
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 23 July 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1326006
This article is part of the Research TopicSurgical Treatment of Thymic Epithelial Tumor and Myasthenia GravisView all 6 articles
A correction has been applied to this article in:
Corrigendum: Case report: A sustained survival benefit of third-line immunotherapy for refractory thymic carcinoma
Thymic carcinoma (TC) is an uncommon type of thymic epithelial tumors. Patients with relapsed or refractory TCs have a poor prognosis. Immune checkpoint inhibitor monotherapy can be applied as a second-line treatment for such cases. This study reported a TC patient who did not respond to conventional chemotherapy and radiotherapy but achieved prolonged partial remission lasting 17 months following the third-line treatment with anti-programmed cell death-1 inhibitor sintilimab. This patient did not experience any serious side effects associated with sintilimab treatment. The above results demonstrated that sintilimab could be a feasible therapeutic option for refractory TC patients.
Thymic carcinoma (TC) is a rare subgroup of aggressive thymic epithelial tumors according to the World Health Organization (WHO) 2015 classification (1). It is highly prone to distant metastases, with a five-year survival rate for stage IV patients of 21.2% (2). The standard treatment for metastatic or recurrent TC is systemic chemotherapy, and paclitaxel plus platinum is the preferred first-line regimen, with an objective response rate (ORR) ranging from 22% to 36% (3). Nevertheless, there is no standard second-line therapy. Previous studies showed that gemcitabine plus capecitabine and pemetrexed had a response rate of 19% to 36% for small sample researches with refractory TC (4, 5). Similarly, lenvatinib and sunitinib showed limited efficacy (6, 7). Therefore, a novel treatment strategy is urgently needed for refractory TC.
The PD-L1 expression level could predict immune efficacy. Pembrolizumab can effectively treat TC, with a median progression-free survival (PFS) time of 3.8 to 6.1 months (8), indicating that immunotherapy might be used as adjuvant therapy for refractory TC. Moreover, the application of immunotherapy in thymic epithelial tumors (TETs) has been quoted in National Comprehensive Cancer Network (NCCN) guidelines (8–11).
Sintilimab (Innovent Biologics, Suzhou, China) is a humanized monoclonal antibody that blocks the interaction between programmed cell death-1 (PD-1) and programmed death ligand 1 (PD-L1). It has been approved in China for the treatment of non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), Hodgkin’s lymphoma, and various solid tumors (12).
We reported a female patient who suffered from refractory TC and showed no response to chemotherapy and radiotherapy (RT). She received sintilimab monotherapy and achieved sustained partial remission (PR) lasting 17 months. Moreover, the patient had almost no sintilimab-related adverse events.
A 53-year-old female was admitted to our hospital in May 2019 due to shortness of breath for two months. The contrast-enhanced chest computed tomography (CT) revealed a large space-occupying lesion involving the heart and large vessels in the anterior mediastinal region. Histopathological stains of the thymic biopsy specimen revealed CK5/6 (+++), P63 (+++), CD117 (+++), CD5 (+++), NapsinA (–), CK7 (-), TTF1 (-), Vimentin (-), LCA (+++), and TdT (-) (Figure 1), indicating squamous cell carcinoma. The PD-L1 expression level was that the tumor proportion score (TPS) was found to be less than 1%, and the combine positive score (CPS) was found to be 1% (Figure 2). Therefore, the patient was diagnosed with Masaoka stage IIIB thymic squamous cell carcinoma. The neoadjuvant chemotherapy regimen of carboplatin AUC 6 plus paclitaxel 175 mg/m2 was administered every three weeks to reduce tumor size and make it resectable. The response evaluation criteria in solid tumors (RECIST) evaluated the treatment effect as stable disease (SD) after three treatment cycles. However, the patient could not tolerate the chemotherapy, and the neoadjuvant treatment was suspended.
