- 1Department of Hematology, Chongqing University Three Gorges Hospital, Chongqing, China
- 2School of Medicine, Chongqing University, Chongqing, China
- 3Department of Medicine, Northwestern University, Chicago, IL, United States
Atypical Chronic Myeloid Leukemia (aCML), a myeloproliferative neoplasm with poor prognosis, was reclassified as aCML by the ICC classification, and as MDS/MPN with neutrophilia by the WHO 2022 classification. Due to the heterogeneity of its clinical features and the lack of unique biomarkers, as well as limited treatment options, aCML currently lacks a standardized treatment protocol. In this case report, we reviewed a young man diagnosed with aCML who achieved complete clinical and hematologic remission subsequent to receiving a therapeutic regimen combining Venetoclax and Azacitidine.
Introduction
Atypical Chronic Myeloid Leukemia (aCML) is a myeloproliferative neoplasm with poor prognosis due to its significant risk of progressing into acute myeloid leukemia(AML) (1). It was reclassified as aCML by the ICC classification, and as MDS/MPN with neutrophilia by the WHO 2022 classification. aCML is characterized by marked leukocytosis/granulocytosis like CML, yet it lacks discernible evidence of the classic t(9;22) BCR-ABL1 translocation as determined through cytogenetic studies, polymerase chain reaction (PCR), or fluorescence situ hybridization (FISH) (2, 3). Due to the heterogeneity of its clinical features and the lack of unique biomarkers, as well as limited treatment options, aCML currently lacks a standardized treatment protocol (4). In this case report, we reviewed a young man diagnosed with aCML who achieved a complete clinical and hematologic remission subsequent to receiving a therapeutic regimen combining Venetoclax and Azacitidine.
Case report
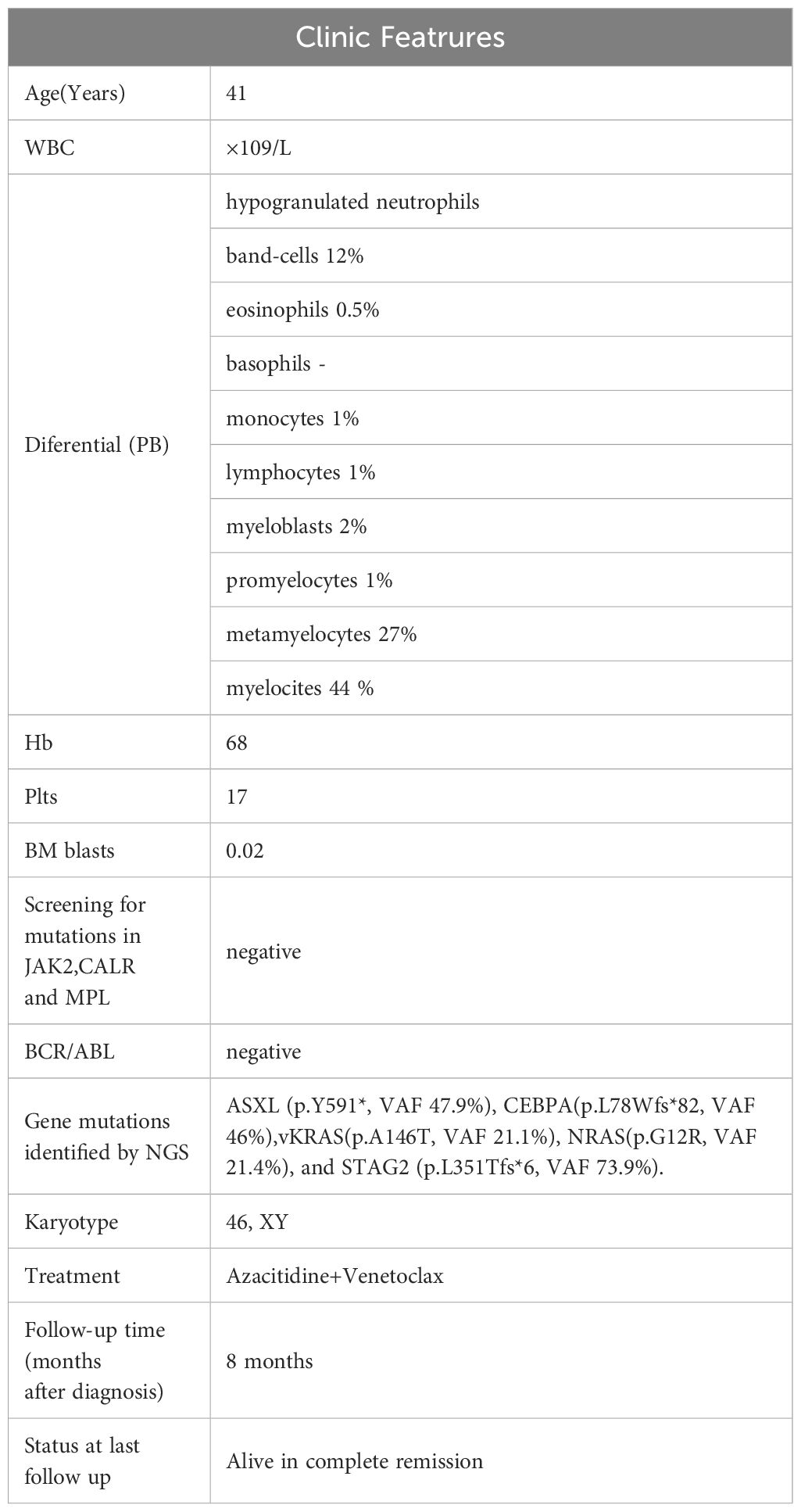
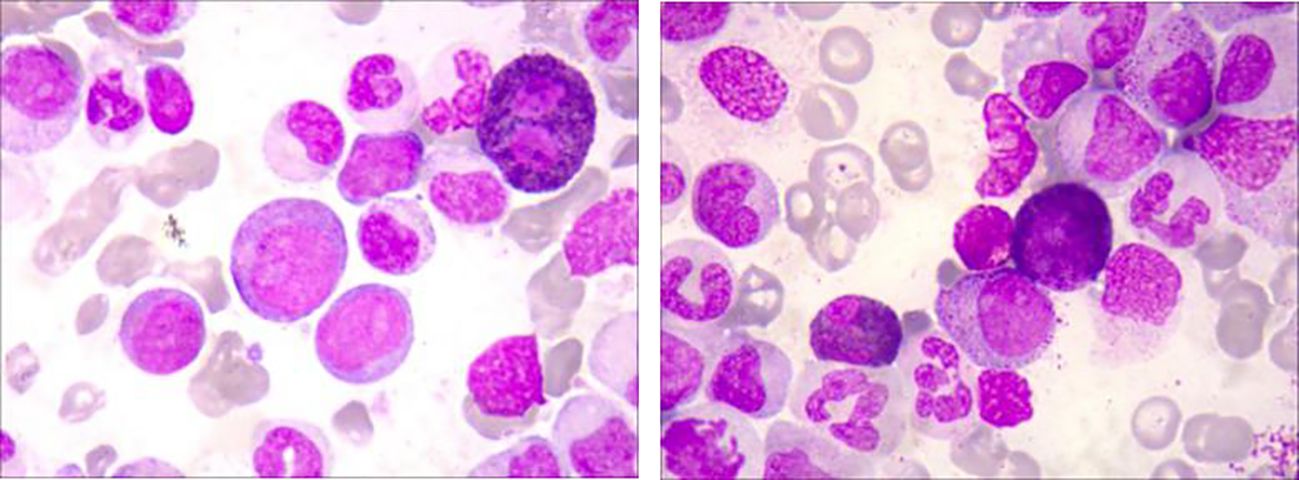
