
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 31 January 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1325524
This article is part of the Research TopicClinical and Translational Research in Prostate Cancer - Volume IIView all 6 articles
Objective: The purpose of this study was to investigate the clinical significance of serum high sensitive C-reactive protein/albumin ratio in primary prostate biopsy.
Methods: Retrospective analysis was done on the clinical data of 1679 patients who had their first transrectal or perineal prostate biopsy at our situation from 2010 to 2018. Prostate cancer (PCa) and benign prostatic hyperplasia (BPH) were the pathologic diagnoses in 819 and 860 cases, respectively. A comparison was made between the HAR differences between PCa and BPH patients as well as the positive prostate biopsy rate differences between groups with increased and normal HAR. The results of the prostate biopsy were examined using logistic regression, and a model for predicting prostate cancer was created. The receiver characteristic curve (ROC) was used to determine the model’s prediction effectiveness. The clinical models integrated into HAR were evaluated for their potential to increase classification efficacy using net reclassification improvement (NRI) and integrated discrimination improvement (IDI). According to the Gleason score (GS) categorization system, prostate cancer patients were separated into low, middle, and high GS groups. The differences in HAR between the various groups were then compared. The prevalence of high GSPCa and metastatic PCa in normal populations and the prevalence of higher HAR in prostate cancer patients were compared using the chi-square test.
Result: Patients with PCa had a median HAR (upper quartile to lower quartile) of 0.0379 (10-3), patients with BPH had a median HAR (0.0137 (10-3)), and the difference was statistically significant (p<0.05). Patients with increased HAR and the normal group, respectively, had positive prostate biopsy rates of 52% (435/839)and 46% (384/840), and the difference was statistically significant (p<0.05). Logistic regression analysis showed that HAR (OR=3.391, 95%CI 2.082 ~ 4.977, P < 0.05), PSA density (PSAD) (OR=7.248, 95%CI 5.005 ~ 10.495, P < 0.05) and age (OR=1.076, 95%CI 1.056 ~ 1.096, P < 0.05) was an independent predictor of prostate biopsy results. Two prediction models are built: a clinical model based on age and PSAD, and a prediction model that adds HAR to the clinical model. The two models’ ROC had area under the curves (AUC) of 0.814 (95%CI 0.78-0.83) and 0.815 (95%CI 0.79-0.84), respectively. When compared to a single blood total PSA (tPSA) with an AUC of 0.746 (95%CI 0.718-0.774), they were all superior. Nevertheless, there was no statistically significant difference (p<0.05) between the two models. We assessed the prediction model integrated into HAR’s capacity to increase classification efficiency using NRI and IDI, and we discovered that NRI>0, IDI>0, and the difference was statistically significant (P>0.05).There was a statistically significant difference in HAR between various GS groups for individuals who had prostate cancer as a consequence of biopsy (p<0.05). The incidence of high GS and metastatic patients was statistically significantly greater (p<0.05) in the HAR elevated group (90.1%and 39.3%, respectively) than in the HAR normal group (84.4% and 12.0%).
Conclusion: Prostate biopsy results that were positive were impacted by HAR, an independent factor that increased with the rate of PCa discovery. Patients with elevated HAR had a greater risk of high GS as well as metastatic PCa among those with recently diagnosed prostate cancer through prostate biopsy.
In the US, prostate cancer (PCa) has the highest incidence and second-highest mortality rate among men. In 2019, it’s anticipated that there will be close to 150,000 new instances of PCa in the country, resulting in more than 31,000 deaths (1). The incidence of PCa in China is gradually rising along with the acceptance of screening techniques (2). An increasing number of studies have established a causal link between inflammation and tumors, and pro-inflammatory cytokines, myeloid cells infiltrating the tumor, and immune cells are important players in nearly all stages of the development of inflammation-induced cancer. According to research by Hanahan et al., inflammation can encourage the transformation of cancerous cells. Furthermore, the tumor microenvironment’s inflammatory response plays a crucial role in tumor angiogenesis, metastasis, immune escape, and treatment resistance in addition to aiding in the growth and survival of cancer cells (3–5).