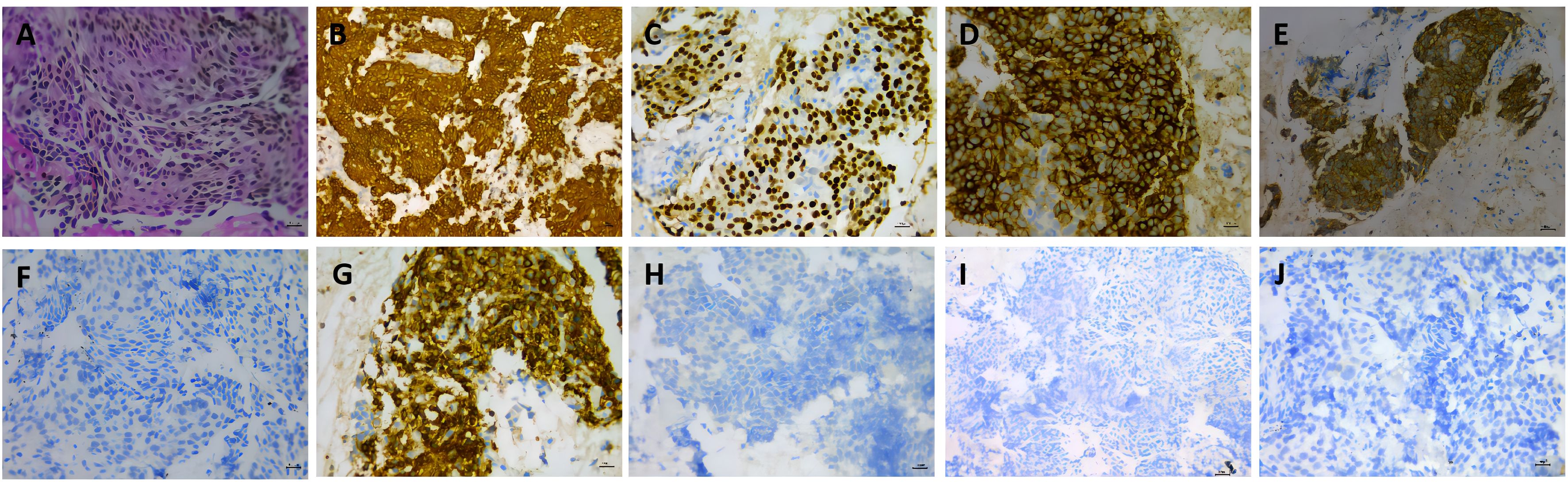
Figure 1. Histopathologic stains from thymic (A–J). (A) hematoxylin and eosin(400X); (B) showed the thymic tumor cells were cytoplasmic positive for CD5/6 (200X); (C) showed the thymic tumor cells were cytoplasmic positive for P63 (400X); (D) showed the thymic tumor cells were cytoplasmic positive for CD117 (400X); (E) showed the thymic tumor cells were cytoplasmic positive for CD5(400X); (F) showed the thymic tumor cells were cytoplasmic negative TTF1(400X); (G) showed the thymic tumor cells were cytoplasmic positive forLCA(400X); (H) showed the thymic tumor cells were cytoplasmic negative TdT(400X); (I) showed the thymic tumor cells were cytoplasmic negative NapsinA(200X); (J) showed the thymic tumor cells were cytoplasmic negative CK7 (400X).
