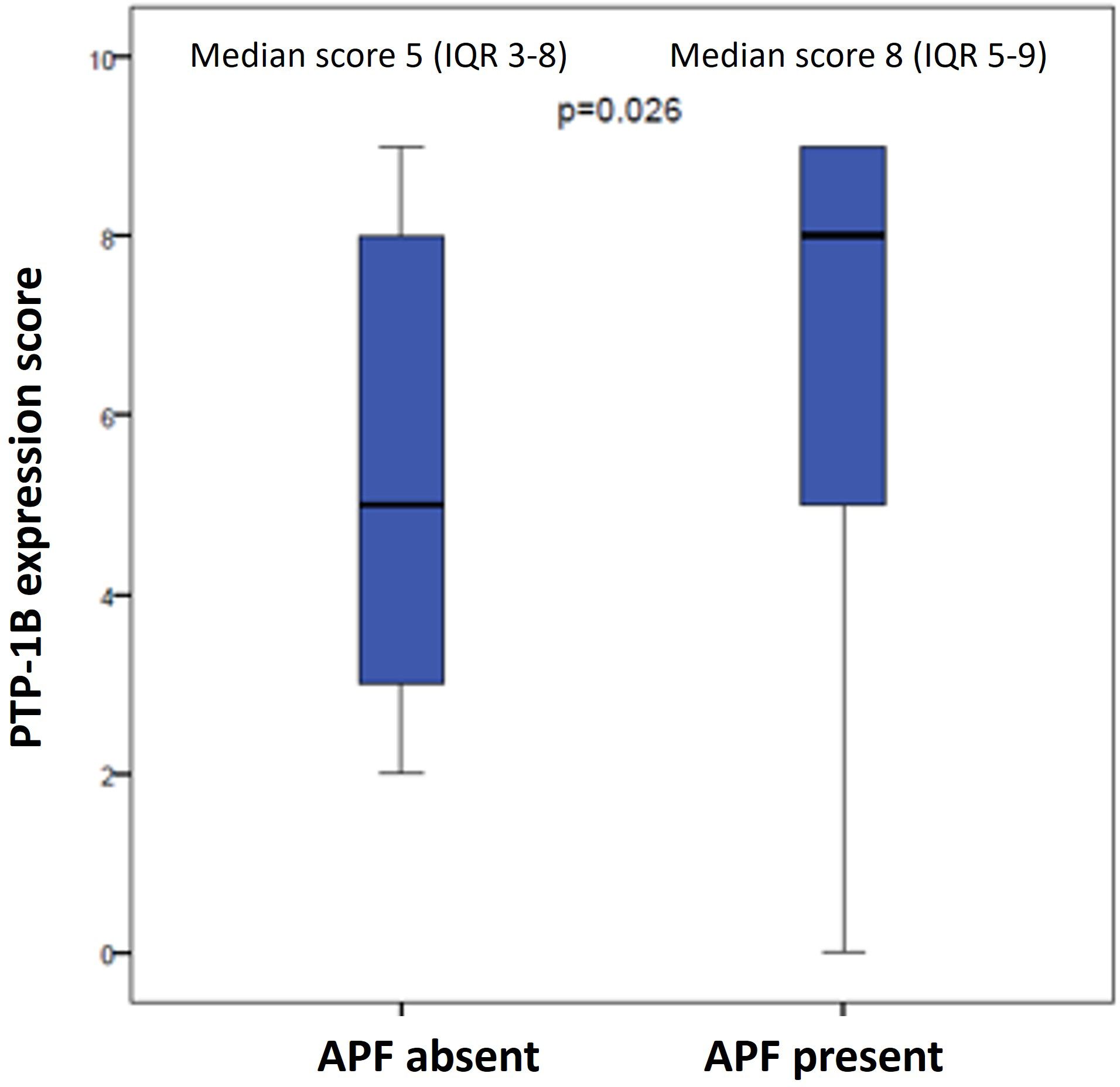
Figure 3 PTP-1B expression score in prostatectomy specimens according to presence or absence of adverse pathological factors (APF).