The human liver produces C-reactive protein (CRP), which is one of the frequently used serum biomarkers to assess a patient’s level of inflammation. Research has indicated that patients with malignant tumors suffer various degrees of CRP elevation (6).The process by which CRP rises in cancer patients is associated with the growth and division of tumor cells, which in turn triggers the activation of inflammatory cells and associated components. One possible explanation is that CRP causes angiogenesis to proceed more quickly in cancer patients by elevating their levels of angiogenesis factor and interleukin circulation (7, 8). A high sensitivity CRP detection technique is desperately needed because traditional CRP detection may measure up to 350 mg/L but is unable to detect a minor increase in CRP. High-sensitivity C-reactive protein (hs-CRP) is the term for the process of accurately detecting extremely low concentrations of CRP in the laboratory using ultra-sensitive detection technologies. CRP is a sensitive indication that can be used to discriminate between low- and high-level inflammation (9).The liver also produces albumin (ALB), which is frequently used as a marker of liver function and malnutrition. The primary job of ALB is to keep the plasma colloid osmotic pressure constant in order to maintain the collective blood volume. Reduced serum albumin levels can be caused by infections, burns, liver illness, nephrotic syndrome, and cancers. Low albumin levels, which indicate a lower nutritional condition, are common in cancer patients and are known to interfere with immune systems such humoral and cellular immunity and phagocytosis (10). Albumin is also linked to immunological response and nutritional status in cancer patients. There are even reports that it can diagnose prostate cancer just as well as PSA (11, 12). It has even been claimed that ALB’s diagnostic efficacy for prostate cancer is not weaker than PSA (13). Growing interest has been given to the CAR ratio (C-reactive protein to albumin ratio) as a novel measure of systemic inflammatory response. When assessing a patient’s inflammatory status, it is more useful than ALB and CRP by itself. The prognosis of pancreatic cancer, hepatocellular carcinoma, non-small cell lung cancer, esophageal cancer, gastric cancer, and prostate cancer has been linked to CAR, according to earlier studies (14–19). High sensitive C-reactive protein (hs-CRP) and albumin levels were assessed in 1679 prostate biopsy patients in order to study the association between HAR—high sensitive C-reactive protein to albumin ratio—and prostate cancer to assess HAR’s importance in prostate biopsy.
An analysis of 1679 patients who were hospitalized to our hospital between 2010 and 2018 and had their first transrectal or perineal prostate biopsy guided by ultrasonography, confirmed by biopsy pathology, and who satisfied the inclusion and exclusion criteria, was done retrospectively. Inclusion criteria (1): Patients with initial prostate biopsy; (2) No chemoradiotherapy or endocrine therapy was received before puncture;(3) pathologically confirmed Pca or BPH;
Exclusion criteria: (1) Patients with tumors at other sites; (2) Patients with blood system diseases; (3) Patients with infection, inflammatory disease, myocardial infarction and other diseases affecting CRP level within one month before surgery; (4) A history of immune system disease; (5) serious liver and kidney function damage. A total of 1679 patients, aged 28-95 years old with an average age of 68 years old, were enrolled. The median tPSA was 13.85 (8.30~ 26.94) ng/ml, and the median PSAD was 0.34 (0.17~ 0.75) ng/(ml·cm3),the median value of HAR was 0.0351 (0.0147 ~ 0.0996)mg/g.There were 1049 cases of transrectal biopsy, 630 cases of perineal biopsy, 860 cases of BPH, and 819 cases of PCa, of which 602 cases (73.5%) were non-metastatic PCa and 217 cases (26.5%) were metastatic PCa. There were 103 patients (12.6%) with Gleason score ≤6, 292 patients (35.6%) with Gleason score 7 (3 + 4 or 4 + 3), and 424 patients (51.8%) with Gleason score ≥8. According to the median HAR, all cases were divided into normal HAR group (≤0.035mg/g) and elevated HAR group (> 0.035mg/g).This study met the convenient review criteria for retrospective studies of the Medical Ethics Committee of the First Affiliated Hospital of Soochow University, review number: (2021) No. 124.
After being admitted, the patient was subjected to regular blood sample, tPSA, blood, blood coagulation, and biochemical tests before being biopsyd. tPSA was measured using the chemiluminescence method, and the XT-4000i blood cell analyzer was used to measure leukocyte (WBC), hemoglobin (HB), neutrophil count (N), lymphocyte count (L), monocyte count (M), and platelet count (PLT). The Japanese SysmexCA-7000 hemagglutination equipment was used to quantify fibrinogen (FIB), and the OLYMPUS5400 automatic biochemical instrument was used to measure hs-CRP, ALB, and triglyceride (TG). Total prostate volume (TPV) is equal to 0.52 times the left-right diameter, anterior-posterior diameter, upper and lower diameter, and PSAD is equal to tPSA/TPV.