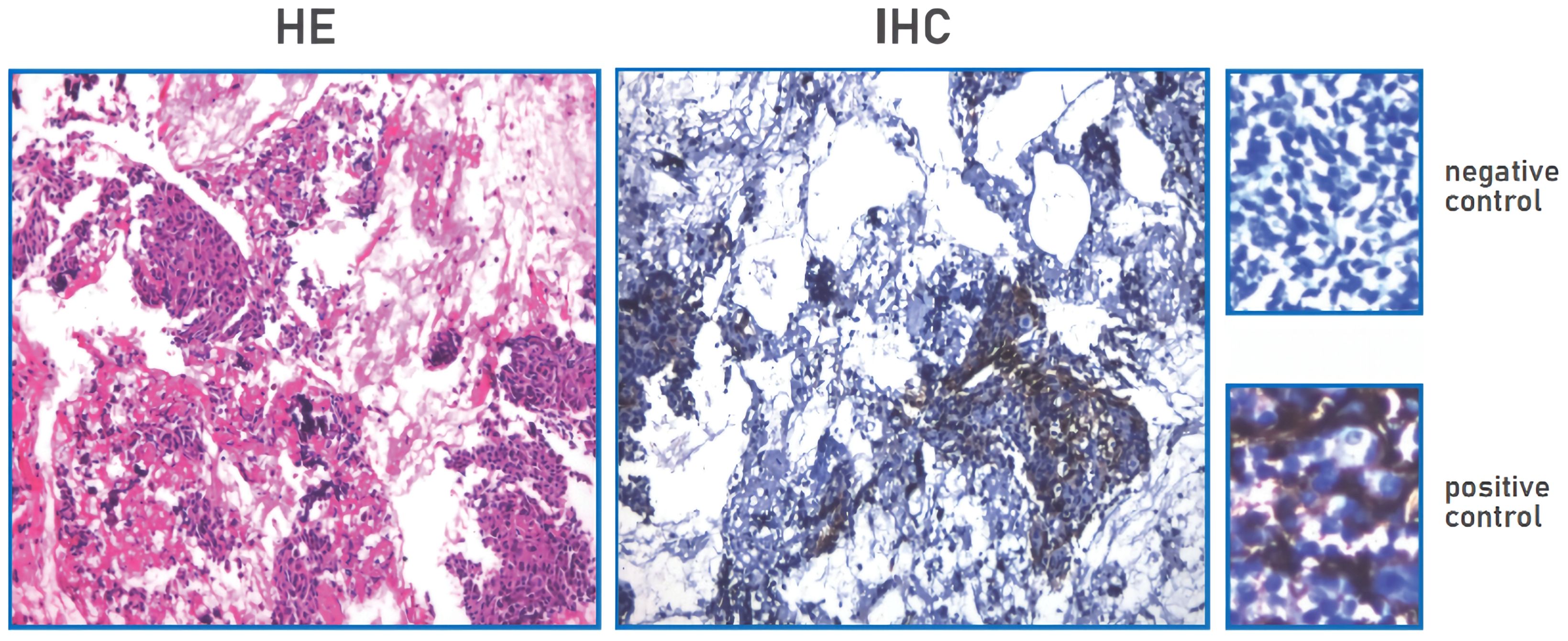
Figure 2. PD-L1 expression TPS negative, TPS<1% (TPS<1%); PD-L1 expression CPS positive, CPS≥1(CPS=1).
The patient’s shortness of breath was significantly worse in December 2020, with a larger anterior mediastinum lesion. Therefore, the case received concurrent chemoradiotherapy with cisplatin (60 mg/m2 on day 1) plus etoposide (120 mg/m2 on days 1-3) plus intensity-modulated radiation therapy (300 Gy each time, 20 times). However, the anterior mediastinum lesion did not significantly shrink, and the patient was deemed to have refractory TC. The patient’s shortness of breath was significantly relieved in April 2021 (3 weeks after initial sintilimab), following administration of 200mg of sintilimab every three weeks. Chest computed tomography (CT) revealed a clearly reduced the size of anterior mediastinum lesion, with a maximum diameter decreasing from 99 mm to 52 mm (Figure 3). Therefore, the treatment response was classified as partial response (PR). No severe side effects appeared during immunotherapy for 17 months (Figure 4).
