
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 08 March 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1331643
This article is part of the Research Topic Radioligand Therapy in Prostate Cancer View all 5 articles
 Francesca Zacchi1,2
Francesca Zacchi1,2 Joan Carles2,3
Joan Carles2,3 Macarena Gonzalez2,3
Macarena Gonzalez2,3 Xavier Maldonado2,3
Xavier Maldonado2,3 Raquel Perez-Lopez2
Raquel Perez-Lopez2 Maria Eugenia Semidey3
Maria Eugenia Semidey3 Joaquin Mateo2,3*
Joaquin Mateo2,3*Despite the development of new therapies in the last few years, metastatic prostate cancer (PCa) is still a lethal disease. Radium-223 (Ra-223) is approved for patients with advanced castration-resistant prostate cancer (CRPC) with bone metastases and no visceral disease. However, patients’ outcomes are heterogenous, and there is lack of validated predictive biomarkers of response, while biomarkers for early identification of patients who benefit from treatment are limited. This case report describes a remarkable and durable response to Ra-223 in a CRPC patient with bone metastases who had rapidly progressed to many previous therapies; this response is now lasting for 5 years even after having stopped backbone androgen deprivation therapy (ADT). Here, we present the clinical course of this exceptional response, as well as comprehensive genomic and histopathology analyses on sequential biopsies acquired before and after therapy. Additionally, we review current knowledge on predictive and response biomarkers to Ra-223 in metastatic prostate cancer.
PCa is one of the most common malignancies in men worldwide and significantly contributes, in the form of metastatic PCa, to increased mortality rates in men globally (1).
Systemic treatments represent the standard approach for metastatic diseases. PCa is driven by androgen receptor (AR)-signaling, making ADT with luteinizing hormone-releasing hormone (LHRH) agonists and AR-targeting drugs, such as abiraterone acetate or AR inhibitors, the mainstay of treatment for advanced PCa.
Beyond AR targeting therapy, taxane-based chemotherapy, and the advent of new drugs such as lutetium-177-PSMA (Prostate Specific Membrane Antigen), Ra-223, and Poly (ADP-ribose) polymerase (PARP) inhibitors, have improved life quality and overall survival (OS) in the setting of metastatic castration-resistant prostate cancer (mCRPC), but metastatic PCa remains a lethal condition (2–7).
Precision medicine, based on tailoring treatment strategies to the molecular make up of individual tumors, holds promise for improving metastatic PCa care; however, currently there are very few validated biomarkers to personalize treatment selection in advanced PCa (8, 9).
Bone is the most common site of metastasis in advanced PCa with a higher prevalence of osteoblastic, rather than osteolytic, metastases (10). These metastatic sites are frequently associated with pain and skeletal-related events, such as pathological fractures, spinal cord compression, or hypercalcemia, which reduce the quality of life and impact survival. The treatment of bone metastases in men with PCa is palliative with the goals to improve survival, relieve pain, improve mobility, and prevent complications (11). Nevertheless, the standard-of-care imaging techniques [e.g., computed tomography (CT) and bone scan (BS)] fail to give an accurate assessment of the burden of bone metastases, as well as to monitor changes in response to treatment; at present, there are no established radiographic criteria for response to systemic therapy in bone disease (12, 13).
Recent studies have shown the high diagnostic performance of new imaging techniques as diffusion-weighted MRI (Magnetic Resonance Imaging) and PSMA-PET (positron emission tomography) to detect bone metastases, exploring their role as response biomarkers during anticancer therapy, but further prospective evaluations are needed (14–16).
Moreover, another important challenge in the treatment of bone metastatic PCa is the different tumour microenvironment (TME) compared with that of soft tissue metastases, resulting in a suboptimal response of bone metastatic CRPC to immune checkpoint therapy (ICT) (17, 18). Recent evidence, as the TGF–β (Transforming growth factor-beta) involvement in the restraining development of Th1 (T-helper type 1) subset or the increased myeloid and neutrophil immune subsets in bone microenvironment after ICT therapy among others, suggest the strategy of targeting the microenvironment associated with bone metastases to improve anti-tumour responses with ICT, leaving this field an open challenge for the future.
Ra-223 is a targeted alpha emitter that acts as a calcium mimetic, forming complexes with hydroxyapatite and selectively binding areas of increased bone turnover such as osteoblastic bone metastases. The alpha particles, with a short path length in tissue and a high linear energy transfer, allow Ra-223 to kill an increased number of cells in its target tissue sparing the surrounding normal tissues (19, 20). In particular, the high-energy alpha-particle radiation induces mainly DNA double-stranded breaks (DSBs) that result in a potent and highly localized cytotoxic effect in the target areas (21). To date, Ra-223 therapy is approved in metastatic CRPC patients with symptomatic bone metastases and no visceral metastases or malignant lymphadenopathy larger than 3cm, while its role in asymptomatic patients is still not well defined (22, 23).
Here, is presented the case of a metastatic CRPC patient affected by few and oligosymptomatic bone metastases, with an exceptional and durable response after 5 years from the starting of Ra-223 therapy and a concomitant suspension of ADT. The patient is still clinically asymptomatic with a persistent radiological response and undetectable PSA (Prostate specific antigen) levels.
A 65-years-old man with an Eastern Cooperative Oncology Group Performance Status 0 (ECOG-PS) without comorbidities was diagnosed with localized PCa and underwent a laparoscopic radical prostatectomy plus a lymphadenectomy in January 2014, confirming an adenocarcinoma, characterized by a Gleason Score (GS) of 9 (4 + 5), pT2N0 (0/14 nodes positive), with affected surgical margins.