GE’s Logiq E9 ultra-high-end color Doppler ultrasound diagnostic system and Hitachi’s prior were used to execute ultrasound-guided transrectal or perineal prostate biopsies, respectively, utilizing an 18G automated biopsy needle made in the US by Bard. In both instances, 1-2 needles were placed into suspicious lesions as part of a 12-point (6-point left and right lobes) biopsy system. Both procedures were carried out by urologists. For the purpose of confirming the existence of bone metastases, all patients who had been diagnosed with prostate cancer underwent a bone scan. Additionally, patients whose diagnoses were unclear underwent MR, CT, or PET-CT scans.
PCa was classified into three groups using the GS grading system suggested in the 2014 International Association of Urology Pathology Consensus: low GS group, or GS grade 1, GS=6 points; middle GS group, GS grade 2 and 3, GS=7 points, including GS=3 + 4 points and GS=4 + 3 points; and high GS group, or GS grade 4 and 5, GS=8 points (20). Two doctors with at least three years of experience in prostate pathology rated the GS scores, and if the results were inconclusive, a pathologist with at least five years of experience was consulted.
SPSS24.0 was used to conduct the statistical analysis. The measures were indicated as median (upper quartile to lower quartile) and the age distribution was normal, represented as Mean ± SD. The rank sum test was used to compare data with skewness distribution between two or more groups; the c² test was used to compare rates; the t test was used to compare data with normality and homogeneity of variance between two groups; the F test was used to compare the mean of multiple groups with homogeneity of variance; and multivariate Analysis of the correlation between each variable and the prostate biopsy results was done using logistic regression analysis. The combination prediction model of prostate cancer and PSA was compared using the ROC and AUC. The model’s performance was assessed using ROC curve analysis, which yielded AUC. The Delong test was utilized to compare the AUC of several models, with a significance level of P<0.05 being statistically significant. The capacity of various models to increase classification efficiency was assessed using NRI and IDI. Positive improvement is indicated by NRI>0 and IDI>0.
The median HAR of 819 patients with PCa was 0.0379mg/g (0.0162 ~ 0.1334mg/g), and that of 860 patients with BPH was 0.0311mg/g (0.0137 ~ 0.0830mg/g). The HAR value of patients with PCa was higher than that of patients with BPH. The difference was statistically significant (P < 0.01). There were also significant differences in age, hs-CRP, ALB, hemolymph to monocyte ratio (LMR), tPSA, TPV, PSAD, and HB between PCa and BPH patients (P < 0.01). There were no statistically significant differences in BMI, WBC, PLT, blood neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), FIB, and TG (P > 0.05), as shown in Table 1.
There were 839 patients with elevated HAR (> 0.0351mg/g), including 435 patients with PCa, with a positive rate of 52%. There were 840 patients with normal HAR (≤0.0351mg/g), including 384 patients with PCa, with a biopsy positive rate of 46%, the difference between the two was statistically significant (χ²=6.319, P < 0.05), as shown in Table 2.
Logistic regression study revealed that the good outcomes of prostate puncture were independently influenced by HAR (OR=3.391, 95%CI 2.082-4.977, P < 0.05), PSAD (OR=7.248, 95%CI 5.005-10.495, P < 0.05), and age (OR=1.076, 95%CI 1.056 ~ 1.096, P < 0.05).,as shown in Table 3.The area under the curve for PCA was estimated by clinical models based on age and PSAD to be 0.814 (95%CI 0.78-0.83). The AUC of a comprehensive model that took age, PSAD, and HAR into account was 0.815 (95%CI 0.79-0.84). They were all better than a single blood total PSA (tPSA) with an AUC of 0.746 (95%CI 0.718-0.774),as shown in Figure 1.The results of the Delong test revealed that there was no statistically significant difference (P>0.05) between the two models. The potential of the comprehensive model to enhance the classification effect was further assessed using NRI and IDI. Positive improvement capacity was observed (NRI>0, IDI>0) in comparison to the single clinical model, and the difference was statistically significant (P<0.05),as shown in Table 4.