A 41-year-old male with an unremarkable medical history had been suffering from abdominal bloating for one month before presenting to our Hematology department. The complete blood count showed a high white blood cell (WBC) count of 210.74×109/L, a low hemoglobin (Hb) level of 68g/L, and a low platelet (PLT) count of 17 ×10^9/L. A manual differential revealed distinct abnormalities in the white blood cell distribution: 95% neutrophils (11% segmented neutrophils, 12% band neutrophils, 27% metamyelocytes, 44% myelocytes, and 1% promyelocytes), 1% monocytes, 2% blast cells and 2% lymphocytes. Physical examination indicated hepatosplenomegaly. A bone marrow (BM) examination and trephine biopsy were performed and revealed hypercellularity with marked myeloid expansion with primary + promyelocytic granulocytes accounting for 7.5% (Figure 1). These granulocytes mainly predominantly exhibited various developmental stages, including middle, late, and rod-shaped forms, with an unbalanced nuclear morphology and decreased or absent cytoplasmic granules. Flow Cytometry analysis uncovered an abnormal myeloid protocell phenotype characterized by CD34+CD117+CD38dimHLA-DRdimCD13+CD33+CD11B-CD15-CD64-CD56-CD7-, accounting for 1.63% of nucleated cells. Karyotype was normal and an initial molecular analysis targeting BCR/ABL1 rearrangement, as well as mutations of CSF3R, JAK2V617F, CARL, and MPL, did not reveal any genetic abnormalities. As a next step, the diagnostic bone marrow sample underwent next generation sequencing (NGS) analysis utilizing the Myeloid Proliferative Neoplasm Sequencing Panel (Illumina), covering 77 genes that are known to be frequently mutated in myeloid malignancies, which unveiled pathogenic variants in ASXL (p.Y591*, VAF 47.9%), CEBPA(p.L78Wfs*82, VAF 46%), vKRAS(p.A146T, VAF 21.1%), NRAS(p.G12R, VAF 21.4%), and STAG2 (p.L351Tfs*6, VAF 73.9%) (Clinic features were showing in Table 1).