Next generation targeted sequencing (NGS) of the diagnostic biopsy shows pathogenic/likely pathogenic alterations in PTEN (exon8:c.968dupA:p.N323fs), TP53 (exon6:c.583A>T:p.I195F) with associated TP53 deletion, AASXL1 (exon12:c.2831dupT:p.L944fs) and FANCL (exon14:c.1096_1099dup:p.T367fs) (Figure 1).
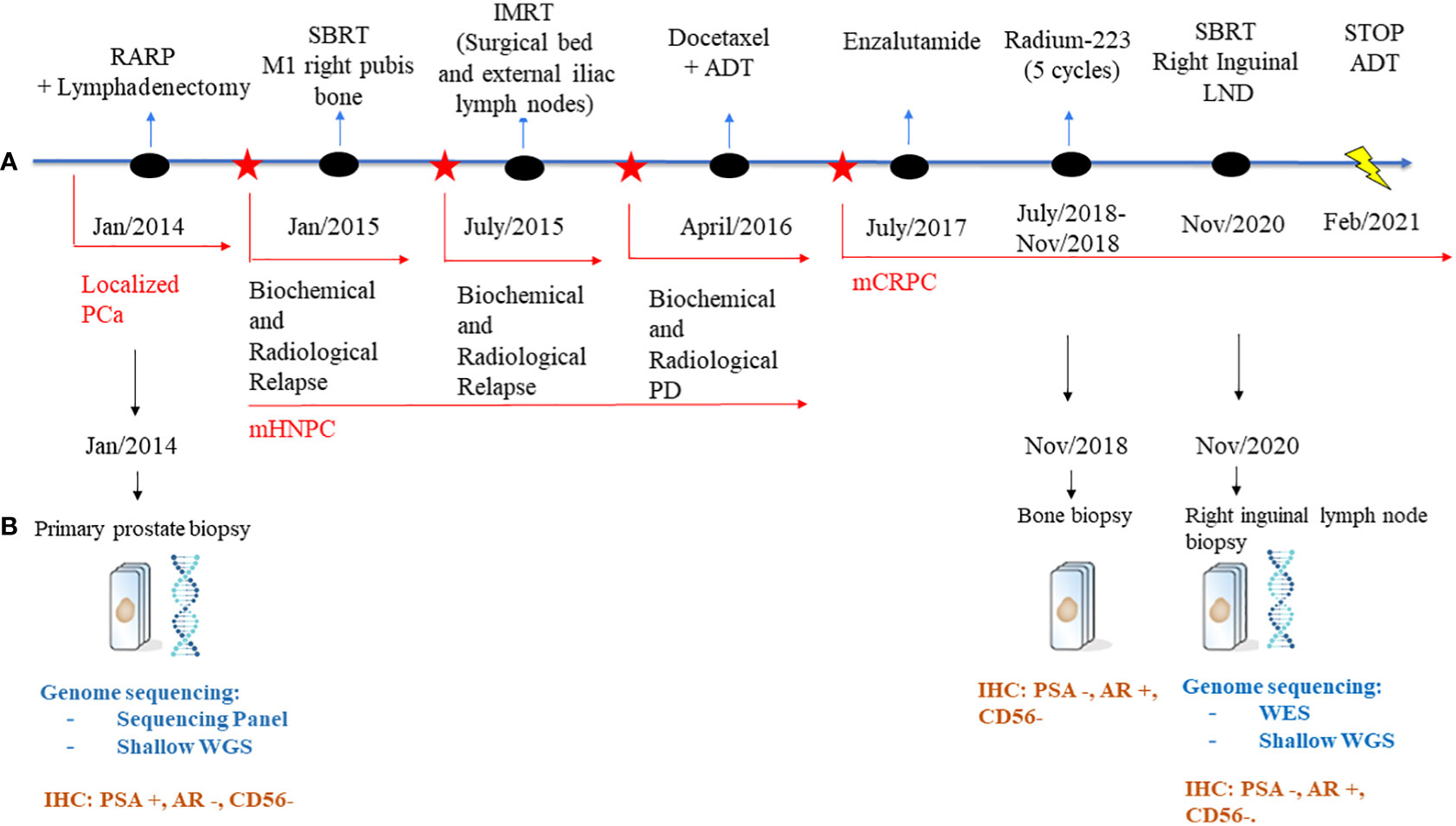
Figure 1 Schematic representation of the patient’s treatment history. (A) Treatment timeline. (B) Genome sequencing and IHC performed on the biopsies throughout the disease course. PCa, Prostate Cancer; RARP, Radical prostatectomy; ADT, adrenal deprivation therapy; PD, progression disease; mHNPC, metastatic hormone naïve prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; LNDs, lymph nodes; NGS, Next generation sequencing; WES, whole genome sequencing; WGS, whole genome sequencing; IHC, immunohistochemistry.
Eleven months after surgery, the patient presented biochemical relapse with a PSA doubling time of 2.5 months; a 18 F-choline PET/CT scan revealed a single metastasis in the right pubis bone for which in January 2015 Stereotactic Body Radiation Therapy (SBRT) was administered (25 Gy in 3 fractions,fr). Nevertheless, the PSA continued to rise and in June 2015 a new 18 F-choline PET/CT scan showed a local relapse on the surgical bed and on external iliac lymph nodes for which received an Intensity-modulated radiation therapy (IMRT with a total dose of 70 Gy) on the sites of relapse.
