
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 17 June 2024
Sec. Cardio-Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1331472
This article is part of the Research TopicCase Reports in Cardio-Oncology: 2023View all 19 articles
Phosphoinositide 3-kinase (PI3K) inhibitors have shown synergistic anticancer effects with endocrine therapy against ER+/PIK3CA-mutated breast cancer. PI3K inhibitors for cancer therapy are becoming more common. There is an increasing need to understand their cardiac adverse events. In this report, we describe the features of near-fatal mixed arrhythmias in a patient who was undergoing a phase Ib clinical study of PI3Kα inhibitor with fulvestrant. Subsequently, the patient survived by cardiopulmonary resuscitation and therefore did not die. This case highlights that PI3K inhibitors can induce QT/QTc prolongation and predispose patients to TdP. The combination of QT/QTc prolongation in combination with prolonged cardiac repolarization, such as an AV block during treatment with PI3Kα inhibitor, may aggravate the occurrence of TdP. It is likely to be a safer strategy to adjust the standard of discontinuing drugs and continuing drugs (QTc interval was <500 and <60 ms at baseline) or choose other types of alternative treatment options. This report provided some ideas for clinicians to identify early and prevent the occurrence of fatal arrhythmias during anticancer treatment.
With different kinds of new anticancer drugs emerging, many heart-related adverse drug reactions (ADRs) have appeared (1). One crucial and potentially harmful marker is prolonged cardiac repolarization, which is reflected by a prolonged QT interval in the ECG (1, 2). Prolongation of the QT can develop into severe ventricular arrhythmia of the torsade de pointes (TdP), which can induce sudden death (3). The phosphoinositide 3-kinase (PI3K) pathway plays a key role in cell growth, survival, apoptosis, metabolism, and myocardial contractility (4, 5). The combination therapy of PI3K inhibitors has presented a better clinical activity particularly in patients who have PIK3CA-mutated tumors (6). PI3K signaling could control cardiac repolarization and increase action potential duration by regulating multiple ion channels (7, 8). However, mixed arrhythmias which are caused by PI3K inhibitors have not been reported previously. This report provides clinical empirical evidence, which we hope will help healthcare practitioners to identify and prevent potential severe arrhythmia during treatment with PI3K inhibitors.
A 73-year-old woman had a radical mastectomy for right breast cancer 6 years earlier and had no other history. This was followed by oral letrozole (2.5 mg/day) for approximately 5 years, oral exemestane (2.5 mg/day) for 8 weeks, oral chidamide (30 mg/3 day) for 4 weeks, and discontinuation of the drug 3 months before admission. The carcinoma recurred after a series of anticancer therapies failed. The patient’s repeated immunohistochemistry test results were as follows: ER (40%, +), PR (-), HER2 (0), KI67 (LI: 40%), and PI3KCA mutation. The initial laboratory tests were in the normal range (Table 1; Figure 1). A 12-lead ECG showed premature ventricular contraction (PVC), frequent bigeminy, and QT/QTc (398/410 ms) (Figure 2A). The patient met the project criteria of a phase Ib study of PI3K inhibitor with fulvestrant in PI3KCA-mutated metastatic breast cancer (project number: HS-10352–102), signed the informed consent, enrolled in the clinical study, and started to take the drug orally (4 mg/day). She took it for 28 days. The patient had no chronic disease, as only the trial drug was taken orally during the study. The ECG revealed frequent PVC, frequent bigeminy, and QT/QTc (440/446 ms) a week after the start of the study (Figure 2A). The laboratory results were in the normal ranges, but the ECG revealed QT/QTc of 410/457 ms, frequent PVC, frequent bigeminy, and second-degree AV block 2 weeks after the study (Figures 1A, 2A). The initial AV block was assessed as CTCAE V5.0 grade 1–2, and the study continued. We still found frequent PVC, frequent bigeminy, second-degree AV block, and QT/QTc (424/471 ms) 3 weeks after the study started (Figure 2A). At 4 weeks, the ECG showed QT/QTc of 524/534 ms and an almost complete AV block (Figure 2A). The second-degree AV block turned to an almost complete AV block, which was assessed as CTCAE V5.0 grade 3. The patient immediately withdrew from the clinical study and had a permanent pacemaker implanted.
