
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 16 February 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1330851
Purpose: This study aims to compare the prognostic outcome of resection (RES) and microwave ablation (MWA) in different tumor burden score (TBS) cohorts.
Patients and Methods: We retrospectively analyzed 479 patients with primary hepatocellular carcinoma (HCC) who underwent RES (n = 329) or MWA (n = 150) with curative intent at our institution. We assessed their overall survival (OS) and progression-free survival (PFS) using the Kaplan–Meier curve. Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) were performed to minimize selection and confounding biases. Multivariate Cox regression was used to define the association between surgical modalities and outcomes.
Results: Following PSM, in the TBS ≤3 cohort, the cumulative 1-, 3-, 5- year OS in the RES and MWA groups were 92.5% vs. 98.8%, 82.7% vs. 90.0%, and 82.7% vs. 83.2% (P = 0.366), respectively. The corresponding PFS rates in the RES and MWA groups were 82.7% vs. 88.0%, 63.6% vs. 68.3% and 55.2% vs. 56.3, respectively (P = 0.218). In the TBS >3 cohort, the cumulative 1-, 3-, 5- year OS between the RES and MWA groups were 92.5% vs. 95.0%, 82.8% vs. 73.2% and 76.3% vs. 55.1%, (P = 0.034), respectively. The corresponding PFS rates in the RES and MWA groups were 78.0% vs. 67.5%, 63.6% vs. 37.5% and 55.2% vs. 37.1%, respectively (P = 0.044). The IPTW analysis showed similar results as shown in PSM analysis. The multivariate Cox regression indicated that the type of surgical modality was not associated with a poorer prognostic outcome in the TBS ≤3 cohort, unlike in the TBS >3 cohort.
Conclusion: TBS, as a discriminator, might help guide treatment decision-making for HCC within the Milan criteria.
Hepatocellular carcinoma (HCC) remains a major clinical challenge considering its relatively high prevalence, rapid progression, and dismal prognosis (1). With improved screening strategies and elevated public awareness, HCC can be detected early and properly managed. Currently, liver resection, liver transplantation, and ablation therapy, such as radiofrequency ablation and microwave ablation (MWA), are the main curative treatments for early-stage HCC (2). Resection was regarded as the standard first-line treatment for those with small primary liver cancer, but some patients are not candidates due to impaired general health status, hepatic insufficiency, and insufficient residual liver volume (3). MWA is an ablation modality that destroys cancer cells using heat from microwave energy. With the rapid advancements and breakthroughs, MWA has become indispensable for managing small HCCs due to its safety, minimal invasiveness, lower expense, and rapid recovery time (4).
Nevertheless, the best management approach for small primary liver cancers eligible for microwave coagulation and liver resection remains controversial. Several studies have compared the prognosis among patients with HCC treated with MWA and surgical resection. Results revealed that MWA was comparable with liver resection in terms of the 1-, 3-, and 5-year OS, and recurrence-free survival (5, 6). However, a systematic review and meta-analysis indicated that the local ablation group demonstrated a significantly higher incidence of local recurrence than the liver resection group (7).
Of note, tumor burden largely indicates the extent of the tumor in HCC and is included in the Barcelona Clinic Liver Cancer (BCLC) and other staging systems (8). Previous studies have shown that differences in tumor characteristics, such as size, and number, affect the outcomes, and prognosis of resection and ablation therapy (9–11). The tumor burden score (TBS), calculated by combining the maximum tumor size and number of lesions, was proposed to predict survival in colorectal liver metastasis (12). TBS was later applied to stratify the prognosis for patients with HCC undergoing resection, local ablation, and transarterial chemoembolization (13–16) and demonstrated a better discriminative ability than the Milan criteria (17). A recent study by Ho et al. showed that TBS was a promising marker to discriminate long-term outcomes in patients with HCC within the Milan criteria undergoing local ablation or transarterial chemoembolization (18). Building on these previous findings, this study aimed to investigate the possible role of preoperative TBS in discriminating the therapeutic outcomes of MWA and liver resection in HCC within the Milan criteria.
We retrospectively collected the data of patients with primary HCC who underwent resection or MWA with curative intent from 2012 to 2019 at the Department of General Surgery, Shengjing Hospital of China Medical University. All patients were diagnosed with HCC preoperatively via contrast ultrasound (US) and enhanced multidetector computed tomography (CT) and magnetic resonance imaging (MRI) accompanied by the alpha-fetoprotein (AFP) test. The inclusion criteria were as follows: (a) within the Milan criteria, (i) single tumor lesion ≤5 cm in diameter or multiple tumor nodules (two to three) and a maximum diameter ≤3 cm, (ii) no vessel or bile duct invasion, and (iii) no lymph node or extrahepatic metastasis; (b) R0 resection; and (c) complete ablation (defined as an ablation zone with a margin [≥5 mm] covering the original tumor size and no HCC features in the imaging test postoperatively). The exclusion criteria were (a) patients with cardiovascular or immune system disease; (b) Child–Pugh score C; (c) repeated carcinoma; (d) ablation combined with resection; (e) loss to follow-up; and (f) lack of laboratory data.