In March 2016, the patient presented a biochemical progression without radiological relapse on 18 F-choline PET/CT scan but with the evidence of two small new bone metastases (D10 and left ischiopubic bone metastases) on whole-body MRI. Because of this metastatic spread, the patient started a systemic treatment with ADT and Docetaxel (baseline PSA: 6.023 ng/dL), completing 6 cycles of chemotherapy and achieving ten months after the end of chemotherapy a complete biochemical and radiological response. However, the patient progressed to a metastatic CRPC status by biochemical criteria in June 2017 (PSA 8.32 ng/mL; testosterone 0.13 ng/dL) and started treatment with the AR inhibitor enzalutamide, achieving as best response stable disease by Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (12), but with continuous rising PSA. Due to lack of symptoms, he continued enzalutamide until radiographic progression after 11 months of treatment. Progression was documented with new bone metastases confirmed on Bone and CT scans: an osteolytic bone metastasis with a soft tissue component in the right ileopubic ramus and an osteoblastic bone metastasis in the right ischiopubic ramus (Figure 2).
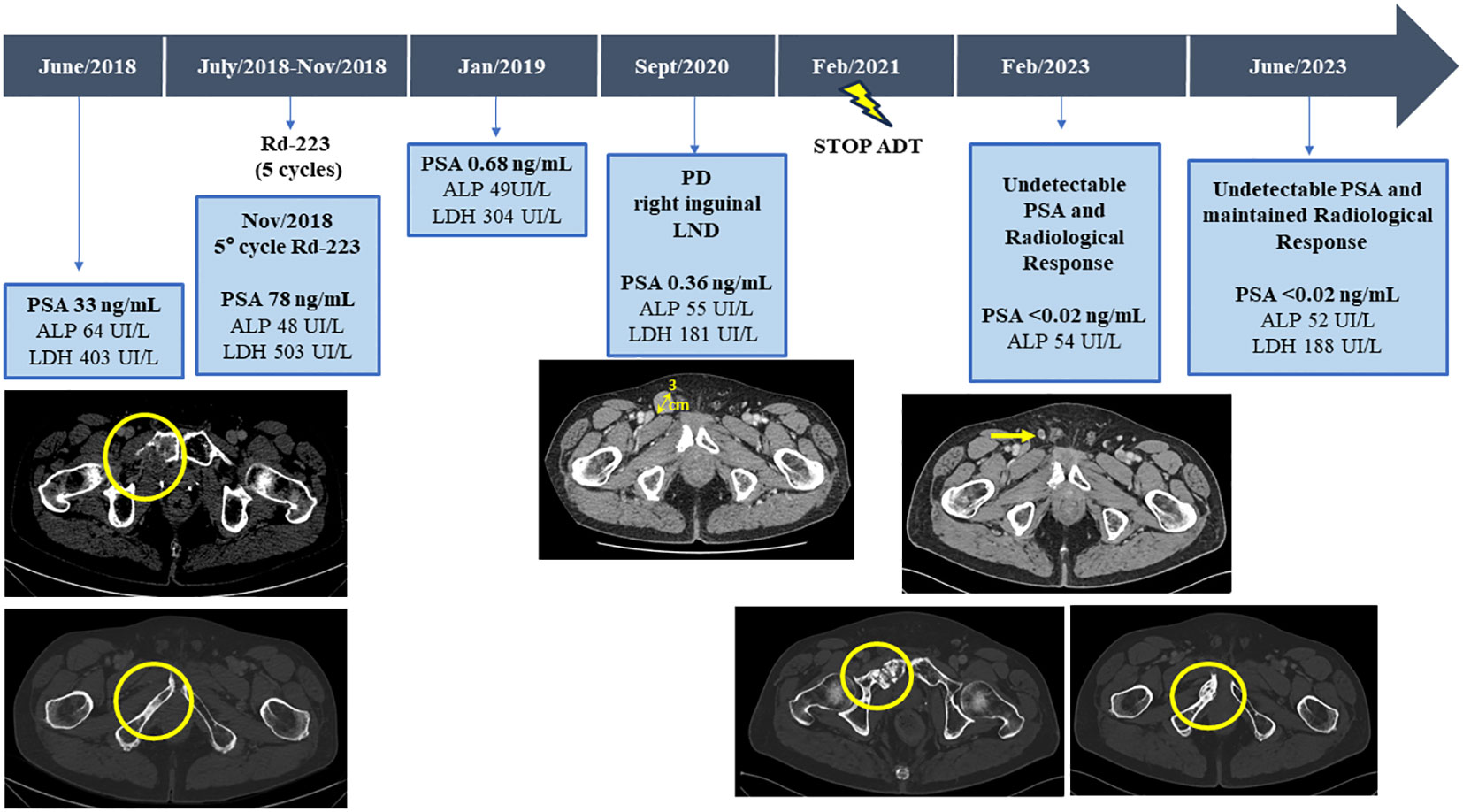
Figure 2 Serum bone biomarkers values (ALP, LDH, and PSA) and CT scans before and after third line with Radium-223 treatment, throughout the disease course. ALP, alkaline phosphatase; LDH, lactic acid dehydrogenase; PSA, Prostate-specific antigen; CT, computed tomography.