In the logistic regression analysis assessing the association of PTP-1B expression with the presence of APFs, two models were applied. The first model, which included biologically associated variables (history of T2DM and metformin use), found that PTP-1B expression remained significantly associated with the presence of APFs (Odds Ratio [OR] 1.21, 95% Confidence Interval [CI] 1.01-1.45, p=0.037), independent of prior T2DM diagnosis or metformin use. The second model, which incorporated known prognostic factors (PSA and Gleason score), did not find an independent association of PTP-1B score with APFs (OR 1.68, 95% CI 0.97-1.41, p=0.11) (Table 4).
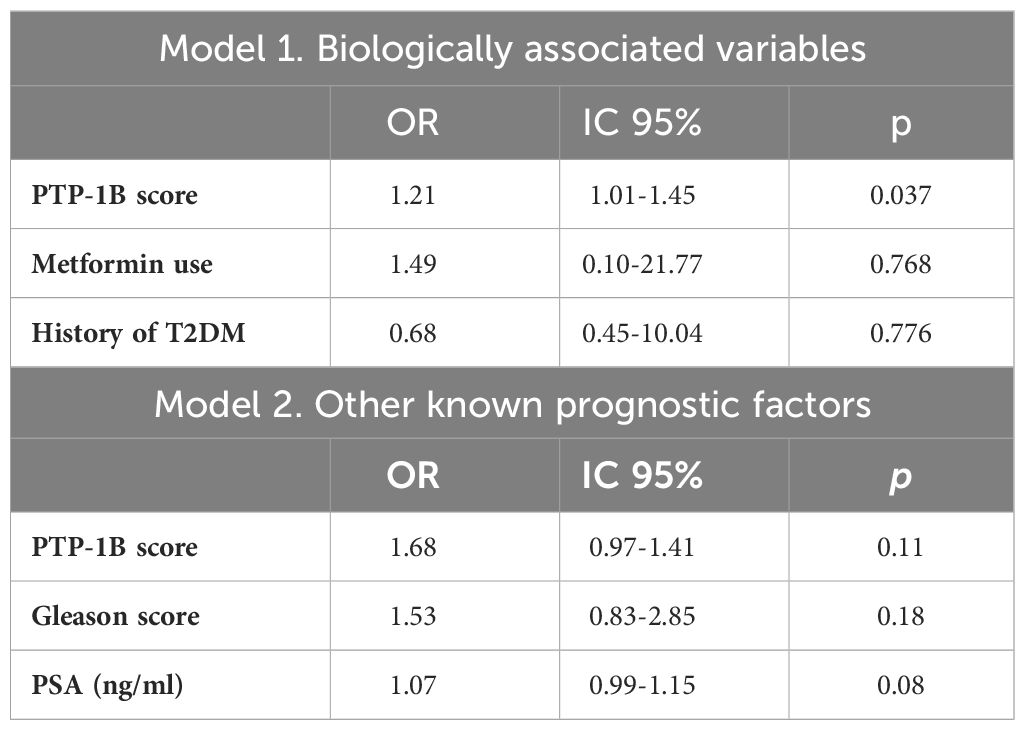
Table 4 Logistic regression models for association with presence of adverse pathological factors in prostatectomy specimens.
To determine the prognostic utility of the PTP-1B IHC scale, we calculated its sensitivity and specificity in classifying cases with either favorable or unfavorable prognosis using 2x2 tables. Receiver Operating Characteristic (ROC) curves were constructed to select the optimal cut-point for the PTP-1B expression score in predicting the presence of APFs, yielding an Area Under the Curve (AUC) of 0.65 (95% CI 0.52-0.78, p=0.03). A cut-off point of 5 was chosen based on sensitivity and specificity analyses (Table 5). Additional ROC curves for PSA and Gleason score demonstrated an AUC of 0.71 (95% CI 0.59-0.82, p=0.03), Combining all predictors (PSA, Gleason score, and PTP-1B score), the AUC was 0.73 (95% CI 0.61-0.84, p=0.001) (Figure 4).