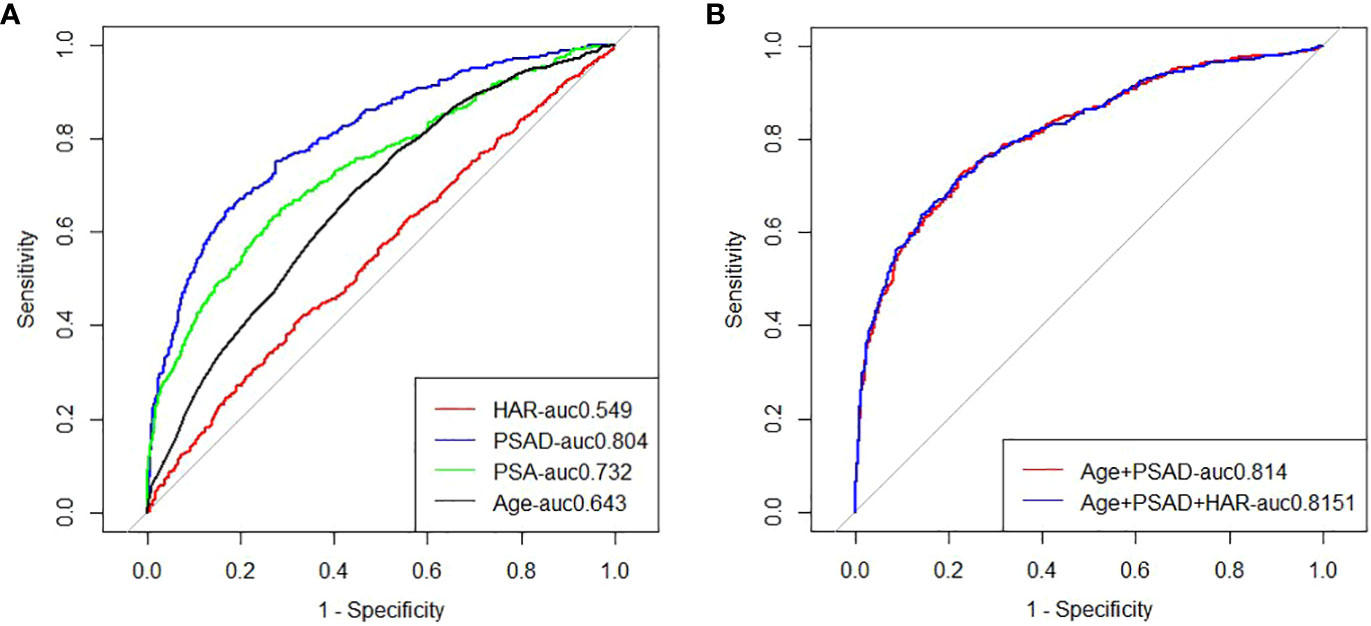
Figure 1 (A) ROC analysis of clinical risk factors predicting prostate biopsy for prostate cancer; (B) ROC analysis of clinical risk factors alone or in combination with HAR to predict prostate cancer from primary prostate biopsy.
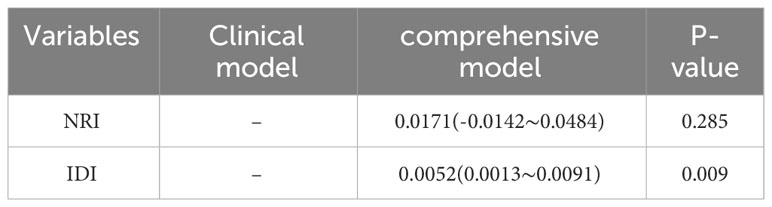
Table 4 Analysis of the increased predictive value of HAR in the diagnosis of Pca by primary prostate biopsy.
Except for Age and LMR, there were statistically significant differences in all indexes among different GS groups (all P < 0.05), as shown in Table 5.
Among the patients with prostate cancer whose initial biopsy results were obtained, the proportion of PCa with high GS in the HAR elevated group was 91.3%, the proportion of PCa with metastatic disease was 53.8%, the proportion of PCa with high GS in the HAR normal group was 85.4%, and the proportion of PCa with metastatic disease was 11.8%, both of which were higher in the HAR elevated group than in the HAR normal group (P < 0.05), as shown in Table 6.