Therefore, with a baseline serum PSA of 33ng/mL, alkaline phosphatase (ALP) of 64UI/L and lactic acid dehydrogenase (LDH) of 403UI/L, the patient started a third line with Ra-223 as part of clinical trial exploring the role of Ra-223 in asymptomatic or pauci-symptomatic patients (EXCAAPE phase II trial) from July 2018 to November 2018. While the possibility of external Beam Radiation Therapy (EBRT) was evaluated, Ra-223 was finally prioritized due to accumulated exposure to radiation in the pelvis from the prior treatments in 2015 and the rapid PSA kinetics. Before starting Ra-223, the patient reported grade 1 lumbar pain as the only symptom, not requiring of painkillers. After the first cycle of Ra-223, he reported acute pain in right hemipelvis, which limited walking for a few days and then improved. The same pattern occurrent after each of the Ra-223 administrations, with temporary increases in pain, compromising mobilization, in the region of the right hemipelvis bone metastasis and concomitant increases in LDH and PSA but stable ALP (PSA 33ng/mL, ALP 71UI/L and LDH 518UI/L before the second cycle of Ra-223; PSA 37ng/mL, LDH 455UI/L and ALP 55UI/L before the third cycle; PSA 47ng/mL, LDH 594 UI/L, ALP 48 UI/L before the fourth cycle and PSA 78ng/mL, ALP 48UI/L and LDH 503UI/L before the fifth cycle). Due to increased pain and rising PSA, new imaging tests were performed after the third cycle of Ra-223, showing stable disease on bone scan but a cortical fracture on the lytic ileopubic lesion with associated soft tissue component on the CT scan.
After 5 cycles of Ra-223, and in the context of increasing pain, it was agreed with the patient to terminate therapy and not to administer the planned 6th cycle of Ra-223. Finally, the bone pain was controlled with non-steroidal anti-inflammatory drugs (NSAIDs), not requiring further radiation therapy.
In January 2019, three months after finishing therapy with Ra-223 and with no other intercurrent therapies, the patient underwent a restaging assessment with a bone and CT scans showing stable disease and an unexpected decrease of PSA (0,68 ng/mL) and started radiological and biochemical follow-ups with a progressive decline of the PSA until a nadir of 0,1 ng/mL in April 2019. A biopsy on the right ileopubic bone lesion was performed prior to the imaging evaluation, confirming the presence of prostate adenocarcinoma cells, with a strong expression in IHC (immunohistochemistry) of Androgen receptor (AR) and NKX3.1, a weak expression of PSA and the negativity of neuroendocrine markers (Chromogranin A, CD56) (Figure 3).
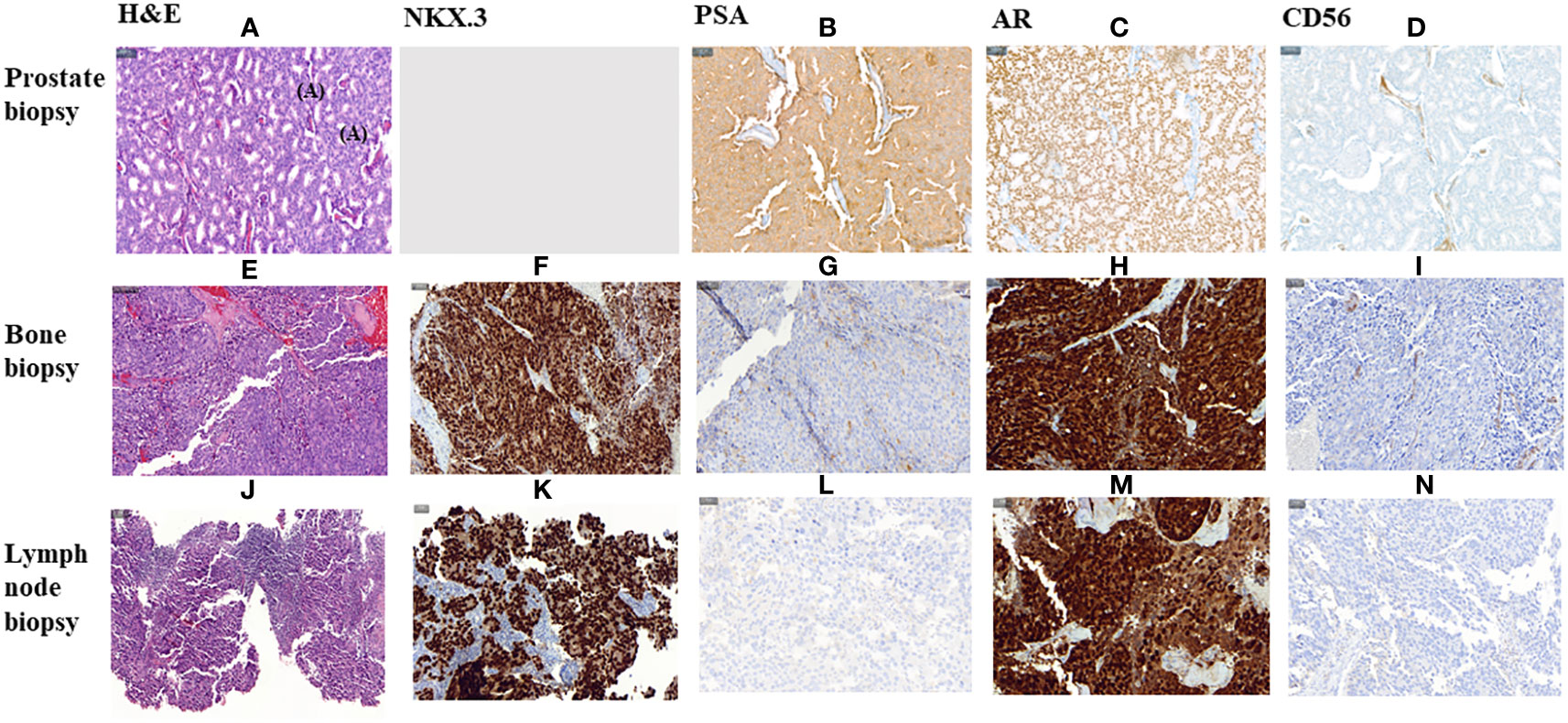
Figure 3 Histological morphology on H&E stains and IHC characteristics of FFPE section of a primitive prostate biopsy, metastatic bone biopsy and metastatic lymph node biopsy from Prostate Cancer adenocarcinoma. (A–I, K–N). magnification 200X. (J) magnification 100X. HE, Haematoxylin and Eosin; IHC, Immunohistochemistry; PSA, Prostate specific antigen; AR, Androgen Receptor.