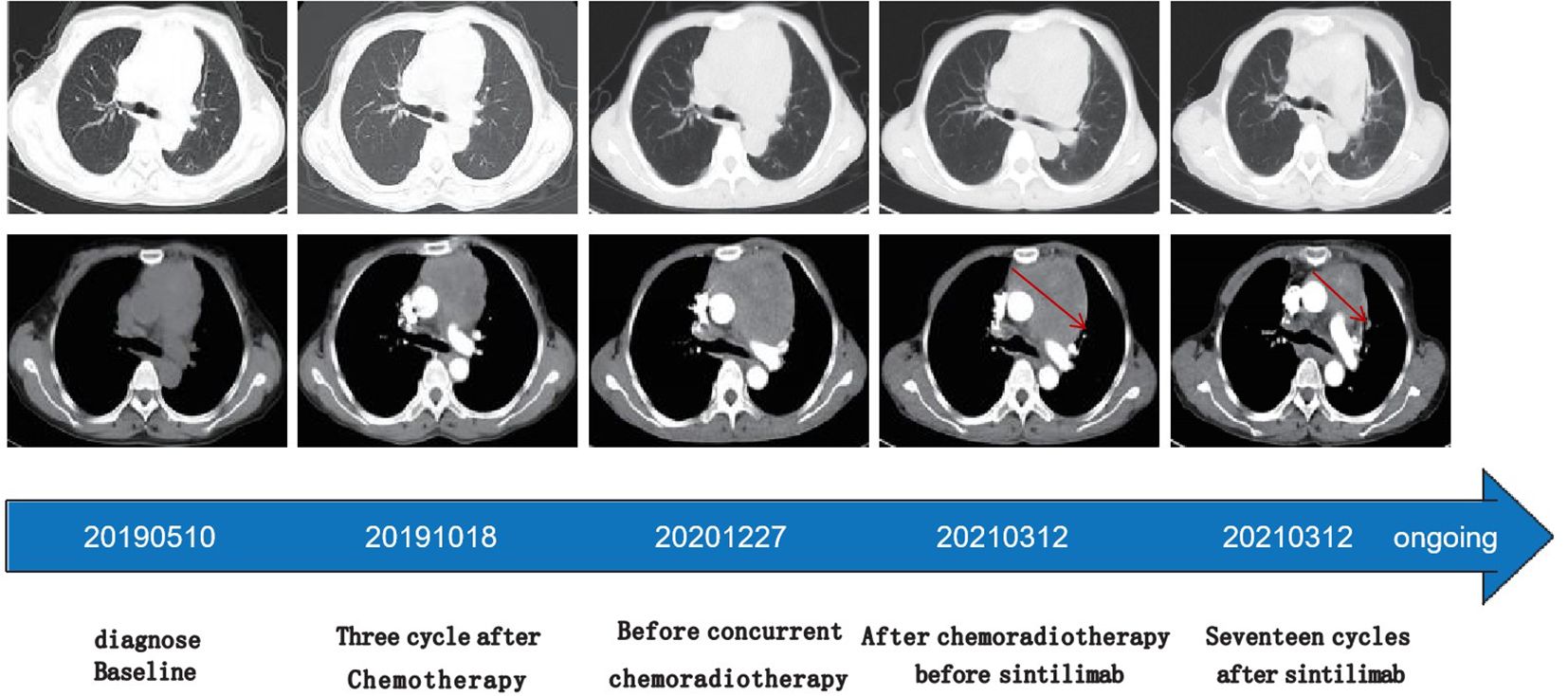
Figure 3. Time line of treatments of the patient and changes in CT scan of thymic carcinoma during treatments Chest computed tomography (CT) revealed a clearly reduced the size of anterior mediastinum lesion indic- ated by red arrows, with a maximum diameter decreasing from 99 mm to 52 mm.