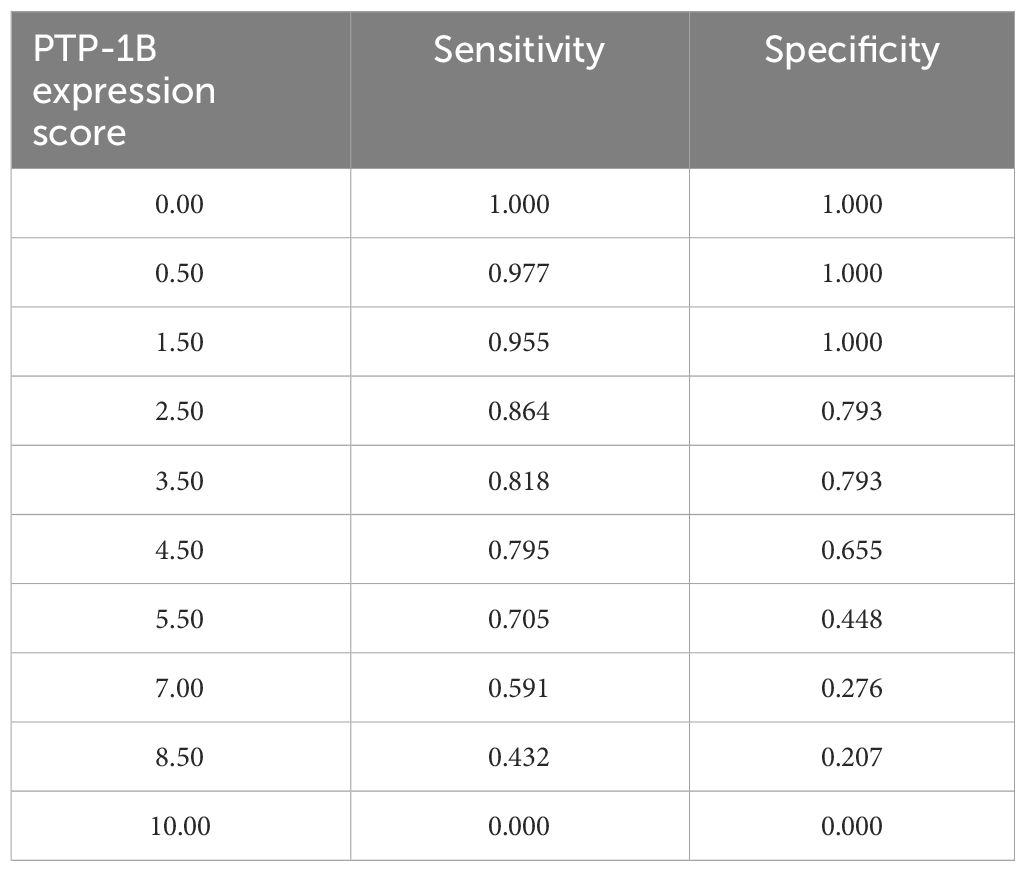
Table 5 Receiver operating characteristic (ROC) curve for identification of the cut-off point of PTP-1B expression score.
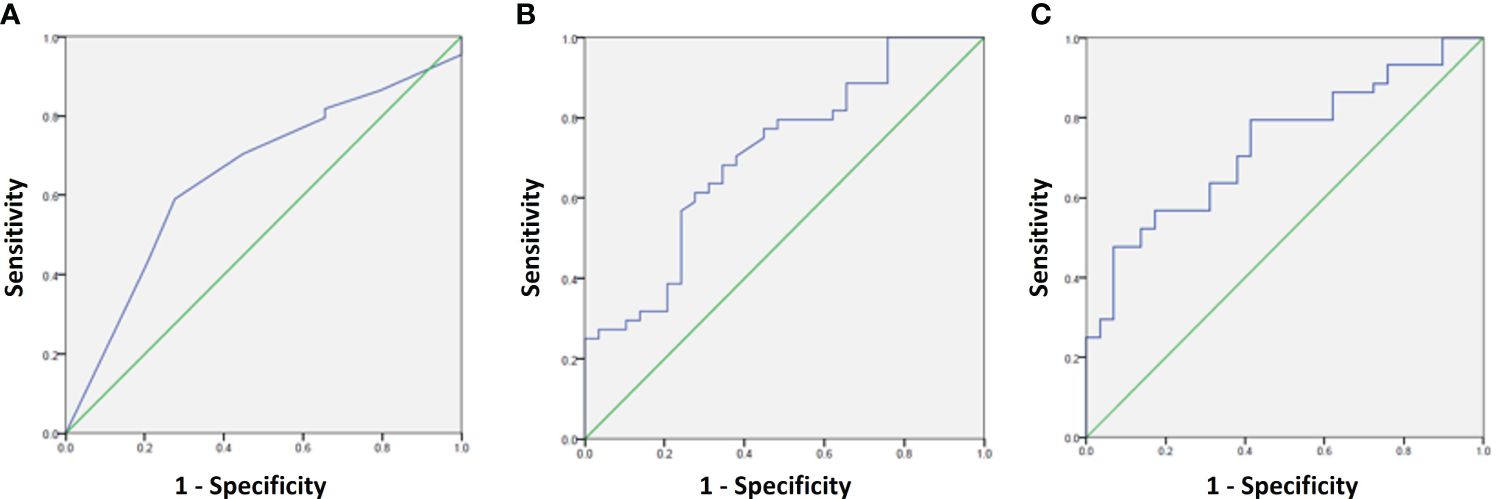
Figure 4 ROC curves for prediction of adverse pathological factors using the following variables: (A) PTP-1B score, (B) PSA + Gleason score, and (C) PSA + Gleason score+ PTP-1B score.
The median overall survival (OS) and cancer-specific survival (CSS) for the entire cohort had not been reached, with estimated 15-year OS at 72% and 15-year CSS at 78%, respectively.
Using the previously selected cut-off point, a PTP-1B expression score ≥6 was not significantly associated with OS (HR 3.15, CI 95% 0.36-26.7, p=0.157) or CSS (HR 3.29, CI 95% 0.36-29.73, p=0.288) (Figure 5).
The quantification of PTP-1B expression by immunohistochemistry is a low-cost test available at most pathology laboratories. Our study shows that the grade of PTP-1B expression is higher in prostatectomy specimens with adverse pathological factors compared to those without adverse pathological factors. This association persisted even when controlling for prior diagnosis of T2DM and metformin use in logistic regression analysis.
Accurate prognostication of adverse pathological factors in localized prostate cancer is of utmost importance to ensure patients receive adequate pretreatment counseling. There are several therapeutic options particularly for low- and intermediate-risk disease, including prostatectomy and radiotherapy with or without ADT. In patients who opt for prostatectomy, however, the pathological specimen might show positive margins or pathological T3 disease, associated with a 13-27% and 40-50% probability of disease recurrence respectively (5, 24–26). Adjuvant radiotherapy for these patients improves metastasis-free survival but carries an increased risk of genitourinary