- 1Department of General Surgery, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, China
- 2Division of Cardiology, The First Affiliated Hospital of Soochow University, Suzhou, China
Background: Serum albumin levels and cancer mortality are closely related, yet large-sample studies encompassing a broad spectrum of cancer types are lacking.
Methods: This study encompassed patients diagnosed with cancer across the continuous 10 cycles of NHANES surveys from 1999 to 2018. The study population was stratified into two groups based on median albumin levels (≤ 4.2g/dL and > 4.2 g/dL) or cancer aggressiveness (well-survived cancers and poorly-survived cancers). Survival rates were estimated using the Kaplan-Meier method. The Cox proportional hazards model was employed to evaluate the association between serum albumin levels and cancer mortality. Restricted cubic spline (RCS) analysis was conducted to assess the nonlinear relationship between serum albumin levels and the risk of cancer mortality.
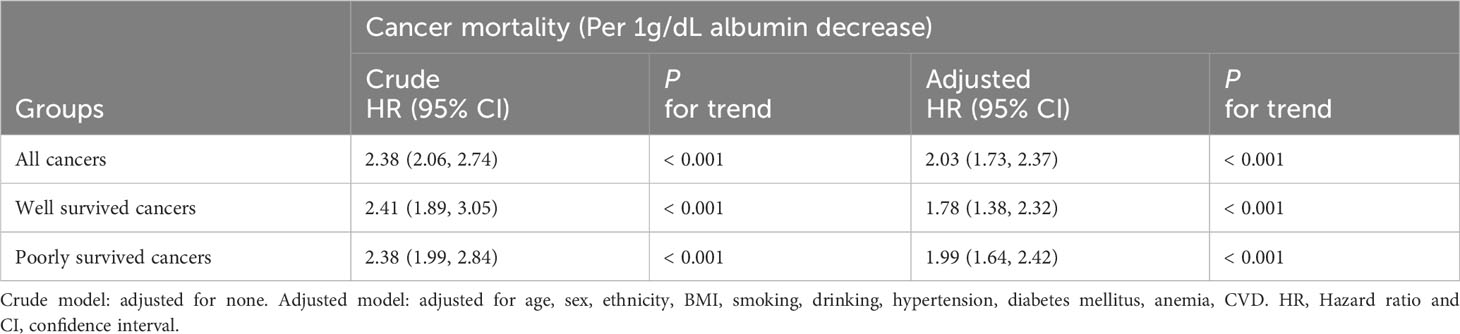
Results: Kaplan-Meier curves demonstrated that patients with albumin levels ≤ 4.2 g/dL exhibited lower survival rates compared to those with levels > 4.2 g/dL, irrespective of cancer aggressiveness. Following adjustment for confounders, decreased albumin levels were associated with an elevated risk of cancer mortality across all groups [all cancers, HR (95%CI) = 2.03(1.73, 2.37); well survived cancers, HR (95%CI) = 1.78(1.38, 2.32); and poorly survived cancers, HR (95%CI) = 1.99(1.64, 2.42)]. RCS analyses revealed a stable nonlinear negative association between albumin levels and cancer mortality in all groups, regardless of confounder adjustment.
Conclusion: Low serum albumin levels predict higher cancer mortality. Furthermore, a nonlinear negative association was observed between serum albumin levels and the risk of cancer mortality.
Introduction
Albumin, the most prevalent protein in the bloodstream, plays a pivotal role in upholding the oncotic pressure of the blood, as well as in the binding and transportation of diverse substances and drugs (1). Additionally, it possesses anti-inflammatory, antioxidant, and antithrombotic attributes (2–5). A multitude of conditions can lead to a reduction in serum albumin levels, including malnutrition, liver injury, renal impairment, elevated catabolism, intestinal fluid loss, and more. Serum albumin levels are commonly employed as a criterion for evaluating patient nutrition status, a parameter intrinsically linked to disease outcome (6). Low serum albumin levels serve as a prognostic indicator for various diseases, including cardiovascular conditions, renal disorders, trauma cirrhosis, and COVID-19 (7–10).