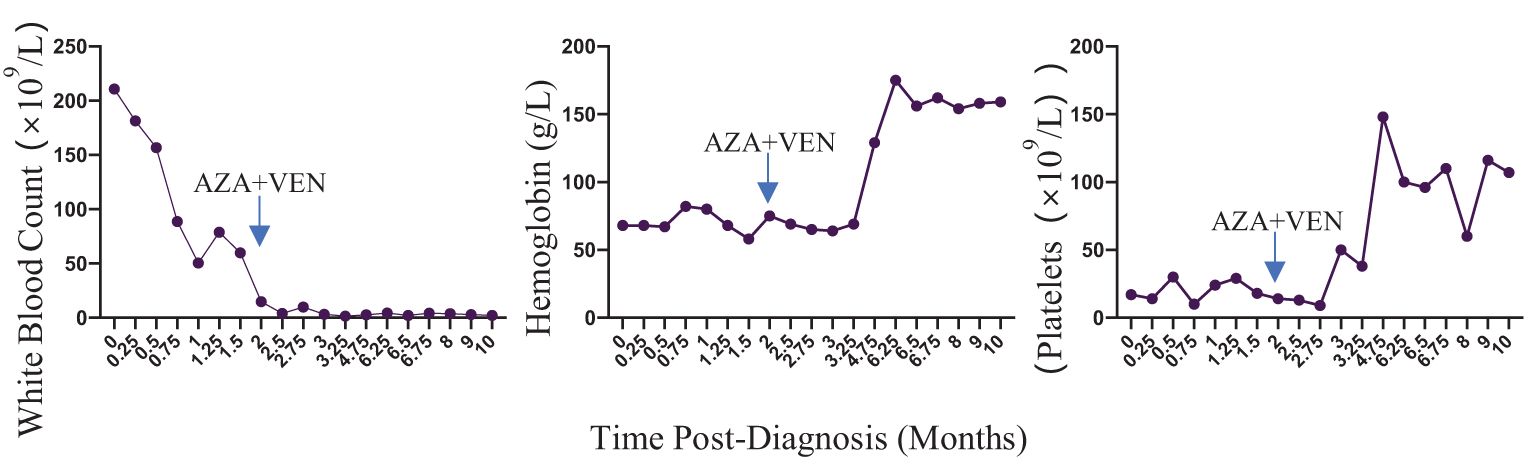
The diagnosis of aCML was made in concordance with the 2022 WHO diagnostic criteria for aCML. Due to the absence of a standardized medical protocol for aCML, a thorough evaluation for stem cell transplantation was recommended, but ultimately declined by the patient. He underwent leukocyte removal to alleviate leukocytosis, reduce blood viscosity and improve microcirculation. He was initially started on both hydroxyurea and imatinib, with concurrent evaluation for imatinib, given the strikingly similar clinical presentation to typical CML. However, imatinib administration was stopped subsequent to a negative result for the BCR/ABL1 rearrangement. Subsequently he received 3 cycles of a 5-day regimen consisting of cytarabine and azacytidine. Unfortunately, the changes of peripheral blood, bone marrow and hepatosplenomegaly did not obviously improve compared with the beginning of the disease, and the cervical lymph nodes were enlarged, indicating suboptimal disease control. Due to the patient’s worsening blood counts and limited alternative treatment options, a therapeutic approach combining venetoclax(400mg daily for 21days) and azacytidine(100mg daily for 7days) was initiated. He had rapid clinical improvements of blood counts (Figure 2), bone marrow, cervical lymph nodes enlargement and hepatosplenomegaly after 1 month of the combination therapy. Then he continued to receive the same combination therapy once a month for. The bone marrow smear showed hyperplasia was active, no primary or promyelocytic granulocytes were seen and the immune residue showed negative after 8 months of the treatment. He continued to maintain complete clinic remission at the latest follow-up after 10 months of the treatment.