The patient continued follow-up visits. A few months later, an osteoporotic fracture in the L5 vertebral body was diagnosed, with no signs of metastatic disease by MRI at that location, and the patient started bisphosphonates (Zometa 4mg every three months).
The PSA values remained <1 during 2019 and 2020, and the patient was mostly asymptomatic. In September 2020, an abnormal right inguinal lymph node was noted at the physical exam at one of the regular follow-up visits, with a PSA of 0,36 ng/mL. CT and bone scan were performed, and the only abnormality beyond the residual images in the bone disease was this palpable 3 cm right inguinal lymph node; an US (ultrasound)-guided biopsy of the node was performed, confirming the diagnosis of metastasis of prostate adenocarcinoma. IHC assessment of the lymph node revealed a strong expression of AR and NKX3.1, and weak expression of PSA (in line with the low PSA levels in serum) and negativity for neuroendocrine markers Chromogranin A and CD56 (Figure 3). Whole-exome sequencing of the lymph node biopsy shows important AR amplification (66 copies) and MYC and FOXA1 amplification, as well as PTEN and FANCA deletions. The original TP53 mutation detected in the primary tumor (p.I195F) was also detected, confirming the common origin (Figure 1).
In November 2020, the patient received radiotherapy on the local lymph node progression (35Gy in 8 fractions), achieving a partial radiological response in the follow up scans (<1cm diameter) with no other evidence of extra-osseus disease; PSA during 2021 remained low (PSA nadir <0.02 ng/mL).
Two and a half years after the last cycle of Ra-223, the patient decided to stop ADT in February 2021, due to concerns about osteoporosis and cardiovascular risks.
Despite the suspension of ADT therapy, 5.5 years after the start of Ra-223 therapy the patient remains asymptomatic, with a maintained radiological response, with no PSA detection in blood (PSA <0.02 ng/mL) and normalization of other disease-related biomarkers (ALP 52UI/L and LDH 188UI/L).
Ra-223 is a life-prolonging agent in patients affected by bone-metastatic CRPC but many open questions remain about its use in clinical practice. In the registration phase III trial (ALSYMPCA), symptomatic bone metastatic CRPC patients who have received best standard of care were treated with Ra-223 and showed a significantly better OS than the placebo group, achieving a median OS of 14.9 months vs 11.3 months (HR 0,70, 95%CI 0,58-0,83). However, the benefit was unclear in the subgroup of patients with less than six bone metastases (23), such as the case presented here. The use of Ra-223 in oligo or asymptomatic patients is further supported by results from international Expanded access program (iEAP), and from a phase II study of our group in asymptomatic patients who progress after first line hormonal therapy in metastatic CRPC demonstrating a PFS of 5.5 months (24, 25).
The optimal time for using this therapy during is a matter of debate since the increase skeletal related events observed in the ERA223 trials (26). Ra-223 is a bone-targeting agent that mimics calcium ions and is adsorbed at bone sites of active mineralization and high osteoblastic turnover (20). For this reason, Ra-223 principally targets PCa metastatic foci where osteoblasts surround PCa cells but can also accumulate in other locations with high bone turnover, such as degenerative bone disease (osteoporosis) or recently (micro)fracture, increasing the risk of fracture.In the case here presented, however, the patient presented a mixture of blastic and lytic metastasis, but the response to therapy was probably homogeneous among them, considering none of the lesions has relapsed after 5 years. This highlights the need for further research for assessing response and progression in bone lesiones, which are limited with current standard-of-care imaging techniques, as well as on the biology of bone metastasis and them mechanisms of action of this agent.
The risk of osteoporosis in metastatic CRPC patients is already increased using continuous ADT. In this case, the patient decided to stop ADT after 3 years of maintained response. While continuous ADT remains the standard-of-care among metastatic CRPC patients, one could argue the role of ADT in patients with exceptionally prolonged responses and no evidence of residual disease is unclear. While these cases are uncommon, and there is no clear agreement upon how to define an “exceptional response”, the fact that the disease has not returned after 2.5 years off-ADT is provoking and goes in line to current research aiming to minimize the impact of testosterone suppression among men with prostate cancer (27, 28). Recent results from the EORTC1333/PEACE III trial (29, 30) have shown that the risks of osteoporosis associated to ADT and Ra-223 can be controlled with appropriate use of bisphosphonates. Therefore, is important to assess the state of bone health and patient’s baseline fracture risk before starting treatment with Ra-223 to evaluate the risk benefit ratio and preventive measures such as the use of VitD, Calcium supplementation, Bisphosphonate or Denosumab.