Empirical evidence corroborates the significance of serum albumin levels as a potent prognostic indicator of cancer-related mortality across various patient cohorts and general populations. The 5-year survival rate for patients with Cardia adenocarcinoma, who had normal albumin levels, was significantly higher compared to those with abnormal albumin levels (38.4% vs 19.1%, P = 0.0003) (11). Colorectal cancer patients with albumin levels above 3.5 g/dL had a significantly higher 5-year survival rate compared to those with levels below 3.5 g/dL (66% vs 34%, P < 0.001) (12). Research into cause-specific mortality within the general community population has shown that low serum albumin levels are linked to increased cancer mortality (13–15). E D’Erasmo and colleagues found that serum albumin levels at admission could predict mortality and clinical outcomes at discharge in elderly patients (16). In a study involving colorectal cancer patients, an increase of 0.1 g/dL in albumin levels was associated with a 7.3% decrease in morbidity and a 15.6% decrease in mortality (17). However, these studies focusing on specific cancer types have limitations, including small sample sizes and a lack of investigation into nonlinear relationships. In this study, we utilized the NHANES (1999-2018) database to enroll a total of 4430 cancer patients in order to analyze the relationship between serum albumin levels and cancer mortality.
Methods
Study population
Data for this study population were obtained from the National Health and Nutrition Examination Survey (NHANES). NHANES, a platform open to the public, used a sophisticated probability sampling design to represent the non-institutionalized civilian population of the United States and was designed to assess the health and nutritional status of the U.S. population. NHANES investigated and collected a wide range of information related to demographics, lifestyle habits, diet, disease, and survival follow-up. The survey protocol was approved by the National Institute of Health Research Ethics Review Board, and all participants signed and provided informed consent. This study included patients who were diagnosed with cancer in the continuous 10 cycles of NHANES survey of 1999-2018. The exclusion criteria were as follows: i) age < 18; ii) non-cancer caused death; and iii) missing data on cancer. The study population was evenly divided into 2 groups based on median albumin (≤ 4.2 and > 4.2 g/dL). In addition, the study population was divided into two groups for comparative analysis based on the aggressiveness of the cancer [well survived cancers (Thyroid, Breast, Prostate and Non-melanoma Skin) and poorly survived cancers (Other cancer types)].
Definition and measurement of variables
The primary outcome was cancer mortality. Survival follow-up began on the day of the participant’s interview and ended on December 31, 2019, with a median follow-up time of 85 months. All patients were diagnosed with cancer before albumin was measured. Patients’ blood samples were collected in the morning after an 8-hour fast, and then serum albumin levels were measured using the DcX800 method. Variables included age, sex, ethnicity, BMI, smoking, drinking, hypertension, diabetes mellitus, anemia, CVD were obtained by physical measurements, questionnaires, and laboratory tests. BMI was evaluated by body mass (kilograms) and body height (m2). The definitions of smoking and drinking referred to the Ministry of Health NZ website (18). The diagnosis of diabetes referred to the most recent ADA criteria. Hypertension was defined as self-reported by asking the question, “Have you been told by a doctor that you have hypertension?”. The diagnosis of anemia referred to the WHO criteria of men <13g/dL and women <12g/dL. The definition of CVD referred to International Classification of Diseases (ICD). Details on the methods of all variables are publicly available on the NHANES website (19).
Statistical analysis
For continuous and categorical variables, baseline data were presented as the mean ± SD and percentages, respectively. The Cochran-Armitage test was used to test for between-group trends. The Kaplan-Meier method was used to estimate survival rates, and log-rank test was used to test whether the survival difference between groups was statistically significant. Univariate and multivariate Cox proportional hazards model were used to assess the association between serum albumin levels and cancer mortality. Restricted cubic spline (RCS)-fitted piecewise logistic model was used to assess the nonlinear association between serum albumin levels and the risk of cancer mortality (hazard ratio, HR). Sensitivity analysis and subgroup analysis were performed to test whether the association between serum albumin levels and cancer mortality was consistent in different subgroups or specific cancer groups. Multiple imputation (Chained equations, 25 times) was used to fill in the missing values. Full analysis was performed using Stata 17 (Stata Corp, TX, US). All tests were two-sided. Statistical significance was considered when P < 0.05.