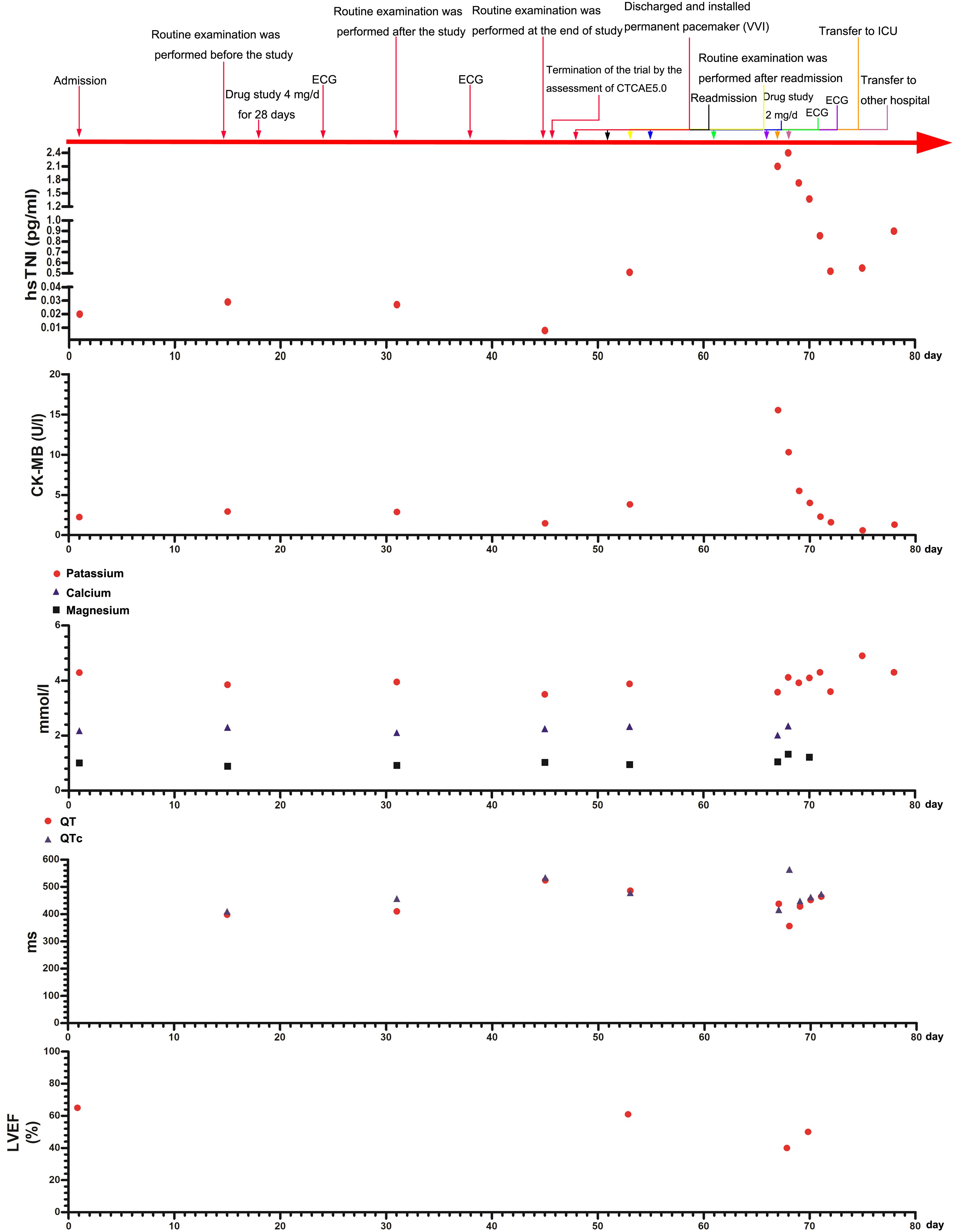
Figure 1 Main clinical and laboratory findings and therapeutic interventions from admission on day 0 to discharge from the ICU on day 78 are depicted. The QT interval correction method is after the Bazett formula. hs-TNI, hypersensitive troponin I; CK-MB, creatine kinase-MB; LVEF, left ventricular ejection fraction.