Demographic characteristics and clinical features included sex; age; body mass index (BMI); AFP level; neutrophil-to-lymphocyte ratio (NLR); hepatopathy; platelet (PLT) count; albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels; prothrombin time (PT); Child–Pugh score; maximum tumor size; tumor number; cirrhosis; hypertension; and hypersplenism. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Shengjing Hospital of China Medical University (2023PS760K). Written informed consent was obtained from all the patients or their representatives. To protect patient privacy, we de-identified all data that can be used to identify patient personal information, such as name, hospital ID, and telephone number.
For the resection group, a team of dedicated liver surgeons performed liver resection. The type and extent of resection were based on the extent of the tumor and hepatic functional reserve. Anatomical resection was the primary option, whereas non-anatomical resection was the secondary option if anatomical resection was not feasible. Intraoperative ultrasonography was used to achieve a resection margin of at least 1 cm. Liver parenchymal dissection was performed using bipolar coagulation forceps (SY-VIIC(Q)-6, Zhejiang, China), harmonic scalpel (HARMONIC SYNERGY® Blades, Ethicon Inc., Cornelia, GA), or the clamp-crushing method with an intermittent Pringle maneuver that was routinely performed within 15 min of ischemia, followed by 5 min of reperfusion.
For the MWA group, MWA was performed using a cooled-shaft system (ECO-100AI10, ECO Microwave System Co, Nanjing, China) with a maximum power of 80 W at 2450 MHz at our institution. The system was equipped with a real-time temperature monitor and cooling circulation technology. The operation was performed by a hepatobiliary surgeon (5–10 years of experience in MWA). All patients underwent US to locate tumor lesions and determine the best treatment strategy. For tumors with a diameter within 2 cm, the antenna was placed at the center of the tumor. For the tumors with a diameter of 2 to 3 cm, the antenna was placed on both sides of the tumor. For the tumors with a diameter exceeding 3 cm, multiple overlapping ablations were performed by repositioning the antenna. The antenna was placed sequentially on different areas of tumors according to the tumor size and shape. The surgeons tried to achieve complete tumor ablation with a margin exceeding 1 cm.
All patients were followed up with CT, MRI, and US 2 months after surgery, and AFP levels were monitored, with follow-up every 3 months thereafter. Treatment options for relapsed patients were determined on the basis of tumor number, size, and location and the patient’s liver function status. Overall survival (OS) was the survival time from the end of the initial surgery to death or the last follow-up. Progression-free survival (PFS) was the survival time from the end of the initial surgery to the first discovery of tumor recurrence or the last follow-up.
According to previous reports, TBS is defined as the distance from the origin of a Cartesian plane and comprises two variables: maximum tumor size (x-axis) and number of tumors (y-axis); thus, TBS2 = (maximum tumor diameter)2 + (number of tumors)2. The cutoff value of TBS was 3.0.
PSM and IPTW were used to minimize selection and confounding biases. The PS was calculated using logistic regression with the following clinical features: sex; age; BMI; NLR; the presence of viral hepatitis; AFP, ALT, and AST levels; PT; Child–Pugh score; the presence of cirrhosis; the presence of hypersplenism.
In the TBS ≤3 cohort, PSM was performed using a ratio of 1:1 via the nearest neighbor matching algorithm with an optimal caliper of 0.2 for MWA versus resection. In the TBS >3 cohort, PSM was performed using a ratio of 1:4 via the nearest neighbor matching algorithm with an optimal caliper of 0.2 for MWA versus resection.
For stabilized IPTW, the weighting coefficient of patients in the MWA and resection groups was PT/PS and (1 − PT)/(1 − PS)(PT = patients in the MWA group/all patients), respectively.
For continuous variables, if they conformed to the normal distribution, the data were presented as the mean ± standard deviation; otherwise, they were presented as the median (quartile 25%, 75%). If the continuous data satisfied normality, a comparison between the two groups was analyzed with the t-test; otherwise, a nonparametric test was used. Fisher’s exact or the chi-squared test was used to compare categorical variables. PFS and OS were assessed using the Kaplan–Meier method with a log-rank test. The association of clinicopathologic variables with PFS and OS was assessed with univariate Cox proportional hazards regression. The multivariate Cox proportional hazards regression model was created with statistically significant (P< 0.05) and clinically relevant (P < 0.1) variables. The “survival” and “survminer” packages were used for survival analyses. The “ggplot2” package was used for plotting. The “RISCA” package was used for IPTW. R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 26.0 (SPSS, Chicago, IL) were used for statistical analyses. A two-tailed P-value of <0.05 was considered statistically significant.