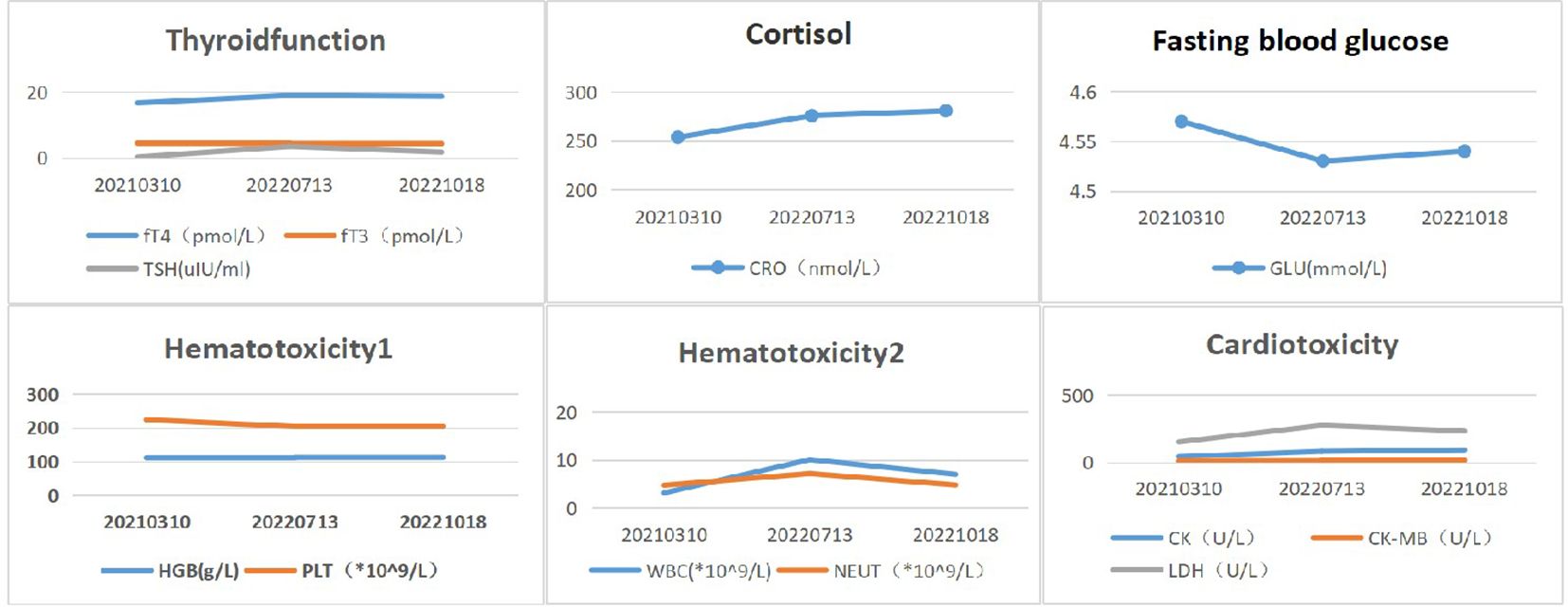
Figure 4. Biochemical and hematological changes after immunotherapy. Reference range:fT4 (8.7-17.3 pmol/L), fT3(2.8-7.1pmol/L), TSH(0.27-4.2uIU/ml), CRO(172-497nmol/L), GLU(3.9- 6.1mmol/L), HGB(115-15 0g/L), PLT(100-300*10^9/L), WBC(3.5-9.5*10^9/L), NEUT(1.8-6.3*10^9/ L), CK(26-140U/ L), CK-MB (0-24 U/L), LDH(140-271U/L).
This patient presented a partial remission for 17 months after receiving 200 mg of third-line sintilimab every three weeks. Sintilimab was not recommended for metastatic or recurrent TC. Our treatment plan was based on the patient’s resistance to chemoradiotherapy, and that sintilumab was the standard treatment for metastatic or recurrent lung squamous cell carcinoma. Moreover, the NCCN guidelines have recommended the anti-PD-1 inhibitor pembrolizumab monotherapy as the second-line treatment of TC. Besides, patients could afford the long-term use of sintilimab due to its economic advantages. This patient did not develop any significant side effects from radiation, chemotherapy, or immunotherapy.