and gastrointestinal toxicity (27). Salvage radiotherapy (i.e. initiated upon evidence of biochemical recurrence) may pose a safe alternative with only a 1 percentage point of change in 5-year event-free survival and a lower proportion of treatment related adverse events, as reported in a pre-planned meta-analysis (28) of three randomized trials studying these treatment options (7, 29, 30).
Since primary radiotherapy is another curative option for localized prostate cancer, it seems logical to optimize the selection of surgical candidates to reduce the necessity for multimodal therapy. However, when evaluated alongside other known prognostic factors such as pre-prostatectomy PSA and biopsy Gleason score, PTP-1B does not seem to be an independent prognostic factor. In line with this, the addition of the PTP-1B expression score to the PSA and Gleason did not significantly improve the sensitivity and specificity to predict the presence of APFs. Our sample was relatively small, and to detect an improvement over already available pre-prostatectomy prognostic factors, our experiment might need to be replicated in a larger cohort.
Hyperglycemia and other components of the metabolic syndrome have been associated with an increased prevalence of adverse pathological features and an increased risk biochemical recurrence of prostate cancer (31). Interestingly, in our study, PTP-1B expression was significantly associated with adverse pathological features, while the presence of diabetes mellitus and metformin usage were not. We did not collect data on pre-prostatectomy glucose levels but, given the low prevalence of insulin use in our study population, and that patients were fit for surgery, it would be expected that our study population had a low rate of uncontrolled hyperglycemia despite 20% of the population having a prior diagnosis of type 2 diabetes mellitus.