The current study enrolled 479 patients (men and women, age range 30–78 years; Figure 1); 329 and 150 patients underwent resection and MWA, respectively. Among 479 patients, 379(79.1%), 50 (10.4%), 10 (2.1%), and 40 (8.4%) patients had HBV-related, HCV-related, coinfection-related, and other HCC. There were 367 (76.6%) patients with cirrhosis and 222 (46.3%) patients with portal hypertension. In the TBS ≤3 cohort, there were 131, and 105 patients in the resection and MWA groups, respectively. In the TBS >3 cohort, there were 198, and 45 patients in the resection and MWA groups, respectively. After PSM, 83 patients each were in the resection and MWA groups in the TBS ≤3 cohort. In the TBS >3 cohort, there were 113, and 40 patients in the resection and MWA groups after PSM, respectively. After stabilized IPTW, in the TBS ≤3 cohort, there were 132.1 and 104.7 patients in the resection and MWA groups, respectively; in the TBS >3 cohort, there were 196.2 and 48.5 patients in the resection and MWA groups, respectively. No significant differences in sex; age; BMI; NLR; the presence of viral hepatitis; PLT count; AFP, ALB, TBIL, ALT, and AST levels; PT; Child–Pugh score; the presence cirrhosis; and the presence of hypersplenism were found whether in the total, PSM, or IPTW cohorts (Tables 1–3).
In the TBS ≤3 cohort, for the resection group, the median follow-up time was 46 months (range 4–118), 18/131 (13.7%) patients died, and 42/131 (36.6%) patients had tumor recurrence during the follow-up period. For the MWA group, the median follow-up time was 48 months (range 6–104), 15/105 (14.3%) patients died, and 42/105 (40%) patients had tumor recurrence during the follow-up period. Before PSM or stabilized IPTW, the 1-, 3-, and 5-year OS rates were 95.3%, 85.7%, and 84.4% in the resection group and 99.9%, 89.8%, and 79.7% in the MWA group, respectively (P = 0.802; Figure 2A). The 1-, 3-, and 5-year PFS rates were 86%, 68.3%, and 59.2% in the resection group and 87.6%, 63.3%, and 49.0% in the MWA group, respectively (P = 0.930; Figure 2B). Following PSM, the 1-, 3-, and 5-year OS rates were 92.5%, 82.7%, and 82.7% in the resection group and 98.8%, 90.0%, and 83.2% in the MWA group, respectively (P = 0.366; Figure 2C); the corresponding PFS rates were 82.7%, 63.6%, and 55.2% and 88.0%, 68.3%, and 56.3% in the resection and MWA groups, respectively (P = 0.218; Figure 2D). Following stabilized IPTW, the 1-, 3-, and 5-year OS rates were 90.3%, 79.5%, and 78.4% in the resection group and 98.9%, 89.5%, and 79.7% in the MWA group, respectively (P = 0.125; Figure 2E); the corresponding PFS rates were 83.4%, 63.5%, and 55.0% and 87.9%, 65.5%, and 52.7% in the resection and MWA groups, respectively (P = 0.361; Figure 2F). In conclusion, there were no significant differences in OS and PFS between the resection and MWA groups in the TBS ≤3 cohort.