An important challenge with Ra-223 therapy is response assessment, considering the predominantly bone disease. Indeed, the RECIST version 1.1 do not define progression or response of bone metastases, as are considered non measurable disease, and Prostate Cancer Clinical Trials Working Group 3 (PCWG3) defines criteria for bone progression on bone scan but fails to establish criteria for bone response assessment (12, 13). Considering Ra-223 is used in late-stage prostate cancer, early readouts of patient benefit are needed to avoid prolonged exposure to inefficient therapies. Decrease of serum PSA is not necessarily expected during Ra-223 therapy and do not seem to be a good marker of patient benefit; this could be explained by the Ra-223 mechanism principally directed towards the bone microenvironment then to the PCa cells directly. In this case, the patient experienced a PSA rise on therapy concomitantly to increased pain, followed by a profound drop in PSA after therapy; this was possible secondary to the lack of PSA expression in his AR positive metastatic biopsies, and not necessarily translating antitumor effect directly. According to the literature, generally low PSA secretors present small cell neuroendocrine features with lower AR transcriptional signature scores. However, this case presented strong expression of AR and NKX3.1, and no genomic loss of KLK3 (the gene encoding for PSA) was detected. Whether this was the result of subclonal selection, or post-genomic mechanism inactivating PSA translation remains unclear in this case (31, 32).
Overall, this further supports that PSA levels are not an appropriate marker to monitor response to Ra-223. Conversely, high basal total level of serum ALP and LDH prior Ra-223 treatment and the correlation with worse outcomes in OS and PFS, suggest their rule as prognostic biomarkers; In addition, the relationship between ALP levels changes and OS during Ra-223 treatment suggest its rule as a response biomarker in metastatic CRPC with bone metastases during Ra-223 (33). Further research into novel imaging techniques such as diffusion-weighted MRI or PET imaging, or other biomarkers such as CTC (circulating tumor cells) or cell-free DNA burden in circulation, to inform clinical decisions when using Ra-223 is also needed (34–36).
There is also a lack of validated predictive biomarkers to identify men who are more likely to derive significant benefit from Ra-223 therapy. Since the induction of DNA damage is the major mechanism of action of Ra-223, it has been hypothesized that Ra-223 could be more effective in patients with genomic defects in DNA repair genes (37, 38). Multiple retrospective studies have explored the efficacy of Ra-223 in metastatic CRPC patients with mutations in DNA repair genes (39–42). Overall, a trend towards more favorable ALP response rates, more durable responses and a more favorable OS figures has been observed in this molecularly defined subset of prostate cancer, but conclusive data is missing, and no prospective validation has been conducted. The case here reported presented loss of the FANCA gene, but the impact of this event in drug sensitivity in unclear. No other alterations in DDR genes were detected in either of the two analyzed biopsies. In these NGS analysis of the post-Ra-223 bone biopsy and lymph node relapse, the main findings were AR amplification, in line with prior use of hormonal therapies, and loss-of-function events in TP53 and PTEN, commonly associated to poor prognosis in prostate cancer, which makes this exceptional response even more remarkable. Indeed, the patient had before experienced relatively short progression-free survival to ADT, docetaxel, and enzalutamide.
Ra-223 represents a therapeutic option within the wide therapeutic armamentarium for the metastatic CRPC patients with bone metastases.
This case presented with few and oligosymptomatic bone metastases and derived exceptionally prolonged benefit from Ra-223 monotherapy allowing even for ADT discontinuation and remaining disease-free after 5 years. This case underscores the lack of validated tools to guide clinical decisions when using this drug, stressing the need for further research on imaging and molecular biomarkers to identify patients who may benefit from Ra-223 treatment, aiming to ultimately improve quality of life and survival of metastatic CRPC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee for Clinical Research at the Vall d’Hebron Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
FZ: Writing – original draft. JC: Writing – review & editing. MG: Writing – review & editing. XM: Writing – review & editing. RP-L: Writing – review & editing. MS: Writing – review & editing. JM: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge funding from CRIS Cancer Foundation (grant TCL_2020-10 to JM) and Fundación AECC (LABAE20019MATE to JM) and FERO Foundation. RP-L is supported by LaCaixa Foundation, a CRIS Foundation Talent Award TALENT19-05, the FERO Foundation, the Instituto de Salud Carlos III-Investigacion en Salud PI18/01395 and PI21/01019 and the Prostate Cancer Foundation 18YOUN19.