The patient had a good prognosis after receiving sintilimab as the third-line treatment. Platinum-based chemotherapy is the standard treatment for advanced, unresectable TC, however, it has a short effect (13). Additionally, the treatment options are limited after the failure of platinum-based chemotherapy. Immune checkpoint inhibitors (ICIs) have recently shown promising efficacy in many solid tumors and have become the standard treatment for many cancers. High PD-L1 expression (36-80%) has been reported in TC cases (14–17), suggesting that the anti-PD-1/PD-L1 inhibitors might effectively treat unresectable TC. Despite having one of the lowest mutation rates among cancers (18), TETs had high PD-L1 levels, which could provide strong evidence for the ICI application (19). PD-L1 positivity (≥ 25% of tumor membrane expression) was frequently found in TETs, and a higher PD-L1 level was associated with longer overall survival rates (20). However, our patient had a PD-L1 level of less than 1%, suggesting that immunotherapy was not just beneficial for metastatic TC patients with PD-L1 positivity. There may be several reasons for this case. On the one hand,PD-L1 expression is not an absolute indicator of immune efficacy. PD-L1 expression exhibits intratumoral heterogeneity, that is to say, expression of PD-L1 may vary in slices of the same tissue. Moreover, the expression of PD-L1 also undergoes dynamic changes with treatment. Therefore, single point and single sampling may not fully evaluate the PD-L1 expression of tumors. On the other hand, it is widely recognized that some biomarkers can be used independently to predict immunotherapy effect of solid tumor. For instance, MSIH and dMMR are considered as biomarkers of immune response in colorectal cancer (21). TMB is regarded as an immune predictor in TC (22). In many other solid tumors, such as ovary, lung, breast cancer and malignant melanoma,CD8+T-TIL,and CD8+TRM cells can be consider as independent predictors of immunotherapy effect (23–26). Nevertheless, more evidences are required to support such a view. Unfortunately, our patient did not undergo genetic testing to understand the relevant mutations, nor did we undergo circulating immune marker testing to predict the effectiveness of immunotherapy. So no genetic information of this patient was available. Therefore, it is unknown whether our patient has genomic alterations which are responded to immunotherapy.
Radiotherapy(RT) has been found to strengthen systemic antitumor responses to immunotherapy, including activating cytotoxic T cells, triggering immunogenic cell death, and enhancing antigen presentation (27). Besides, it could increase PD-L1 expressions and promote effector T cells infiltration (28). Therefore, some cancer patients following RT manifested significant immunogenic patterns (29). This might explain why this case had a poor response to RT alone but responded well to the combination of RT plus immunotherapy. This may also be the reason why the patient in this case, despite having low PDL1 expression, has a good therapeutic effect with PD-L1. Sintilimab is an anti-PD-1 inhibitor. Previous research showed that RT plus immunotherapy yielded a better treatment effect in NSCLC patients (30). Prior case reports showed that sintilimab combined with radiotherapy could effectively treat HCC patients (31). However, there is no data on the treatment effect of immunotherapy after radiotherapy for TC.
Immunotherapy is a promising treatment in refractory TC patients. However, several studies have revealed that immune-related adverse events (irAE) were found in 15% to 62% of TET patients, such as those with myasthenia gravis (3-14%), hypothyroidism (13%), and myocarditis (5%) (8, 9, 11). Our patient received third-line sintilimab with a median PFS of 17 months and no significant irAEs.
The combined or sequential therapy of anti-PD-1 and RT might be a treatment option in refractory TC. Nevertheless, the finding should be evaluated in dedicated prospective studies.
The shortness of breath had already affected my daily life and work, causing me great anxiety. This treatment successfully addressed my difficulties; the doctor explanation and professional treatment group during my hospitalization helped me to largely relieved my fear of carcinoma. I was very relieved that the Immunotherapy process went smoothly without complications, and I consider that I received a very successful treatment. I would like to share my medical history and I have signed an informed consent form.