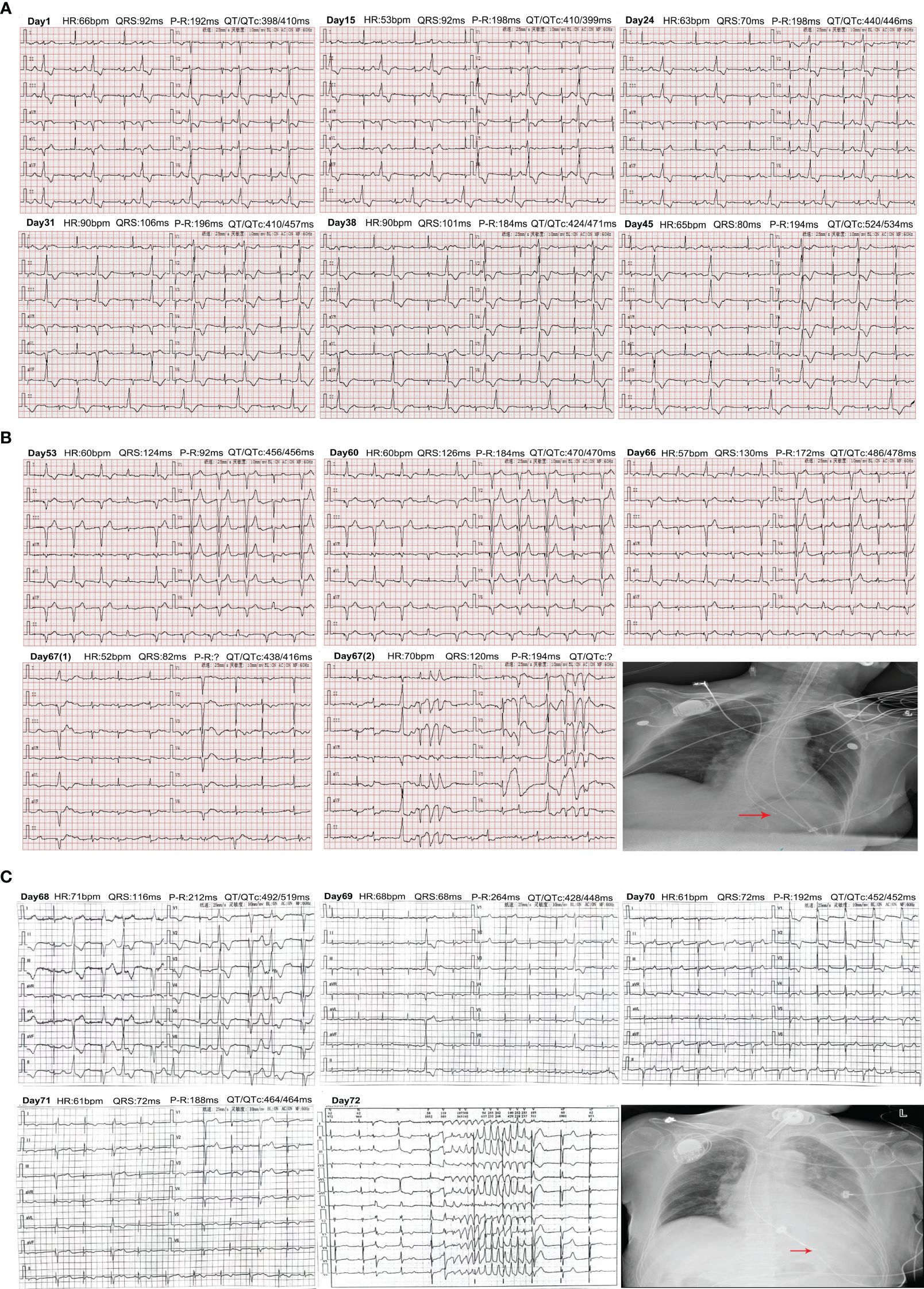
Figure 2 Changes of electrocardiogram from admission to discharge. (A) Changes in the ECG from admission to before the patient was installed. Days 1–24: ECG showed QT/QTc interval gradually prolongation. Days 31–45: ECG displayed worsening QT/QTc interval and atrioventricular block. (B) Changes in the ECG from enrolling in the study again to transfer to our hospital ICU. Days 53–66: ECG showed gradual prolongation of the QT/QTc and worsening of AV block. Day 67: Changes of ECG after being transferred to the ICU. The chest X-ray revealed that the location of the cardiac pacemaker was normal after CPR. (C) Changes in the ECG and chest X-ray in the patient after transfer to another hospital’s ICU. Days 68–71: ECG changes from days 1 to 4. Day 75: The occurrence of TdP was recorded on the 5th day. The chest X-ray displayed that the location of the cardiac pacemaker was normal.