JM has served as an advisor for AstraZeneca, Amunix/Sanofi, Daichii-Sankyo, Janssen, MSD; Pfizer, Roche, and Nuage Therapeutics. He is PI for grants to institutions by AstraZeneca, Pfizer, and Amgen. JC received consulting and speaker’s fees from Bayer, Johnson & Johnson, Bristol-Myers Squibb, Astellas Pharma, Pfizer, Sanofi, MSD Oncology, Roche, AstraZeneca and Asofarma, and research support from AB Science, Aragon Pharmaceuticals, Arog Pharmaceuticals INC, Astellas Pharma., Astrazeneca AB, Aveo Pharmaceuticals INC, Bayer AG, Blueprint Medicines Corporation, BN Immunotherapeutics INC, Boehringer Ingelheim, Bristol-Myers Squibb International Corporation BMS, Clovis Oncology INC, Cougar Biotechnology INC, Deciphera Pharmaceuticals LLC, Exelixis INC, F. Hoffmann-La Roche LTD, Genentech INC, Glaxosmithkline, SA, Incyte Corporation, Janssen-Cilag International NV, Karyopharm Therapeutics INC, Laboratoires Leurquin Mediolanum SAS, Lilly SA, Medimmune, Millennium Pharmaceuticals INC., Nanobiotix SA, Novartis Farmacéutica SA, Pfizer, S.L.U, Puma Biotechnology INC, Sanofi-Aventis SA, SFJ Pharma LTD. II, Teva Pharma S.L.U.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate cancer epidemiology and risk factors-A systematic review. Eur Urol. (2023) 84:191–206. doi: 10.1016/j.eururo.2023.04.021
2. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. (2019) 37:2974–86. doi: 10.1200/JCO.19.00799
3. Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. (2021) 39:2294–303. doi: 10.1200/JCO.20.03488
4. James ND, Clarke NW, Cook A, Ali A, Hoyle AP, Attard G, et al. Abiraterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year follow-up results from the STAMPEDE randomised trial (NCT00268476). Int J Cancer. (2022) 151:422–34. doi: 10.1002/ijc.34018
5. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. (2022) 27:5730. doi: 10.3390/molecules27175730
6. Fizazi K, Gillessen S, ESMO Guidelines Committee. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann Oncol. (2023) 34:557–63. doi: 10.1016/j.annonc.2023.02.015
7. Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. (2023) 185:178–215. doi: 10.1016/j.ejca.2023.02.018
8. Hamid AA, Huang HC, Wang V, Chen YH, Feng F, Den R, et al. Transcriptional profiling of primary prostate in metastatic hormone sensitive prostate cancer and association with clinical outcomes: correlative analysis of the E3805 CHAARTED trial. Ann Oncol. (2021) 32:1157–66. doi: 10.1016/j.annonc.2021.06.003
9. Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. (2012) 487:239–43. doi: 10.1038/nature11125
10. Sartor O, DiBiase S. Bone metastases in advanced prostate cancer: management. Post TW, editor. Waltham, MA: UpToDate (2023).
11. Ryan C, Stoltzfus KC, Horn S, Chen H, Louie AV, Lehrer EJ, et al. Epidemiology of bone metastases. Bone. (2022) 158:115783. doi: 10.1016/j.bone.2020.115783
12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
13. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. (2016) 34:1402–18. doi: 10.1200/JCO.2015.64.2702
14. Perez-Lopez R, Nava Rodrigues D, Figueiredo I, Mateo J, Collins DJ, Koh DM, et al. Multiparametric magnetic resonance imaging of prostate cancer bone disease: correlation with bone biopsy histological and molecular features. Invest Radiol. (2018) 53:96–102. doi: 10.1097/RLI.0000000000000415
15. Shagera QA, Karfis I, Kristanto P, Spyridon S, Diamand R, Santapau A, et al. PSMA PET/CT for response assessment and overall survival prediction in patients with metastatic castration-resistant prostate cancer treated with androgen receptor pathway inhibitors. J Nucl Med. (2023) 64:1869–75. doi: 10.2967/jnumed.123.265874
16. Perez-Lopez R, Mateo J, Mossop H, Blackledge MD, Collins DJ, Rata M, et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: a pilot study. Radiology. (2017) 283:168–77. doi: 10.1148/radiol.2016160646
17. Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. (2019) 179:1177–90.e13. doi: 10.1016/j.cell.2019.10.029
18. Subudhi SK, Siddiqui BA, Aparicio AM, Yadav SS, Basu S, Chen H, et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer. (2021) 9:e002919. doi: 10.1136/jitc-2021-002919
19. Bruland ØS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. (2006) 12:6250s–7s. doi: 10.1158/1078-0432.CCR-06-0841
20. Sindhu KK, Nehlsen AD, Stock RG. Ra-223 for metastatic castrate-resistant prostate cancer. Pract Radiat Oncol. (2022) 12:312–6. doi: 10.1016/j.prro.2022.03.004
21. Henriksen G, Breistøl K, Bruland ØS, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. (2002) 62:3120–5.
22. European Medicines Agency. Ra-223 dichloride (2013). Available at: https://www.ema.europa.eu/en/documents/product-information/xofigo-epar-product-information_en.
23. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. (2013) 369:213–23. doi: 10.1056/NEJMoa1213755
24. Carles J, Alonso-Gordoa T, Mellado B, Méndez-Vidal MJ, Vázquez S, González-Del-Alba A, et al. Radium-223 for patients with metastatic castration-resistant prostate cancer with asymptomatic bone metastases progressing on first-line abiraterone acetate or enzalutamide: A single-arm phase II trial. Eur J Cancer. (2022) 173:317–26. doi: 10.1016/j.ejca.2022.06.057
25. Heidenreich A, Gillessen S, Heinrich D, Keizman D, O'Sullivan JM, Carles J, et al. Radium-223 in asymptomatic patients with castration-resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer. (2019) 19:12. doi: 10.1186/s12885-018-5203-y
26. Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:408–19. doi: 10.1016/S1470-2045(18)30860
27. Arafa AT, Blader LR, Ramakrishna K, Engle J, Ryan CJ, Zorko NA, et al. Clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with abiraterone with or without ongoing androgen deprivation therapy: A retrospective case-control study. Prostate. (2023) 83:1279–84. doi: 10.1002/pros.24589
28. Ohlmann CH, Jäschke M, Jaehnig P, Krege S, Gschwend J, Rexer H, et al. LHRH sparing therapy in patients with chemotherapy-naïve, mCRPC treated with abiraterone acetate plus prednisone: results of the randomized phase II SPARE trial. Prostate Cancer Prostatic Dis. (2022) 25:778–84. doi: 10.1038/s41391-022-00533-6
29. Maughan BL, Kessel A, McFarland TR, Sayegh N, Nussenzveig R, Hahn AW, et al. Radium-223 plus enzalutamide versus enzalutamide in metastatic castration-refractory prostate cancer: final safety and efficacy results. Oncologist. (2021) 26:1006–e2129. doi: 10.1002/onco.13949
30. Gillessen S, Choudhury A, Rodriguez-Vida A, Nole F, Diaz EG, Roumeguere TA, et al. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. J Clin Oncol. (2021) 39(Suppl 15). doi: 10.1200/JCO.2021.39.15_suppl.5002
31. Aggarwal R, Romero GR, Friedl V, Weinstein A, Foye A, Huang J, et al. Clinical and genomic characterization of Low PSA Secretors: a unique subset of metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis. (2021) 24:81–7. doi: 10.1038/s41391-020-0228-0
32. Han H, Lee HH, Choi K, Moon YJ, Heo JE, Ham WS, et al. Prostate epithelial genes define therapy-relevant prostate cancer molecular subtype. Prostate Cancer Prostatic Dis. (2021) 24:1080–92. doi: 10.1038/s41391-021-00364-x
33. Van der Zande K, Oyen WJG, Zwart W, Bergman AM. Radium-223 treatment of patients with metastatic castration resistant prostate cancer: biomarkers for stratification and response evaluation. Cancers (Basel). (2021) 13:4346. doi: 10.3390/cancers13174346
34. Parker C, Tunariu N, Tovey H, Alonzi R, Blackledge MD, Cook GJR, et al. Radium-223 in metastatic castration resistant prostate cancer: whole body diffusion-weighted MRI to assess response. JNCI Cancer Spectr. (2023) 7:pkad077. doi: 10.1093/jncics/pkad077
35. Probst S, Bjartell A, Anand A, Skamene T, Ferrario C. Interval changes in PSMA PET/CT during radium-223 therapy for metastatic bone disease from castration-resistant prostate cancer. Nucl Med Mol Imaging. (2022) 56:188–95. doi: 10.1007/s13139-022-00754-6
36. Carles J, Castellano D, Méndez-Vidal MJ, Mellado B, Saez MI, González Del Alba A, et al. Circulating tumor cells as a biomarker of survival and response to radium-223 therapy: experience in a cohort of patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. (2018) 16:e1133–9. doi: 10.1016/j.clgc.2018.07.013
37. Merseburger AS, Waldron N, Ribal MJ, Heidenreich A, Perner S, Fizazi K, et al. Genomic testing in patients with metastatic castration-resistant prostate cancer: A pragmatic guide for clinicians. Eur Urol. (2021) 79:519–29. doi: 10.1016/j.eururo.2020.12.039
38. Chung JS, Morgan TM, Hong SK. Clinical implications of genomic evaluations for prostate cancer risk stratification, screening, and treatment: a narrative review. Prostate Int. (2020) 8:99–106. doi: 10.1016/j.prnil.2020.09.001
39. Castro E, Lozano R, Saez MI, De Giorgi U, Aragon I, Romero-Laorden N, et al. Impact of germline mutations in homologous recombination (HR) genes on the response to Radium-223 for metastatic castration resistant prostate cancer (mCRPC). Ann Oncol. (2019) 30(Suppl 5):v343–4. doi: 10.1093/annonc/mdz248.033
40. Steinberger AE, Cotogno P, Ledet EM, Lewis B, Sartor O. Exceptional duration of radium-223 in prostate cancer with a BRCA2 mutation. Clin Genitourin Cancer. (2017) 15:e69–71. doi: 10.1016/j.clgc.2016.09.001
41. Isaacsson Velho P, Qazi F, Hassan S, Carducci MA, Denmeade SR, Markowski MC, et al. Efficacy of radium-223 in bone-metastatic castration-resistant prostate cancer with and without homologous repair gene defects. Eur Urol. (2019) 76:170–6. doi: 10.1016/j.eururo.2018.09.040
42. Van der Doelen MJ, Isaacsson Velho P, Slootbeek PHJ, Pamidimarri Naga S, Bormann M, van Helvert S, et al. Impact of DNA damage repair defects on response to radium-223 and overall survival in metastatic castration-resistant prostate cancer. Eur J Cancer. (2020) 136:16–24. doi: 10.1016/j.ejca.2020.05.001
Keywords: Radium-223, bone response, bone metastases, metastatic castration-resistant prostate cancer, prognosis
Citation: Zacchi F, Carles J, Gonzalez M, Maldonado X, Perez-Lopez R, Semidey ME and Mateo J (2024) Case report: Exceptional and durable response to Radium-223 and suspension of androgen deprivation therapy in a metastatic castration-resistant prostate cancer patient. Front. Oncol. 14:1331643. doi: 10.3389/fonc.2024.1331643
Received: 01 November 2023; Accepted: 01 February 2024;
Published: 08 March 2024.
Edited by:
Arun Azad, Peter MacCallum Cancer Centre, AustraliaReviewed by:
Vincenza Conteduca, University of Foggia, ItalyCopyright © 2024 Zacchi, Carles, Gonzalez, Maldonado, Perez-Lopez, Semidey and Mateo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joaquin Mateo, am1hdGVvQHZoaW8ubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.