Discussion
The age of onset for aCML predominantly occurs around 60 years of age, with approximately half of the patients exhibiting concurren hepatosplenomegaly, increased WBC count, anemia and thrombocytopenia. Due to the low incidence, specific molecular abnormalities associated with aCML have yet to be identified to date, leading to significant challenges in the clinical diagnosis of this condition. Our patient was diagnosed with aCML defined by his BCR-ABL1(-) status in the setting of marked leukocytosis/granulocytosis and prominent dysgranulopoiesis, and granulocytic dysplasia, according to the 2022 WHO diagnostic criteria for aCML.
aCML is a rare disease, and standard treatment approaches are lacking both domestically and internationally. Patients should be treated promptly when they manifest progressive leukocytosis, anemia, thrombocytopenia, spleen enlargement, or other disease-associated symptoms (2, 5). Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) is recommended as a preferred option for younger patients with a suitable donor; however, the optimal timing of transplantation remains controversial (6). Given that aCML exhibits numerous resemblances to other chronic myeloid disease, its treatment strategy can be referred to Myelodysplastic Syndromes (MDS) or Myeloproliferative Neoplasms (MPN). This can involve the use of agents like hydroxyurea or interferon to mitigate tumor burden and alleviate splenomegaly-related symptoms. Regrettably, these interventions tend to yield suboptimal results, particularly in advanced stages of the disease (2). Based on the good efficacy of demethylated drugs such as decitabine or azacitidine in Chronic Myelomonocytic Leukemia (CMML), some studies have tried to use these drugs in aCML treatment (7). So far, it has been reported that the complete response (CR) rate of treating aCML with decitabine alone can reach 40%, but the long-term survival benefit still needs to be confirmed by larger sample analyses and longer follow-up observations (8). Notable elevations in leukocyte count, combined with anemia, thrombocytopenia and splenomegaly, prompted the necessity for treatment, but the economic conditions for transplantation were not available, and the effectiveness of adding hydroxyurea to reduce leukocytes proved limited. In order to minigrate disease progression and improve patient survival, azacitidine induction therapy was administered.
With the continuous development of molecular diagnostic technologies, there has been an increasing detection of mutated genes in aCML, leading to a deeper understanding of this condition, which, in turn, holds significant implications for its treatment (9). Some clinical reports have shown that Ruxolitinib can improve the prognosis of aCML patients (10). However, in the case of this patient who presented with anemia and thrombocytopenia and exhibited no mutations in the CSF3R or JAK2 genes, the use of Ruxolitinib was not considered. B-cell lymphoma-2 (BCL-2), a key regulator of the mitochondrial apoptotic pathway, is overexpressed in many hematological malignancies, especially within leukemia stem cells (11). Overexpression of BCL-2 has been shown to reduce the survival rate of AML cells and is associated with chemotherapy resistance (12). Venetoclax is currently the only approved BCL-2 inhibitor. As a BH3 analogue, venetoclax can simulate the selective binding of BH3-only pro-apoptotic protein to BH3 domain of anti-apoptotic protein in BCL-2 family, effectively inhibit BCL-2, and affect the permeability of mitochondrial outer membrane, leading to the rapid initiation of apoptosis (12, 13). In vitro studies have illuminated that venetoclax can increase the sensitivity of AML cells to demethylation drugs (14). Furthermore, demethylation drugs can reduce the level of anti-apoptotic protein MCL-1 and delay the emergence of venetoclax resistance (15). Therefore, the patient was treated with azacytidine combined with the BCL-2 targeting drug venetoclax, and the patient had obtained CR, and minimal residual disease (MRD) turned negative early to the follow-up cut-off point, the survival time of the patient was 10 months, and the prognosis was significantly improved. Even though this therapeutic regimen combining Venetoclax and Azacitidine could promote complete clinical and hematologic remission for, but the long-term benefit is unknown, so allo-transplantation should also be initialed as soon as possible to achieve long-term survival.
Conclusion
The diagnosis of aCML is difficult, and only morphological changes may cause certain missed diagnosis in clinic. The relentless progress in molecular biology technology has led to the continual detection of MDS/MPN-related molecular markers, thereby enriching our comprehension of aCML. The patient, aged 41, had no transplantation conditions. Remarkable clinical efficacy was achieved after treatment with azacitidine combined with venetoclax, with good hematological and molecular responses and high safety. However, the long-term clinical outcome still needs to be further followed up and evaluated.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The case report was approved by the medical research center. The patient has provided written informed consent for the publication of this case report.