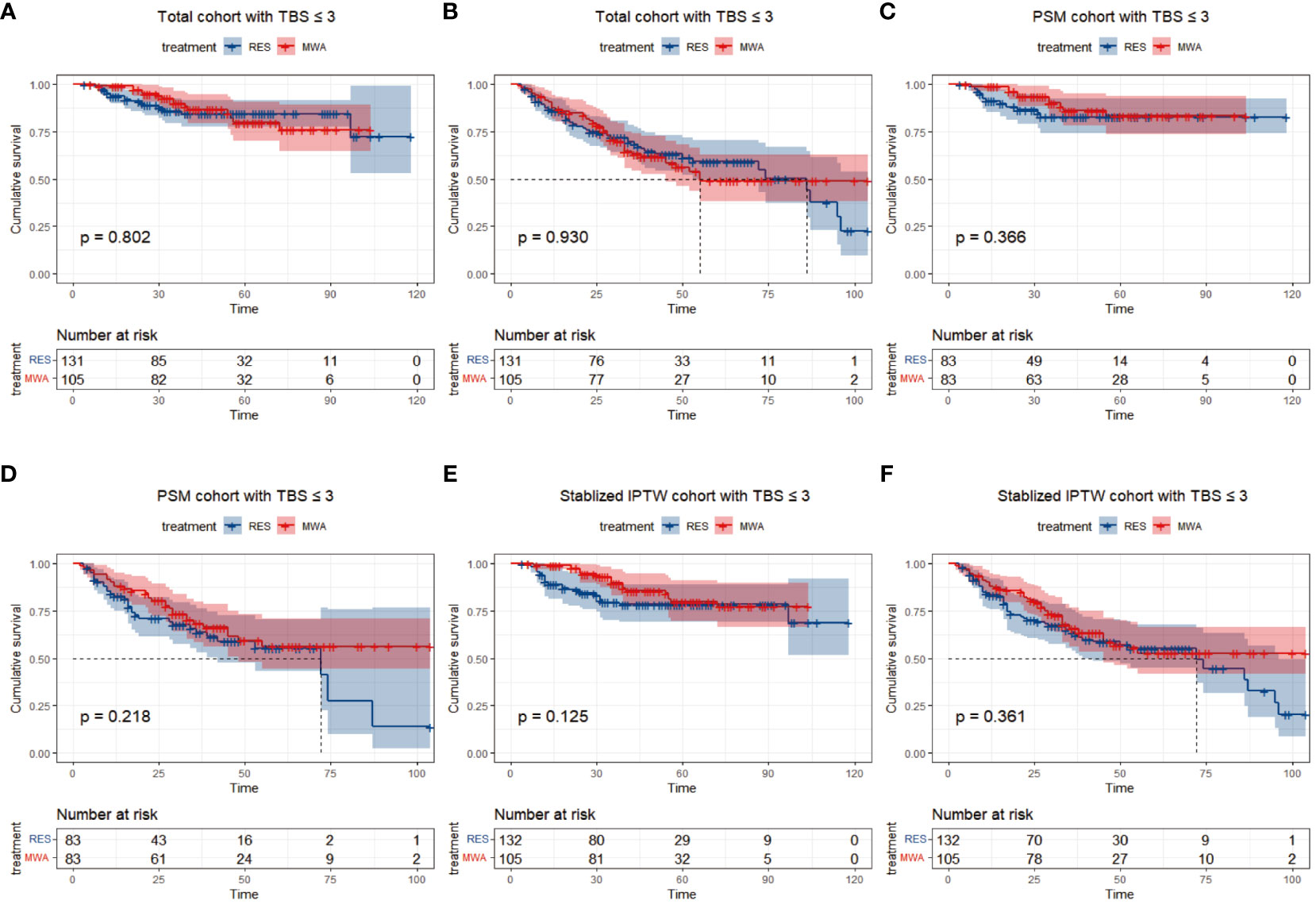
Figure 2 Differences of and overall survival (A) and progression-free survival (B) between RES group versus MWA group in total cohort with TBS ≤ 3. Differences of and overall survival (C) and progression-free survival (D) between RES group versus MWA group in PSM cohort with TBS ≤ 3. Differences of and overall survival (E) and progression-free survival (F) between RES group versus MWA group in Stabilized IPTW with TBS ≤ 3.
In the TBS >3 cohort, for the resection group, the median follow-up time was 44 months (range 1–104), 32/198 (16.2%) patients died, and 80/198 (40.4%) patients had tumor recurrence during the follow-up period. For the MWA group, the median follow-up time was 62 months (range 4–105), 19/45 patients (42.2%) died, and 30/45 (66.7%) patients had tumor recurrence during the follow-up period. Before PSM or stabilized IPTW, the 1-, 3-, and 5-year OS rates were 94.0%, 86.0%, and 79.7% in the resection group and 95.6%, 72.7%, and 51.3% in the MWA group, respectively (P < 0.001; Figure 3A). The 1-, 3-, and 5-year PFS rates were 79.4%, 61.2%, and 50.5% in the resection group and 68.9%, 36.3%, and 30.8% in the MWA group, respectively (P = 0.008; Figure 3B). Following PSM, the 1-, 3-, and 5-year OS rates were 92.5%, 82.8%, and 76.3% in the resection group and 95.0%, 73.2%, and 55.1% in the MWA group, respectively (P = 0.034; Figure 3C); the corresponding PFS rates were 78.0%, 61.6%, and 48.6% and 67.5%, 37.5%, and 31.7% in the resection and MWA groups, respectively (P = 0.044; Figure 3D). Following stabilized IPTW, the 1-, 3-, and 5-year OS rates were 94.1%, 85.9%, and 79.1% in the resection group and 89.8%, 77.7%, and 58.9% in the MWA group, respectively (P = 0.027; Figure 3E); the corresponding PFS rates were 79.8%, 62.4%, and 50.9% and 68.0%, 35.8%, and 33.9% in the resection and MWA groups, respectively (P = 0.036; Figure 3F). In conclusion, there were significant differences in OS and PFS between the resection and MWA groups in the TBS >3 cohort.