Chronic inflammation and malignancies are intimately connected, and CRP is one of the most researched inflammatory indicators (21). In past research, we discovered that patients with high hs-CRP were more likely to have a prostate biopsy result that was positive and to have bone metastases (22, 23). According to a recent prospective study called PROCA-life, PCa risk and prognosis were worse in male patients with elevated hs-CRP (24). In this study, hs-CRP was also significantly greater in the PCa group than in the BPH group, and it was also significantly higher in the PCa group with high GS than in the PCa group with low and middle GS. Currently, it is thought that tumors and inflammation interact and have an impact on one another: Numerous factors that cause chronic inflammation, including amyloid (which causes physical damage), high-fat diet consumption, obesity, chemical damage, and intestinal and urinary tract microbes all contribute to the occurrence and development of prostate cancer in various ways (25). Most of these inflammatory reactions involve CRP. Inflammatory cytokines like interleukin-1 and interleukin-6, which act on the liver to produce hs-CRP, can be produced by inflammatory cells in the tumor microenvironment (26). According to the well-known Swedish AMORIS trial, ALB levels were found to be favorably associated with GS 4 + 3 prostate cancer and negatively associated with high-risk or metastatic prostate cancer 14 years before diagnosis (27). ALB in this study was shown to be significantly lower in the PCa group than the BPH group and to be significantly lower in the PCa group with high GS than the PCa group with low and intermediate GS. Most studies indicate that albumin is a key indication of the prognosis of tumor patients and that it is the primary reflection of the nutritional condition of tumor patients. The prognosis of patients is often poor when the nutritional status is poor at the advanced stage of the malignancy. Although nutritional status is typically unaffected before or in the early stage of tumor incidence, certain studies have revealed that albumin declines before or in the early stage of tumor occurrence (27, 28). We hypothesize that albumin contributes to the development of tumors or to its early stages. Currently, there are primarily two hypotheses that potentially account for this association between albumin and tumors: First, inflammatory mediators make capillaries more permeable, which results in intravascular albumin leakage into the tissue space (29). Second, decreased albumin production may be caused by interleukin-6, tumor necrosis factor, and acute phase reactants (30). In their initial investigation into the connection between CAR and prognosis in castration-resistant prostate cancer (CRPC), Kazumasa Komura et al. discovered that individuals with high CAR had shorter tumor-specific survival for CRPC patients treated with abiraterone or enzalutamide. In terms of predicting both overall and tumor-specific survival, CAR is an independent predictor.
For the first time, the relationship between HAR prior to the initial prostate biopsy and the biopsy results was examined in this study. It was discovered that HAR prior to the biopsy was highly correlated with the prostate biopsy positive rate, Gleason score, and distant metastasis of prostate cancer, indicating that HAR may be linked to the aggressiveness and malignancy of prostate cancer. Our findings confirm the two aforementioned theories about albumin and tumor, and they also explain why patients with high HAR in other studies had a worse prognosis. Selection bias could have resulted from this study’s single-center retrospective design and lack of external cohort validation. Serum HAR levels can be influenced by a wide range of circumstances. However, because these confounding factors could not have been controlled for in this investigation, the results may be biased. Second, in order to increase the prediction efficacy of the model, we neglected to incorporate prostate MRI and free/total PSA in our analysis because of the lengthy duration and dearth of clinical data. The lack of follow-up among the prostate cancer patients made it impossible to assess the correlation between HAR and the disease’s prognostic value or its association with survival, progression, or recurrence. Therefore, in order to confirm the findings of this investigation and provide greater insight into the diagnostic and prognostic utility of HAR for patients with prostate cancer, larger sample sizes, multi-center studies, and prospective designs are required in the future.