Author contributions
HC: Writing – original draft. YL: Writing – original draft. YY: Writing – review & editing. XX: Writing – original draft. NW: Writing – review & editing, Project administration.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the Chongqing University Medical and Industrial Integration Project , Project number 2022CDJYGRH-012 and Natural Science Foundation of Chongqing, Project number cstc2020jcyj-msxmX0969.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Crisà E, Nicolosi M, Ferri V, Favini C, Gaidano G, Patriarca A. Atypical chronic myeloid leukemia: where are we now? Int J Mol Sci (2020) 21(18):6862. doi: 10.3390/ijms21186862
2. Patnaik MM, Tefferi A. Atypical chronic myeloid leukemia and myelodysplastic/myeloproliferative neoplasm, not otherwise specified: 2023 update on diagnosis, risk stratification, and management. Am J Hematol (2023) 98(4):681–9. doi: 10.1002/ajh.26828
3. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
4. Wang JS, Elghawy O, Kurpiel BR, Douvas MG. Diagnosis and management of atypical chronic myeloid leukemia with a t(2;13)(q33;q12) translocation. Case Rep Hematol (2022) 2022:4628183. doi: 10.1155/2022/4628183
5. Diamantopoulos PT, Viniou NA. Atypical chronic myelogenous leukemia, BCR-ABL1 negative: diagnostic criteria and treatment approaches. Front Oncol (2021) 11:722507. doi: 10.3389/fonc.2021.722507
6. Onida F, de Wreede LC, van Biezen A, Eikema DJ, Byrne JL, Iori AP, et al. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: a retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol (2017) 177(5):759–65. doi: 10.1111/bjh.14619
7. Marumo A, Mizuki T, Tanosaki S. Atypical chronic myeloid leukemia achieving good response with azacitidine. Indian J Cancer (2019) 56(4):354–5. doi: 10.4103/ijc.IJC_506_18
8. Cheng J, Zhang H, Ma HZ. Decitabine combined with CAG for the treatment of atypical chronic myeloid leukemia: a case report and literature review. Transl Cancer Res (2020) 9(8):5015–9. doi: 10.21037/tcr-19-1806
9. Castellino A, Santambrogio E, Rapezzi D, Massaia M. Atypical chronic myeloid leukemia: new developments from molecular diagnosis to treatment. Medicina (Kaunas) (2021) 57(10):1104. doi: 10.3390/medicina57101104
10. Dao KT, Gotlib J, Deininger MMN, Oh ST, Cortes JE, Collins RH Jr, et al. Efficacy of ruxolitinib in patients with chronic neutrophilic leukemia and atypical chronic myeloid leukemia. J Clin Oncol (2020) 38(10):1006–18. doi: 10.1200/JCO.19.00895
11. Alam M, Ali S, Mohammad T, Hasan GM, Yadav DK, Hassan MI. B cell lymphoma 2: A potential therapeutic target for cancer therapy. Int J Mol Sci (2021) 22(19):10442. doi: 10.3390/ijms221910442
12. Wei Y, Cao Y, Sun R, Cheng L, Xiong X, Jin X, et al. Targeting bcl-2 proteins in acute myeloid leukemia. Front Oncol (2020) 10:584974. doi: 10.3389/fonc.2020.584974
13. Zhou JD, Zhang TJ, Xu ZJ, Gu Y, Ma JC, Li XX, et al. BCL2 overexpression: clinical implication and biological insights in acute myeloid leukemia. Diagn Pathol (2019) 14:68. doi: 10.1186/s13000-019-0841-1
14. DiNardo CD, . Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
Keywords: atypical chronic myeloid leukemia, Venetoclax, Azacitidine, combination therapy, allo-transplantation
Citation: Chen H, Wang N, Li Y, Xie X and Yang Y (2024) A case report of atypical chronic myeloid leukemia with complete hematological and major molecular response to Venetoclax/Azacitidine treatment. Front. Oncol. 14:1327834. doi: 10.3389/fonc.2024.1327834
Received: 25 October 2023; Accepted: 22 January 2024;
Published: 25 March 2024.
Edited by:
Mohamed A. Yassin, Qatar University, QatarReviewed by:
Ahmet Emre Eskazan, Istanbul University-Cerrahpasa, TürkiyeLucia Gozzo, University of Catania, Italy
Copyright © 2024 Chen, Wang, Li, Xie and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Yang, c3h6eHlhbmd5aUAxNjMuY29t; Xiaohong Xie, MTE0MzU4NTIyMkBxcS5jb20=
 Hongxia Chen
Hongxia Chen Ning Wang1
Ning Wang1 Yi Yang
Yi Yang