We implanted a single-chamber permanent pacemaker (VVI). The ECG revealed QT/QTc of 456/456 ms, normal pacemaker sensing, and an almost complete AV block (Figure 2B). The laboratory results were also in the normal range. CTCAE V5.0 grade 3 turned to grades 1–2, so we adjusted the dose of the PI3K inhibitor (2 mg/day) and kept her on it for 28 days (Figure 1). We still observed a gradual prolongation of the QT/QTc (470/470 ms) after a week, QT/QTc (486/478 ms) after 2 weeks, and a consistently complete AV block (Figure 2B). The patient suddenly lost consciousness with no pulse after 12 days of dose adjustment. Continuous cardiopulmonary resuscitation (CPR) was performed, and the patient was transferred to the ICU. The patient’s circulation eventually recovered with short episodes of ventricular tachycardia (VT), and TdP frequently occurred (Figure 2B). The chest X-ray result revealed that the location of the cardiac pacemaker was normal (Figure 2B). We achieved hemodynamic stability with a continuous infusion of magnesium sulfate (4 mg/min) and isoprenaline (1–3 μg/min), and the patient maintained a normal potassium level.
The patient was transferred to the ICU of another hospital for further antiarrhythmic therapy on the next day. The echocardiography showed a weakened systolic function and LVEF of approximately 40% (Figure 1). The laboratory tests results are shown in Figure 1. The ECG showed prolongation of the QT/QTc, frequent PVC, and almost complete AV block for 4 days (Figure 2C). The patient still had occasional short episodes of TdP on the 5th day, but the myocardial enzymes were gradually declining, and the chest X-ray result revealed that the location of the cardiac pacemaker was normal (Figure 2C). Finally, the patient recovered consciousness, was weaned from mechanical ventilation, and was discharged from the ICU after 2 weeks.
Numerous clinical reports have shown that PI3K inhibitors combined with endocrine therapy in HR+/HER2- advanced breast carcinoma could prolong progression-free survival (PFS) and enhance the overall response rate (ORR), clinical benefit rate (CBR), and median overall survival (OS) compared with control treatments (9, 10). According to FDA drug instructions, monitoring blood glucose and glycosylated hemoglobin before and after treatment with PI3K inhibitors is important (11). One study reported an incidence of hyperglycemia of 65%, an incidence of diarrhea of 58%, and a non-infectious pneumonia onset of 1.8% during alpelisib treatment. However, its adverse cardiovascular drug reactions are not reported in the clinic.
The patient had no chest pain or other cardiac symptoms, but the occurrence of fatal arrhythmias seems to be traceable. She could be observed to have experienced a second-degree AV block approximately 2 weeks after the start of the study, and the second-degree AV block worsened to almost a complete AV block throughout the study. Whether PI3K inhibitors induce AV block is unknown and has rarely been reported. The effect of PI3K inhibitors on AV block is permanent or temporary and requires extensive clinical data. We also observed a gradual prolongation of the QT/QTc during the study. ECG revealed a QT/QTc (524/534 ms) of approximately 120 ms above baseline and complete AV block after 4 weeks. We observed the QT/QTc (456/456 ms) to be below 60 ms from baseline and complete AV block after the pacemaker was implanted. Her cardiac risk seemed to have been removed, only to develop cardiac arrest when she had an adjusted drug dose. We recorded three ECG changes of QT/QTc gradual prolongation, which eventually led to cardiac arrest. There is no threshold of QTc prolongation at which TdP is certain to occur. The QTc greater than 500 ms has been associated with a twofold to threefold higher risk for TdP, and each 10-ms increase contributes to approximately 5% to 7% exponential increase in risk (12). Therefore, it appears that it is difficult to predict when to stop the PI3K therapy. We found (1, 2) no evidence showing that fulvestrant can lead to QT prolongation, so we can speculate that PI3K inhibitors lead to the prolongation of the QT/QTc. Whether it is correlated with the dose of the drug is unknown. The drug effects on cardiac conduction function require further clinical data and pharmacokinetic studies.