Serum PSA detection is cost-effective, practical, and less invasive. It has a high sensitivity but a low specificity, which leads to more unnecessary biopsies, particularly as the number of instances of clinically meaningless PCa rises year after year. Although it may be connected to the aberrant rise in PSA in the majority of the cases in this study, PSA is not a standalone factor in this study that influences the outcomes of prostate biopsy. Prostate inflammation, prostate volume, and transrectal digital examination of the prostate are additional factors contributing to the rise in cases of prostate cancer. Some investigations even reported that there was no statistical difference in PSA between prostate cancer and prostate hyperplasia in men with prostate biopsy (31). Occasionally, PSA struggles to discriminate these disorders efficiently. Although the development of multi-parameter magnetic resonance and targeted biopsy technology in recent years has increased the detection rate of clinically significant PCa (32, 33), these two technologies cannot currently be used in the majority of primary hospitals due to financial constraints and technical limitations. Therefore, tPSA, PSAD, and age should also be taken into account while evaluating the outcomes of prostate biopsy in clinical work. HAR is another option.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the risk of the study is minimal and the consent of the subject cannot be obtained. This study met the convenient review criteria for retrospective studies of the Medical Ethics Committee of the First Affiliated Hospital of Soochow University, review number: (2021) No. 124.
HY conceived the idea. HY designed the study. XC, YL, GX, GL and XZ collected and analyzed the data. XC,YL and YH provided methodology. XC and YL drafted the manuscript. HY and XC reviewed and corrected the manuscript. All authors contributed to the article and approved the submitted version.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
2. Liu Y, Uemura H, Ye D, Lee JY, Chiong E, Pu YS, et al. Prostate cancer in Asia: design of a patient registry to inform real-world treatments, outcomes, and quality of life. Prostate Int (2019) 7:108–13. doi: 10.1016/j.prnil.2018.12.001
3. Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer (2014) 110:1409–12. doi: 10.1038/bjc.2014.90
4. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev (2013) 39:534–40. doi: 10.1016/j.ctrv.2012.08.003
5. Mimeault M, Batra SK. Development of animal models underlining mechanistic connections between prostate inflammation and cancer. World J Clin Oncol (2013) 4:4–13. doi: 10.5306/wjco.v4.i1.4
6. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers (Basel) (2020) 12. doi: 10.3390/cancers12071986
7. Lee S, Choe JW, Kim HK, Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol (2011) 21:161–8. doi: 10.2188/jea.je20100128
8. Sciarra A, Gentilucci A, Salciccia S, Pierella F, Del Bianco F, Gentile V, et al. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: a critical review. J Inflammation (Lond) (2016) 13:35. doi: 10.1186/s12950-016-0143-2
9. Moutachakkir M, Lamrani Hanchi A, Baraou A, Boukhira A, Chellak S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann Biol Clin (Paris) (2017) 75:225–9. doi: 10.1684/abc.2017.1232
10. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr (2019) 43:181–93. doi: 10.1002/jpen.1451
11. Man YN, Chen YF. Systemic immune-inflammation index, serum albumin, and fibrinogen impact prognosis in castration-resistant prostate cancer patients treated with first-line docetaxel. Int Urol Nephrol (2019) 51:2189–99. doi: 10.1007/s11255-019-02265-4
12. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J (2010) 9:69. doi: 10.1186/1475-2891-9-69
13. Kaya C, Caliskan S, Sungur M, Aydin C. HALP score and albumin levels in men with prostate cancer and benign prostate hyperplasia. Int J Clin Pract (2021) 75:e13766. doi: 10.1111/ijcp.13766
14. Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol (2017) 24:561–8. doi: 10.1245/s10434-016-5579-3
15. Shen Y, Wang H, Li W, Chen J. Prognostic significance of the CRP/Alb and neutrophil to lymphocyte ratios in hepatocellular carcinoma patients undergoing TACE and RFA. J Clin Lab Anal (2019) 33:e22999. doi: 10.1002/jcla.22999
16. Ni XF, Wu J, Ji M, Shao YJ, Xu B, Jiang JT, et al. Effect of C-reactive protein/albumin ratio on prognosis in advanced non-small-cell lung cancer. Asia Pac J Clin Oncol (2018) 14:402–9. doi: 10.1111/ajco.13055