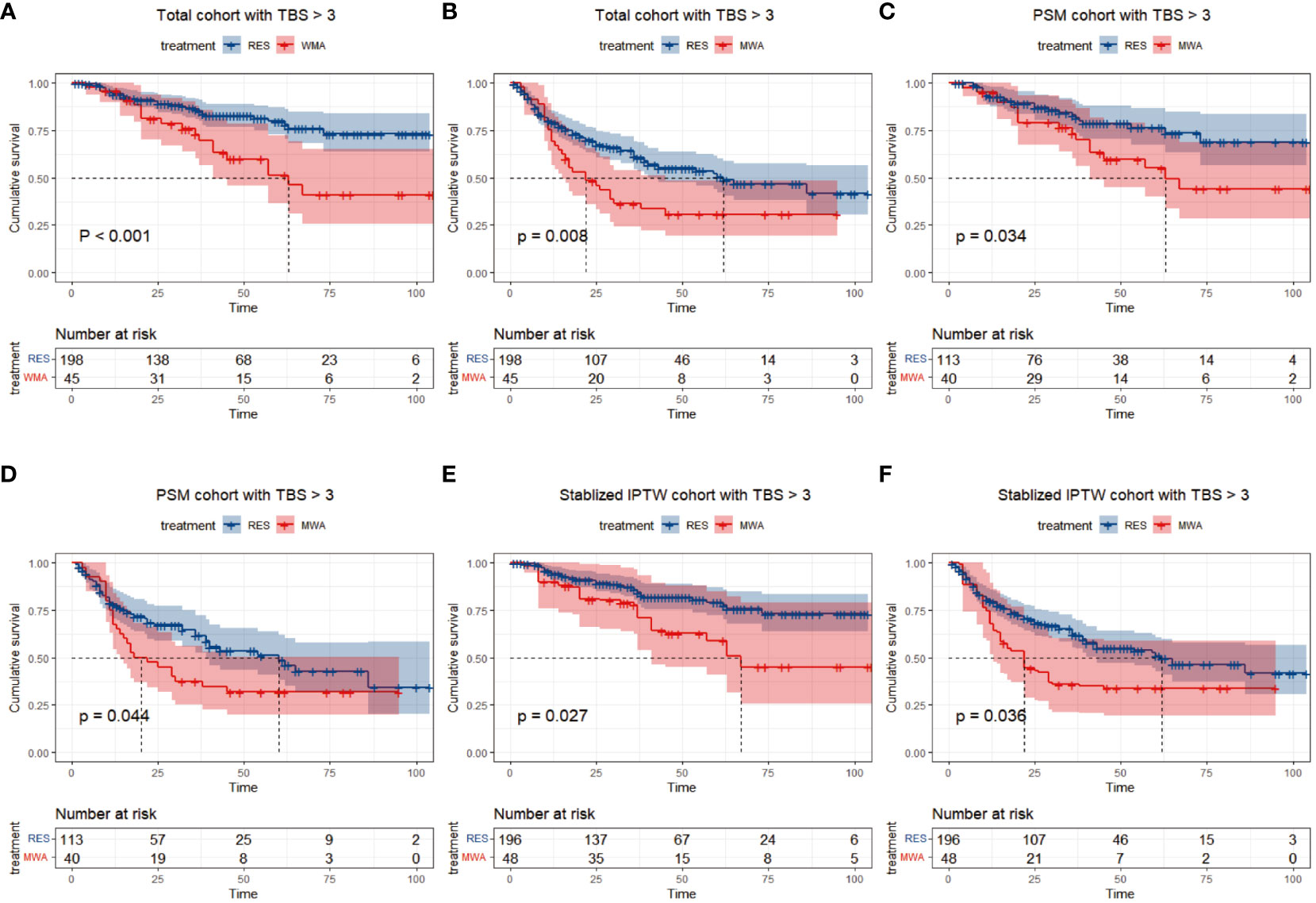
Figure 3 Differences of and overall survival (A) and progression-free survival (B) between RES group versus MWA group in total cohort with TBS > 3. Differences of and overall survival (C) and progression-free survival (D) between RES group versus MWA group in PSM cohort with TBS >3. Differences of and overall survival (E) and progression-free survival (F) between RES group versus MWA group in Stabilized IPTW cohort with TBS > 3.