The study first reported that third-line sintilimab monotherapy could achieve effective disease control and sustained survival benefit in a TC patient, offering a treatment choice for unresectable TC patients with low PD-L1 expressions. Nevertheless, more clinical trials are required to clarify the safety and effectiveness of sintilimab immunotherapy in TC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MM: Writing – original draft, Writing – review & editing. BY: Writing – original draft, Writing – review & editing. JL: Writing – review & editing. YB: Writing – review & editing. LL: Writing – review & editing. SC: Writing – review & editing. SH: Writing – review & editing. HM: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guizhou Provincial Health Commission (2024GZWJKJXM0704,Grant gzwkj2023-115), The Natural Science Foundation of Guizhou Provincial Scientific and Technology Department Grant (Grant No. 2022(626), Grant No. 2021(386)), and the National Natural Science Foundation of China (81960513).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
TC, Thymic carcinoma; TETs, thymic epithelial tumors; PD-1, programmed cell death-1; PD-L1, programmed death ligand 1; RT, Radiotherapy.
1. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 world health organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. (2015) 10:1383–95. doi: 10.1097/JTO.0000000000000654
2. Ye C, Bao M, Li H, Liu X, Jiang G, Wang W. Surgery in Masaoka stage IV thymic carcinoma: a propensity-matched study based on the SEER database. J Thorac Dis. (2020) 12:659–71. doi: 10.21037/jtd
3. Hirai F, Yamanaka T, Taguchi K, Daga H, Ono A, Tanaka K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:363–8. doi: 10.1093/annonc/mdu541
4. Palmieri G, Buonerba C, Ottaviano M, Federico P, Calabrese F, Von Arx C, et al. Capecitabine plus gemcitabine in thymic epithelial tumors:final analysis of a Phase II trial. Future Oncol. (2014) 10:2141–7. doi: 10.2217/fon.14.144
5. Gbolahan OB, Porter RF, Salter JT, Yiannoutsos C, Burns M, Chiorean EG, et al. A phase II study of pemetrexed in patients with recurrent thymoma and thymic carcinoma. J Thorac Oncol. (2018) 13:1940–8. doi: 10.1016/j.jtho.2018.07.094
6. Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. (2015) 16:177–86. doi: 10.1016/S1470-2045(14)71181-7
7. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. (2020) 21:843–50. doi: 10.1016/S1470-2045(20)30162-5
8. Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. (2019) 37:2162–70. doi: 10.1200/JCO.2017.77.3184
9. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. (2018) 19:347–55. doi: 10.1016/S1470-2045(18)30062-7
10. Katsuya Y, Horinouchi H, Seto T, Umemura S, Hosomi Y, Satouchi M, et al. Single-arm,multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer. (2019) 113:78–86. doi: 10.1016/j.ejca.2019.03.012
11. Rajan A, Heery CR, Thomas A, Mammen AL, Perry S, O'Sullivan Coyne G, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody(Avelumab) treatment in advanced thymoma. J Immunother Cancer. (2019) 7:269. doi: 10.1186/s40425-019-0723-9
12. Hoy SM. Sintilimab: first global approval. Drugs. (2019) 79:341–6. doi: 10.1007/s40265-019-1066-z
13. He Y, Ramesh A, Gusev Y, Bhuvaneshwar K, Giaccone G. Molecular predictors of response to pembrolizumab in thymic carcinoma. Cell Rep Med. (2021) 2:100392. doi: 10.1016/j.xcrm.2021.100392
14. Padda SK, Riess JW, Schwartz EJ, Tian L, Kohrt HE, Neal JW, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. (2015) 10:500–8. doi: 10.1097/JTO.0000000000000429
15. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe S, Tsuta K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. (2015) 88:154–9. doi: 10.1016/j.lungcan.2015.03.003
16. Yokoyama S, Miyoshi H, Nishi T, Hashiguchi T, Mitsuoka M, Takamori S, et al. Clinicopathologic and prognostic implications of programmed death ligand 1 expression in thymoma. Ann Thorac Surg. (2016) 101:1361–9. doi: 10.1016/j.athoracsur.2015.10.044