17. Tamagawa H, Aoyama T, Tamagawa A, Komori K, Maezawa Y, Kano K, et al. Influence of the preoperative C-reactive protein-to-albumin ratio on survival and recurrence in patients with esophageal cancer. Anticancer Res (2020) 40:2365–71. doi: 10.21873/anticanres.14205
18. Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric cancer patients. World J Surg (2018) 42:1819–25. doi: 10.1007/s00268-017-4400-1
19. Uchimoto T, Komura K, Fujiwara Y, Saito K, Tanda N, Matsunaga T, et al. Prognostic impact of C-reactive protein-albumin ratio for the lethality in castration-resistant prostate cancer. Med Oncol (2019) 37:9. doi: 10.1007/s12032-019-1332-7
20. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol (2016) 40:244–52. doi: 10.1097/PAS.0000000000000530
21. Michels N, van Aart C, Morisse J, Mullee A, Huybrechts I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit Rev Oncol Hematol (2021) 157:103177. doi: 10.1016/j.critrevonc.2020.103177
22. Chen Y, Li G, Xie G, Zhang X, Yin H, Hu Q, et al. The correlation between high sensitive C-reactive protein and bone metastasis of patients with newly diagnosed prostate cancer. Chin J Urol (2016) 37:772–6. doi: 10.3760/cma.j.issn.1000-6702.2016.10.012
23. Stikbakke E, Richardsen E, Knutsen T, Wilsgaard T, Giovannucci EL, McTiernan A, et al. Inflammatory serum markers and risk and severity of prostate cancer: The PROCA-life study. Int J Cancer (2020) 147:84–92. doi: 10.1002/ijc.32718
24. de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, et al. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer (2020) 20:455–69. doi: 10.1038/s41568-020-0267-9
25. Groblewska M, Mroczko B, Sosnowska D, Szmitkowski M. Interleukin 6 and C-reactive protein in esophageal cancer. Clin Chim Acta (2012) 413:1583–90. doi: 10.1016/j.cca.2012.05.009
26. Arthur R, Williams R, Garmo H, Holmberg L, Stattin P, Malmström H, et al. Serum inflammatory markers in relation to prostate cancer severity and death in the Swedish AMORIS study. Int J Cancer (2018) 142:2254–62. doi: 10.1002/ijc.31256
27. Sejima T, Iwamoto H, Masago T, Morizane S, Yao A, Isoyama T, et al. Low pre-operative levels of serum albumin predict lymph node metastases and ultimately correlate with a biochemical recurrence of prostate cancer in radical prostatectomy patients. Cent Eur J Urol (2013) 66:126–32. doi: 10.5173/ceju.2013.02.art3
28. Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet (1985) 1:781–4. doi: 10.1016/s0140-6736(85)91447-3
29. Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol (1994) 95:366–72. doi: 10.1111/j.1365-2249.1994.tb07005.x
30. Erdogan A, Polat S, Keskin E, Turan A. Is prostate volume better than PSA density and free/total PSA ratio in predicting prostate cancer in patients with PSA 2.5-10 ng/mL and 10.1-30 ng/mL? Aging Male (2020) 23:59–65. doi: 10.1080/13685538.2019.1578741
31. Hou J, Xi Q, Pu J, Huang C, Ouyang J, Li G, et al. The value of transrectal ultrasound and magnetic resonance imaging fusion targeted prostate biopsy in biopsy-naive men. Chin J Urol (2017) 38:469–72. doi: 10.3760/cma.j.issn.1000-6702.2017.06.016
32. Mo X, Li Ga Cai X, Zhang X, Tang J, Pu J, et al. Clinical significance of serum high sensitive C-reactive protein in patients undergone prostate biopsy. Chin J Urol (2014) 35:461–4. doi: 10.3760/cma.j.issn.1000-6702.2014.06.017
33. Lee AYM, Yang XY, Lee HJ, Law YM, Huang HH, Lau WKO, et al. Multiparametric MRI-ultrasonography software fusion prostate biopsy: initial results using a stereotactic robotic-assisted transperineal prostate biopsy platform comparing systematic vs targeted biopsy. BJU Int (2020) 126:568–76. doi: 10.1111/bju.15118
Keywords: high sensitive C-reactive protein-to-albumin ratio, prostate cancer, primary prostate biopsy, Gleason score, BPH
Citation: Chen X, Li Y, Li G, Zhang X, Xie G, Huang Y and Yin H (2024) Clinical significance of serum high sensitive C-reactive protein/albumin ratio in primary prostate biopsy. Front. Oncol. 14:1325524. doi: 10.3389/fonc.2024.1325524
Received: 21 October 2023; Accepted: 09 January 2024;
Published: 31 January 2024.
Edited by:
Sifeng Qu, Shandong University, ChinaReviewed by:
Kenneth Chen, Singapore General Hospital, SingaporeCopyright © 2024 Chen, Li, Li, Zhang, Xie, Huang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huming Yin, aHVsb3ZlMTIzQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.