Multivariate analysis showed that ALB >35 g/L (hazard ratio [HR] 95CI% 0.25 [0.12–0.52], P < 0.001) and cirrhosis (HR 95CI% 2.69 [1.23–5.91], P = 0.013) were associated with a better OS, and male sex (HR 95CI% 1.65 [1.01–2.69], P = 0.047) and NLR >1.05 (HR 95CI% 3.09 [1.34–7.09], P = 0.008) were associated with a poorer PFS in the TBS ≤3 cohort (Supplementary Table 1). Additionally, in the TBS >3 cohort, age ≥60 years (HR 95CI% 2.12 [1.21–3.71], P = 0.008), TBIL >17.1 µmol/L (HR 95CI% 1.78 [1.01–3.15], P = 0.048), and PLT <100 ∗ 109 (HR 95CI% 2.87 [1.61–5.3], P < 0.001) were associated with a poorer OS, and viral hepatitis (HR 95CI% 2.59 [1.19–5.60], P = 0.016) and TBIL >17.1 µmol/L (HR 95CI% 1.68 [1.13–2.51], P = 0.011) were associated with a poorer PFS (Supplementary Table 1).
In the TBS ≤3 cohort, the type of surgical modality was not associated with poorer OS or PFS, no matter how many factors were adjusted (Supplementary Figure 1A). In contrast, in the TBS >3 cohort, the type of surgical modality was associated with poorer OS or PFS, no matter how many factors were adjusted (Supplementary Figure 1B).
HCC is a highly heterogeneous disease in terms of biological and clinical behavior (19). Systemic therapy has been shown to prolong the survival of patients with advanced stage HCC (20, 21). The 2022 version of BCLC staging demonstrates that both ablation and liver resection are clinically effective treatment options and have comparable therapeutic effects for very early and early-stage HCC (2). MWA has become an increasingly used local ablation modality. Its theoretical benefits include high thermal efficiency and a larger ablation zone compared with radiofrequency ablation (22). To date, the best approach to the management of HCC within the Milan criteria, eligible for both microwave coagulation and liver resection, remains controversial.
According to previous studies, tumor morphology has been validated as a strong predictor of recurrence and poorer survival outcomes (23, 24). Sun et al. reported that no marked difference was found in the 1-, 3-, and 5-year OS rates and the 1-year disease-free survival (DFS) rate between the MWA and resection groups. Additionally, no significant differences were observed in the OS and DFS rates between the two groups with solitary HCC ≤3 cm and in the OS rate for solitary HCC 3–5 cm (25). Nevertheless, the DFS for solitary HCC 3–5 cm in the resection group was significantly higher compared with that in the MWA group in the study cited above. Interestingly, a study demonstrated that in the subgroup of BCLC-0, no significant differences in PFS or OS were observed between the MWA and liver resection groups. Conversely, in the subgroup of BCLC-A, the liver resection group had a significant increase in PFS compared with the MWA group (26). Of note, a cohort study by Dou et al. demonstrated that no differences were observed regarding OS and DFS in HCC ≤4.0 cm after MWA or surgical resection. For HCC 4.1–5.0 cm, MWA had lower OS (P = 0.01) and DFS rates (P = 0.01) than surgical resection (27). These results indicate that tumor burden might be a reliable tool to differentiate the prognosis in patients with HCC after MWA and resection.
Tumor burden is considered one of the most important prognostic predictors of HCC (14, 17, 28). Traditionally, the tumor diameter, and number of nodules are used to assess tumor burden. Although the use of arbitrary cutoff categorical (tumor size) or ordinal (tumor number) values is a convenient way to assess disease burden, it has limitations with regard to statistical power compared with continuous variables (12). Previous studies have suggested the use of total tumor volume and diameter, which are continuous variables, to assess tumor burden (29, 30). However, these two scores are too complicated because of the requirement of all tumor number and size information. The use of the Pythagorean theorem, with TBS as a single and continuous variable rather than a dichotomous variable to indicate disease burden in HCC, has recently been proposed to minimize the heterogeneity in tumor nodule size and number. Previous studies demonstrate that TBS has a better predictive ability for outcomes compared with the established Milan or up-to-7 criteria (12, 17). Additionally, some studies suggest TBS as a discriminator for some treatment decisions in HCC (18, 31).