We might find some clues to prevent the occurrence of fatal arrhythmia in this case. Firstly, we should fully assess the medical history, including older age (>65 years), sex, a congenital long QT syndrome (LQTS), a high baseline QTc, hypothyroidism, and induced QTc prolonged by other drugs (1, 2). Secondly, the risk classification of anticancer drugs and supportive drugs should also be fully estimated, and evaluation of the risk of QTc prolongation is necessary for moderate–high risk drugs (13, 14). Thirdly, monitoring the ECG and electrolyte levels is also crucial and the easiest way to get it. The latter report (1) also emphasized the standard of discontinuing the drugs when the QTc interval was ≥500 or ≥60 ms above baseline until the QTc interval was <500 or <60 ms above baseline. Electrolyte imbalances directly increase the risk of developing TdP, just as several drugs that cause QTc prolongation corrected the target value for serum K+ from 4 mEq/L to the upper limit of normal, and serum Ca2+ and Mg2+ within the normal range was safer (15).
We found a special phenomenon in which both the QT/QTc interval and AV block were gradually exacerbated in this case. We also noticed that the patient had frequent bigeminy before the pacemaker was implanted. There are reports that bigeminy can be another arrhythmia contributing to torsades (16). Although we cannot predict whether a patient will experience sudden cardiac death, the mixed arrhythmias should be noticed as early as possible. Some reports (17, 18) have shown that AV block-induced cardiac electrical and structural remodeling predisposes the heart to TdP in an AV dog model. Bhattad et al. (2023) (19) described that tachycardia usually shortens the QTc interval, but the adjustment is not instantaneous. Bradycardia increases the risk of QTc prolongation and therefore the risk for torsade de point. It is therefore logical that a patient with increasing AV block picture and subsequent bradycardia has an increased risk for torsade de points. Bradycardia and torsade de points are connected. The mechanism by which AV block and PI3K inhibitors induce TdP is that both can lead to prolonged cardiac repolarization and further promote action potential prolongation (18). We observed that the patient still experienced multiple short episodes of TdP when the QTc interval was <500 and <60 ms at baseline. Thus, we believe that the previous standard of discontinuing drugs and continuing drugs no longer seems to be safe.
The report stresses a comprehensive assessment of risk factors for QT prolongation before anticancer therapy. Frequent recording of ECG changes and electrolyte levels is necessary during the treatment. When the QT was gradually prolonged along with this type, arrhythmias of cardiac repolarization and action potential prolongation appear together. It should be of great concern to healthcare professionals. It is likely to be safer to choose other types of alternative treatment options or downregulate the standard for discontinuing and restarting drugs. No more data were collected from the other patients participating in the trial. We only report this one case, and there was no serum concentration monitoring in this case. We frequently monitored the patients’ ECG and found three kinds of arrhythmias. It seems that it could provide some proof for clinicians to identify early and prevent the occurrence of fatal arrhythmias during anticancer treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquires can be directed to the corresponding authors.