In this study, we found a weak positive correlation between PTP-1B expression and Gleason score, which might be related to PTP-1B expression in neuroendocrine cells of prostate cancer in humans, since the prevalence of neuroendocrine differentiation increases in tumors of higher grade (32). This supports the association between a higher PTP-1B expression score and poorer prognosis, despite PTP-1B expression not being an independent predictor for adverse pathological features. In addition, in recent years, there has been renewed interest in PTP-1B as a druggable target (33). A dual inhibitor of PTP-1B and TC-PTP has shown to induce anti-tumor immunity in animal models of cancer resistant to PD-1 blockade (34) and is currently being investigated in a phase 1 study of advanced solid tumors (ClinicalTrials.gov identifier NCT04777994). Therefore, as future directions for this study, we plan to construct this immunostaining panel, including immune-related markers and analyze its association with adverse pathological features and other outcomes.
Our survival analysis did not show a significant difference in OS or CSS. However, this study was underpowered for this analysis, and median OS and CSS were not reached despite a long follow-up, which is expected in localized prostate cancer. Replication in a larger cohort that also includes patients who received primary radiotherapy could perhaps provide deeper insights into the relationship between PTP-1B expression and long-term outcomes.
Quantification of expression of PTP-1B can be achieved with other methods, such as Western blot or PCR (19, 35). Nevertheless, we decided to use immunohistochemistry since it is a readily available technique, even in resource-limited settings. However, it is important to recognize that expression by IHC does not necessarily reflect the production of a functional protein. PTP-1B in its inactive form is ligated to the endoplasmic reticulum near the nucleus, and in the cytoplasm near the cell membrane in its active form. The location of the latter allows it to interact with the insulin receptor, and other receptor tyrosine kinases such as JAK2 and c-Src.
Due to the intricate relationships of intracellular signaling processes, an immunostaining panel including other markers implicated in migration, invasion and proliferation of prostate cancer (36) might improve on already described clinical markers. Potential candidates for such markers could be PTEN, RB1, NKX3, TP53, TMPRSS2, ATM, MYC, enolase, DLX2, Ki67, and estrogen receptor (37, 38). Loss of PTEN, higher expression of Ki67 and overexpression of MYC in high-risk prostate cancer prostatectomy specimens have been associated with worse progression-free survival (39).
In addition to the previously mentioned, we acknowledge as additional limitations of this study its retrospective design and the extended observation period which limited the availability and quality of archival prostatectomy specimens, which underscores the necessity for continued research.
In conclusion, PTP-1B expression, as determined by immunohistochemistry, was higher in patients with adverse pathological factors in prostatectomy specimens, independently of T2DM or prior metformin use. However, PTP-1B expression does not add additional prognostic value to previously known prognostic factors such as PSA and Gleason score.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MB: Conceptualization, Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. SU-R: Data curation, Investigation, Writing – review & editing. HV-A: Visualization, Writing – original draft, Writing – review & editing. MM-P: Investigation, Writing – original draft. HV: Investigation, Writing – review & editing. EL-R: Formal analysis, Writing – review & editing. YA-F: Formal analysis, Writing – review & editing. MD-G: Data curation, Formal analysis, Investigation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We thank Fundación Aramont AC y Canales de Ayuda AC for their financial support of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. (1998) 280:969–74. doi: 10.1001/jama.280.11.969
3. Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:1119–34. doi: 10.1016/j.annonc.2020.06.011
4. Maubon T, Branger N, Bastide C, Lonjon G, Harvey-Bryan KA, Validire P, et al. Impact of the extent of extraprostatic extension defined by Epstein's method in patients with negative surgical margins and negative lymph node invasion. Prostate Cancer Prostatic Dis. (2016) 19:317–21. doi: 10.1038/pcan.2016.24
5. Salomon L, Anastasiadis AG, Johnson CW, McKiernan JM, Goluboff ET, Abbou CC, et al. Seminal vesicle involvement after radical prostatectomy: predicting risk factors for progression. Urology. (2003) 62:304–9. doi: 10.1016/S0090-4295(03)00373-X
6. Alkhateeb S, Alibhai S, Fleshner N, Finelli A, Jewett M, Zlotta A, et al. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. (2010) 183:145–50. doi: 10.1016/j.juro.2009.08.132
7. Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet. (2020) 396:1413–21. doi: 10.1016/S0140-6736(20)31553-1
8. Hunter T. The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease. Philos Trans R Soc Lond B Biol Sci. (1998) 353:583–605. doi: 10.1098/rstb.1998.0228
9. Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. (2001) 5:416–23. doi: 10.1016/S1367-5931(00)00223-4
10. Ventura JJ, Nebreda AR. Protein kinases and phosphatases as therapeutic targets in cancer. Clin Transl Oncol. (2006) 8:153–60. doi: 10.1007/s12094-006-0005-0
11. Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. (2002) 2:489–95. doi: 10.1016/S1534-5807(02)00148-X
12. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. (1999) 283:1544–8. doi: 10.1126/science.283.5407.1544
13. Liu F, Chernoff J. Protein tyrosine phosphatase 1B interacts with and is tyrosine phosphorylated by the epidermal growth factor receptor. Biochem J. (1997) 327:139–45. doi: 10.1042/bj3270139
14. Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. (2000) 275:41439–46. doi: 10.1074/jbc.M004852200
15. Lessard L, Stuible M, Tremblay ML. The two faces of PTP1B in cancer. Biochim Biophys Acta. (2010) 1804:613–9. doi: 10.1016/j.bbapap.2009.09.018
16. López-Zelada KA, Garibay-Díaz JC, Escobar-Arriaga E, León-Rodríguez E, de la Peña-López R, Esparza-López J, et al. PTP1B silencing reduces cell proliferation in primary cultures of breast cancer. Gaceta Mexicana Oncología. (2014) 13:144–51.
17. Soysal S, Obermann EC, Gao F, Oertli D, Gillanders WE, Viehl CT, et al. PTP1B expression is an independent positive prognostic factor in human breast cancer. Breast Cancer Res Treat. (2013) 137:637–44. doi: 10.1007/s10549-012-2373-1
18. Davidson B, Bock AJ, Holth A, Nymoen DA. The phosphatase PTPN1/PTP1B is a candidate marker of better chemotherapy response in metastatic high-grade serous carcinoma. Cytopathology. (2021) 32:161–8. doi: 10.1111/cyt.12921
19. Wang N, She J, Liu W, Shi J, Yang Q, Shi B, et al. Frequent amplification of PTP1B is associated with poor survival of gastric cancer patients. Cell Cycle. (2015) 14:732–43. doi: 10.1080/15384101.2014.998047
20. Hoekstra E, Das AM, Swets M, Cao W, van der Woude CJ, Bruno MJ, et al. Increased PTP1B expression and phosphatase activity in colorectal cancer results in a more invasive phenotype and worse patient outcome. Oncotarget. (2016) 7:21922–38. doi: 10.18632/oncotarget.v7i16
21. Labbé DP, Nowak DG, Deblois G, Lessard L, Giguère V, Trotman LC, et al. Prostate cancer genetic-susceptibility locus on chromosome 20q13 is amplified and coupled to androgen receptor-regulation in metastatic tumors. Mol Cancer Res. (2014) 12:184–9. doi: 10.1158/1541-7786.MCR-13-0477
22. Lessard L, Labbé DP, Deblois G, Bégin LR, Hardy S, Mes-Masson AM, et al. PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer. Cancer Res. (2012) 72:1529–37. doi: 10.1158/0008-5472.CAN-11-2602
23. Wu C, Zhang L, Bourne PA, Reeder JE, di Sant'Agnese PA, Yao JL, et al. Protein tyrosine phosphatase PTP1B is involved in neuroendocrine differentiation of prostate cancer. Prostate. (2006) 66:1125–35. doi: 10.1002/(ISSN)1097-0045