17. Yokoyama S, Miyoshi H, Nakashima K, Shimono J, Hashiguchi T, Mitsuoka M, et al. Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res. (2016) 22:4727–34. doi: 10.1158/1078-0432.CCR-16-0434
18. Radovich M, Pickering CR, Felau I, Ha G, Zhang H, Jo H, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell. (2018) 33:244–58. doi: 10.1016/j.ccell.2018.01.003
19. Jakopovic M, Bitar L, Seiwerth F, Marusic A, Krpina K, Samarzija M. Immunotherapy for thymoma. J Thorac Dis. (2020) 12:7635–41. doi: 10.21037/jtd
20. Arbour KC, Naidoo J, Steele KE, Ni A, Moreira AL, Rekhtman N, et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PloS One. (2017) 12:e0182665. doi: 10.1371/journal.pone.0182665
21. Zhang X, Wu T, Cai X, Dong Jakopovic M, Bitar L, Seiwerth F, et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol. (2022) 13:795972. doi: 10.3389/fimmu.2022.795972
22. Girard N, Basse C, Schrock A, Ramkissoon S, Killian K, Ross JS. Comprehensive genomic profiling of 274 thymic epithelial tumors unveils oncogenic pathways and predictive biomarkers. Oncologist. (2022) 27:919–29. doi: 10.1093/oncolo/oyac115
23. Zheng QM, Li YY, Wang YP, Li GX, Zhao MM, Sun ZG. Association between CD8+tumor-infiltrating lymphocytes and prognosis of non-small cell lung cancer patients treated with PD-1/PD-L1 inhibitors: a systematic review and meta-analysis. Expert Rev Anticancer Ther. (2023) 23:643–59. doi: 10.1080/14737140.2023.2208351
24. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. (2020) 21:1346–58. doi: 10.1038/s41590-020-0769-3
25. Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discovery. (2020) 10:1808–25. doi: 10.1158/2159-8290.CD-20-0522
26. Damei I, Trickovic T, Mami-Chouaib F, Corgnac S. Tumor-resident memory T cells as a biomarker of the response to cancer immunotherapy. Front Immunol. (2023) 14:1205984. doi: 10.3389/fimmu.2023.1205984
27. Darragh LB, Oweida AJ, Karam SD. Overcoming resistance to combination radiation-immunotherapy: A focus on contributing pathways within the tumor microenvironment. Front Immunol. (2019) 9:3154. doi: 10.3389/fimmu.2018.03154
28. Weissferdt A, Fujimoto J, Kalhor N, Rodriguez J, Bassett R, Wistuba II, et al. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod Pathol. (2017) 30:826–33. doi: 10.1038/modpathol.2017.6
29. Yu WD, Sun G, Li J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. (2019) 452:66–70. doi: 10.1016/j.canlet.2019.02.048
30. Li S, Chen K, Yang M, Hlaing SS, Chen M, Gu P, et al. Radiotherapy improves the outcomes of immunotherapy with Sintilimab in non-small-cell lung cancer: A real-world analysis. Front Immunol. (2022) 13:991431. doi: 10.3389/fimmu.2022.991431
31. Liang X, Liu Q, Yao W, Zhu S. Encouraging efficacy of a comprehensive therapy consisting of sintilimab, bevacizumab biosimilar IBI305, hypo-fractionated intensity-modulated radiotherapy, and oxaliplatin for a maxillary metastasis from hepatocellular carcinoma: A case report and literature review. Front Oncol. (2022) 12:941454. doi: 10.3389/fonc.2022.941454
Keywords: sintilimab, thymic carcinoma(TC), therapeutic option, case report, anti-PD-1
Citation: Meng M, Yu B, Luo J, Bai Y, Li L, Chen S, He S and Ma H (2024) Case report: A sustained survival benefit of third-line immunotherapy for refractory thymic carcinoma. Front. Oncol. 14:1326006. doi: 10.3389/fonc.2024.1326006
Received: 22 October 2023; Accepted: 08 July 2024;
Published: 23 July 2024.
Edited by:
Piergiorgio Muriana, San Raffaele Scientific Institute (IRCCS), ItalyReviewed by:
Yongfeng He, Cornell University, United StatesCopyright © 2024 Meng, Yu, Luo, Bai, Li, Chen, He and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Ma, bWFodWFiQDE2My5jb20=; Sisi He, c2lzaWhlMTIxOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.