The studies involving humans were approved by the institution of Hubei Cancer Hospital for scientific research paper. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LZ: Conceptualization, Project administration, Supervision, Writing – review & editing. YZ: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. GC: Methodology, Resources, Writing – review & editing. FZ: Validation, Visualization, Writing – original draft. SL: Conceptualization, Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Giraud EL, Ferrier KRM, Lankheet NAG, Desar IME, Steeghs N, Beukema RJ, et al. The QT interval prolongation potential of anticancer and supportive drugs: a comprehensive overview. Lancet Oncol. (2022) 23(9):e406–15. doi: 10.1016/S1470–2045(22)00221–2
2. Salem JE, Nguyen LS, Moslehi JJ, Ederhy S, Lebrun-Vignes B, Roden DM, et al. Anticancer drug-induced life-threatening ventricular arrhythmias: a World Health Organization pharmacovigilance study. Eur Heart J. (2021) 42:3915–28. doi: 10.1093/eurheartj/ehab362
3. Salvi V, Karnad DR, Panicker GK, Kothari S. Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol. (2010) 159:34–48. doi: 10.1111/j.1476-5381.2009.00427.x
4. Tian X, Wu L, Jiang M, Zhang Z, Wu R, Miao J, et al. Downregulation of GLYAT facilitates tumor growth and metastasis and poor clinical outcomes through the PI3K/AKT/snail pathway in human breast cancer. Front Oncol. (2021) 11:641399. doi: 10.3389/fonc.2021.641399
5. Patel VB, Zhabyeyev P, Chen X, Wang F, Paul M, Fan D, et al. PI3Kα-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease. Nat Commun. (2018) 9:5390. doi: 10.1038/s41467-018-07812-8
6. Juric D, Janku F, Rodon J, Burris HA, Mayer IA, Schuler M, et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: A phase 1b clinical trial. JAMA Oncol. (2019) 5:e184475. doi: 10.1001/jamaoncol.2018.4475
7. Lu Z, Wu CYW, Jiang YP, Ballou LM, Clausen C, Cohen IS, et al. Suppression of Phosphoinositide 3-Kinase Signaling and Alteration of Multiple Ion currents in drug-induced Long QT syndrome. Sci Transl Med. (2012) 4:131ra50. doi: 10.1126/scitranslmed.3003623
8. Cohen IS, Lin RZ, Ballou LM. Acquired long QT syndrome and phosphoinositide 3-kinase. Trends Cardiovasc Med. (2017) 27:451–9. doi: 10.1016/j.tcm.2017.05.005
9. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo H, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
10. André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. (2021) 32:208–17. doi: 10.1016/j.annonc.2020.11.011
11. Novartis Pharmaceuticals Corporation. Highlights of prescribing information, PIQRAY (Alpelisib) tablets, for oral use. Initial U.S.: EB/OL (2022). Available at: https://www.accessdata.fda.gov/drugsatfdadocs/label/2019/212526s000lbl.pdf.
13. European Medicines Agency. ICH Topic E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (2005). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-14-clinical-evaluation-qt/qts-interval-prolongation-proarrhythmic-potential-non-antiarrhythmic-drugs-step-5_en.pdf (Accessed Nov 12, 2021).
14. United States Food and Drug Administration. E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (2012). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e14-clinical-evaluation-qtqtc-interval-prolongation-and-proarrhythmic-potential-non-antiarrhythmic-0 (Accessed Nov 12, 2021).
15. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. (2020) 17:474–502. doi: 10.1038/s41569–020-0348–1
16. Lerma C, Lee CF, Glass L, Goldberger AL. The rule of bigeminy revisited: analysis in sudden cardiac death syndrome. J Electrocardio. (2007) 40:78–88. doi: 10.1016/jelectrocard.2006.04.011
17. Van Bavel JJA, Pham C, Beekman HDM, Houtman MJC, Bossu A, Sparidans RW, et al. PI3K/mTOR inhibitor omipalisib prolongs cardiac repolarization along with a mild proarrhythmic outcome in the AV block dog model. Front Cardiovasc Med. (2022) 9:956538. doi: 10.3389/fcvm.2022.956538
18. Volders PG, Sipido KR, Vos MA, Spätjens RL, Leunissen JD, Carmeliet E, et al. Downregulation of delayed rectifier K (+) currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. (1999) 100:2455–61. doi: 10.1161/01.CIR.100.24.2455
Keywords: phosphoinositide 3-kinase inhibitor, QT/QTc, atrioventricular block, torsade de pointes, electrocardiogram
Citation: Zhang L, Zheng Y, Chen G, Zhao F and Li S (2024) Case report: Phosphoinositide 3-kinase inhibitor with fulvestrant in a patient with ER+/HER2- metastatic breast carcinoma induced fatal arrhythmias: a preventable event? Front. Oncol. 14:1331472. doi: 10.3389/fonc.2024.1331472
Received: 01 November 2023; Accepted: 01 May 2024;
Published: 17 June 2024.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Tareg Bey, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2024 Zhang, Zheng, Chen, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Li, bGlzaGk4OTA0MjdAMTYzLmNvbQ==; Fang Zhao, Y2VkaWxhbmlkQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.