This study noted that TBS is a feasible marker to discriminate long-term outcomes, and we noted that TBS may provide a differential influence in selecting resection or MWA for HCC within the Milan criteria. In the TBS ≤3 cohort, there was no significant difference in PFS and OS between the two groups. However, in the TBS >3 cohort, PFS, and OS rates were higher in the resection group than in the MWA group. After PSM or stabilized IPTW, similar results were observed in the TBS ≤3 and TBS >3 cohorts. Additionally, after multivariate Cox regression model adjustments, surgical modalities were not associated with a poorer prognosis in the low TBS cohort but were associated with a poorer prognosis in the high TBS cohort.
Our study findings have some limitations. First, this was a retrospective single-center clinical study. Although the IPTW and PSM analyses were conducted to reduce selection and confounding biases, potential flaws may still exist. Second, the patient number in the MWA group was small for the TBS >3 cohort. Third, we have no reliable data on the treatment of patients with recurrent disease and it is a possible additional confounder in the analysis of long-term survival and PFS. In addition, TBS = 3 was not the best cutoff value of prognostic outcomes in our study. Hence, future multi-center prospective studies are needed to be validated.
In conclusion, preoperative TBS as a discriminator might help guide treatment decision-making for HCC within the Milan criteria and in the group with TBS >3, surgery can be considered in patients requiring a less extensive surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of Shengjing Hospital of China Medical University (2023PS760K). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CD: Funding acquisition, Resources, Supervision, Writing – review & editing. ZW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. KX: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. FX: Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by the grants from 2021 Scientific Research Funding Project of Education Department of Liaoning Province (LJKZ0748 to CD) and 345 Talent Project.
The authors are grateful to CD for her help with the preparation of figures in this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1330851/full#supplementary-material
AFP, Concentrations of a-fetoprotein; ALB, Albumin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; CT, Computed tomography; DFS, Disease-free survival; HCC, Hepatocellular carcinoma; IPTW, Inverse Probability of Treatment Weighting; LR, Liver resection; MWA, Microwave ablation; MRI, Magnetic resonance imaging; NLR, Neutrophil-to-lymphocyte ratio; OS, Overall survival; PFS, Progression-free survival; PLT, Platelet count; PSM, Propensity Score Matching; PT, Prothrombin time; RES, Resection; TBIL, Total bilirubin; TBS, Tumor burden score; US, Ultrasound.
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. (2019) 16:589–604 doi: 10.1038/s41575-019-0186-y.
2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
3. Prodeau M, Drumez E, Duhamel A, Vibert E, Farges O, Lassailly G, et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol. (2019) 71:920–9. doi: 10.1016/j.jhep.2019.06.003
4. Xu Z, Xie H, Zhou L, Chen X, Zheng S. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst). (2019) 2019:8619096. doi: 10.1155/2019/8619096
5. Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H, et al. Hepatic resection versus operative microwave ablation for single hepatocellular carcinoma ≤5 cm: A propensity score-matched analysis. Surgery. (2019) 166:254–62. doi: 10.1016/j.surg.2019.05.007
6. Liu W, Zou R, Wang C, Qiu J, Shen J, Liao Y, et al. Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis. Ther Adv Med Oncol. (2019) 11:1758835919874652. doi: 10.1177/1758835919874652
7. Shin SW, Ahn KS, Kim SW, Kim T-S, Kim YH, Kang KJ, et al. Liver resection versus local ablation therapies for hepatocellular carcinoma within the milan criteria: A systematic review and meta-analysis. Ann Surg. (2021) 273:656–66. doi: 10.1097/SLA.0000000000004350
8. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. (2019) 156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065
9. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. (2019) 70:866–73. doi: 10.1016/j.jhep.2018.12.027
10. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
11. Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O, et al. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. (2020) 2:100134. doi: 10.1016/j.jhepr.2020.100134
12. Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, et al. The tumor burden score: A new "Metro-ticket" Prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. (2018) 267:132–41. doi: 10.1097/SLA.0000000000002064