24. Kordan Y, Salem S, Chang SS, Clark PE, Cookson MS, Davis R, et al. Impact of positive apical surgical margins on likelihood of biochemical recurrence after radical prostatectomy. J Urol. (2009) 182:2695–701. doi: 10.1016/j.juro.2009.08.054
25. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. (1997) 24:395–406. doi: 10.1016/S0094-0143(05)70386-4
26. Eggener SE, Roehl KA, Smith ND, Antenor JA, Han M, Catalona WJ. Contemporary survival results and the role of radiation therapy in patients with node negative seminal vesicle invasion following radical prostatectomy. J Urol. (2005) 173:1150–5. doi: 10.1097/01.ju.0000155158.79489.48
27. Shaikh MP, Alite F, Wu MJ, Solanki AA, Harkenrider MM. Adjuvant radiotherapy versus wait-and-see strategy for pathologic T3 or margin-positive prostate cancer: A meta-analysis. Am J Clin Oncol. (2018) 41:730–8. doi: 10.1097/COC.0000000000000358
28. Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. (2020) 396:1422–31. doi: 10.1016/S0140-6736(20)31952-8
29. Kneebone A, Fraser-Browne C, Duchesne GM, Fisher R, Frydenberg M, Herschtal A, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. (2020) 21:1331–40. doi: 10.1016/S1470-2045(20)30456-3
30. Sargos P, Chabaud S, Latorzeff I, Magné N, Benyoucef A, Supiot S, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol. (2020) 21:1341–52. doi: 10.1016/S1470-2045(20)30454-X
31. Liu Z, Zhu X, He J, Lu J. Metabolic syndrome and its components predict the biochemical recurrence and adverse pathological features of patients following radical prostatectomy: a propensity score matching study. BMC Cancer. (2023) 23:50. doi: 10.1186/s12885-023-10507-z
32. Merkens L, Sailer V, Lessel D, Janzen E, Greimeier S, Kirfel J, et al. Aggressive variants of prostate cancer: underlying mechanisms of neuroendocrine transdifferentiation. J Exp Clin Cancer Res. (2022) 41:46. doi: 10.1186/s13046-022-02255-y
33. Wilson NS, Huntington ND. Small molecule. Big biology. Dual phosphatase inhibitor enters the immunotherapy fray. Immunol Cell Biol. (2024) 102:8–11. doi: 10.1111/imcb.12711
34. Baumgartner CK, Ebrahimi-Nik H, Iracheta-Vellve A, Hamel KM, Olander KE, Davis TGR, et al. The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity. Nature. (2023) 622:850–62. doi: 10.1038/s41586-023-06575-7
35. Gao L, Zhang X, Wang FR, Cao MF, Zhang XJ, Sun NN, et al. Chronic ethanol consumption up-regulates protein-tyrosine phosphatase-1B (PTP1B) expression in rat skeletal muscle. Acta Pharmacol Sin. (2010) 31:1576–82. doi: 10.1038/aps.2010.161
36. Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. (2011) 470:214–20. doi: 10.1038/nature09744
37. Green WJ, Ball G, Hulman G, Johnson C, Van Schalwyk G, Ratan HL, et al. KI67 and DLX2 predict increased risk of metastasis formation in prostate cancer-a targeted molecular approach. Br J Cancer. (2016) 115:236–42. doi: 10.1038/bjc.2016.169
38. Grindstad T, Skjefstad K, Andersen S, Ness N, Nordby Y, Al-Saad S, et al. Estrogen receptors α and β and aromatase as independent predictors for prostate cancer outcome. Sci Rep. (2016) 6:33114. doi: 10.1038/srep33114
Keywords: prostate cancer, PTP-1B, prostatectomy, prognostic factors, protein expression
Citation: Bourlon MT, Urbina-Ramirez S, Verduzco-Aguirre HC, Mora-Pineda M, Velazquez HE, Leon-Rodriguez E, Atisha-Fregoso Y and De Anda-Gonzalez MG (2024) Differences in the expression of the phosphatase PTP-1B in patients with localized prostate cancer with and without adverse pathological features. Front. Oncol. 14:1334845. doi: 10.3389/fonc.2024.1334845
Received: 07 November 2023; Accepted: 01 April 2024;
Published: 19 April 2024.
Edited by:
Mimma Rizzo, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, ItalyReviewed by:
Gaetano Pezzicoli, University of Bari Aldo Moro, ItalyCopyright © 2024 Bourlon, Urbina-Ramirez, Verduzco-Aguirre, Mora-Pineda, Velazquez, Leon-Rodriguez, Atisha-Fregoso and De Anda-Gonzalez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria T. Bourlon, bWFpdGVib3VybG9uQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.