13. Tsilimigras DI, Mehta R, Paredes AZ, Moris D, Sahara K, Bagante F, et al. Overall tumor burden dictates outcomes for patients undergoing resection of multinodular hepatocellular carcinoma beyond the Milan criteria. Ann Surg. (2020) 272:574–81. doi: 10.1097/SLA.0000000000004346
14. Tsilimigras DI, Moris D, Hyer JM, Bagante F, Sahara K, Moro A, et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. (2020) 107:854–64. doi: 10.1002/bjs.11464
15. Ho SY, Liu P-H, Hsu C-Y, Huang Y-H, Liao J-I, Su C-W, et al. Tumor burden score as a new prognostic surrogate in patients with hepatocellular carcinoma undergoing radiofrequency ablation: Role of albumin-bilirubin (ALBI) grade vs easy ALBI grade. Expert Rev Gastroenterol Hepatol. (2022) 16:903–11. doi: 10.1080/17474124.2022.2117156
16. Ho SY, Liu P-H, Hsu C-Y, Ko C-C, Huang Y-H, Su C-W, et al. Tumor burden score as a new prognostic marker for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Gastroenterol Hepatol. (2021) 36:3196–203. doi: 10.1111/jgh.15593
17. Vitale A, Lai Q, Farinati F, Bucci L, Giannini EG, Napoli L, et al. Utility of tumor burden score to stratify prognosis of patients with hepatocellular cancer: Results of 4759 cases from ITA.LI.CA study group. J Gastrointest Surg. (2018) 22:859–71. doi: 10.1007/s11605-018-3688-y
18. Ho SY, Liu P-H, Hsu C-Y, Huang Y-H, Liao J-I, Su C-W, et al. Radiofrequency Ablation versus Transarterial Chemoembolization for Hepatocellular Carcinoma within Milan Criteria: Prognostic Role of Tumor Burden Score. Cancers (Basel). (2022) 14(17):4207. doi: 10.3390/cancers14174207
19. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236]. J Hepatol. (2019) 70:817. doi: 10.1016/j.jhep.2019.01.020
20. Sangro B, Nishida N, Kudo M. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:525–43. doi: 10.3390/cancers15072070
21. Dai H, Fan Q, Wang C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Explor (Beijing). (2022) 2:20210157. doi: 10.1002/EXP.20210157
22. Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency ablation and microwave ablation in liver tumors: An update. Oncologist. (2019) 24:e990–e1005. doi: 10.1634/theoncologist.2018-0337
23. Hewitt DB, Brown ZJ, Pawlik TM. The role of biomarkers in the management of colorectal liver metastases. Cancers (Basel). (2022) 14(19):4602. doi: 10.3390/cancers14194602
24. Xu X-F, Xing H, Han H, Li Z-L, Lau W-Y, Zhou Y-H. Patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg. (2019) 154:209–17. doi: 10.1001/jamasurg.2018.4334
25. Sun Q, Shi J, Ren C, Du Z, Shu G, Wang Y, et al. Survival analysis following microwave ablation or surgical resection in patients with hepatocellular carcinoma conforming to the Milan criteria. Oncol Lett. (2020) 19:4066–76. doi: 10.3892/ol.2020.11529
26. Tong Y, Cai R, Li J-X, Chang D-H, Wang L-Z, Cai W-W, et al. Liver resection versus microwave ablation for hepatocellular carcinoma in ideal candidates for ablation per Barcelona Clinic Liver Cancer staging: a propensity score matching and inverse probability of treatment weighting analysis. Aliment Pharmacol Ther. (2022) 56:1602–14. doi: 10.1111/apt.17263
27. Dou J, Cheng Z, Han Z, Liu F, Wang Z, Yu X, et al. Microwave ablation vs. surgical resection for treatment naïve hepatocellular carcinoma within the Milan criteria: a follow-up of at least 5 years. Cancer Biol Med. (2021) 19:1078–88. doi: 10.20892/j.issn.2095-3941.2020.0625
28. Ho SY, Liu P-H, Hsu C-Y, Huang Y-H, Liao J-I, Su C-W, et al. A new tumor burden score and albumin-bilirubin grade-based prognostic model for hepatocellular carcinoma. Cancers (Basel). (2022) 14(3):649. doi: 10.3390/cancers14030649
29. Toso C, Meeberg G, Hernandez-Alejandro R, Dufour J-F, Marotta P, Majno P, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. (2015) 62:158–65. doi: 10.1002/hep.27787
30. Galdino-Vasconcelos MR, Silva Feijó M, Metzker Ferro H, Ramalho Gomes AC, De Almeida Santos ME, Ferreira G, et al. Preoperative alpha-fetoprotein and radiological total tumor diameter as predictors of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. (2022) 54:1333–40. doi: 10.1016/j.transproceed.2022.02.065
Keywords: liver resection, microwave ablation, hepatocellular carcinoma, tumor burden score, prognosis
Citation: Wei Z, Xie K, Xu F and Dai C (2024) The tumor burden score may be a discriminator in microwave ablation versus liver resection for hepatocellular carcinoma within the Milan criteria: a propensity score matching and inverse probability of treatment weighting study. Front. Oncol. 14:1330851. doi: 10.3389/fonc.2024.1330851
Received: 31 October 2023; Accepted: 30 January 2024;
Published: 16 February 2024.
Edited by:
Giuseppe Zimmitti, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Chao Li, Eastern Hepatobiliary Surgery Hospital, ChinaCopyright © 2024 Wei, Xie, Xu and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoliu Dai, Y211ZGFpY2hhb2xpdTIwMjNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.