Results
Baseline characteristics
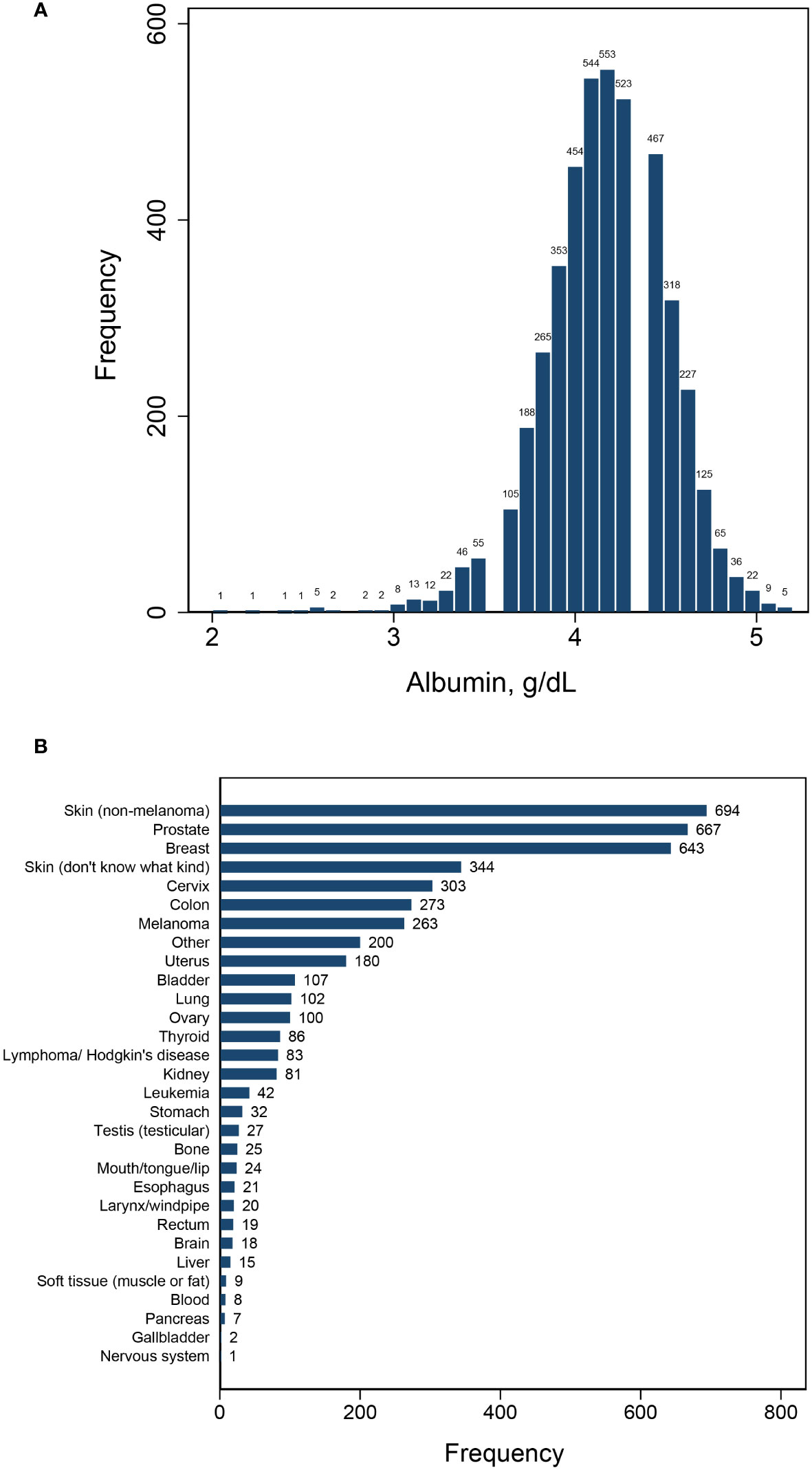
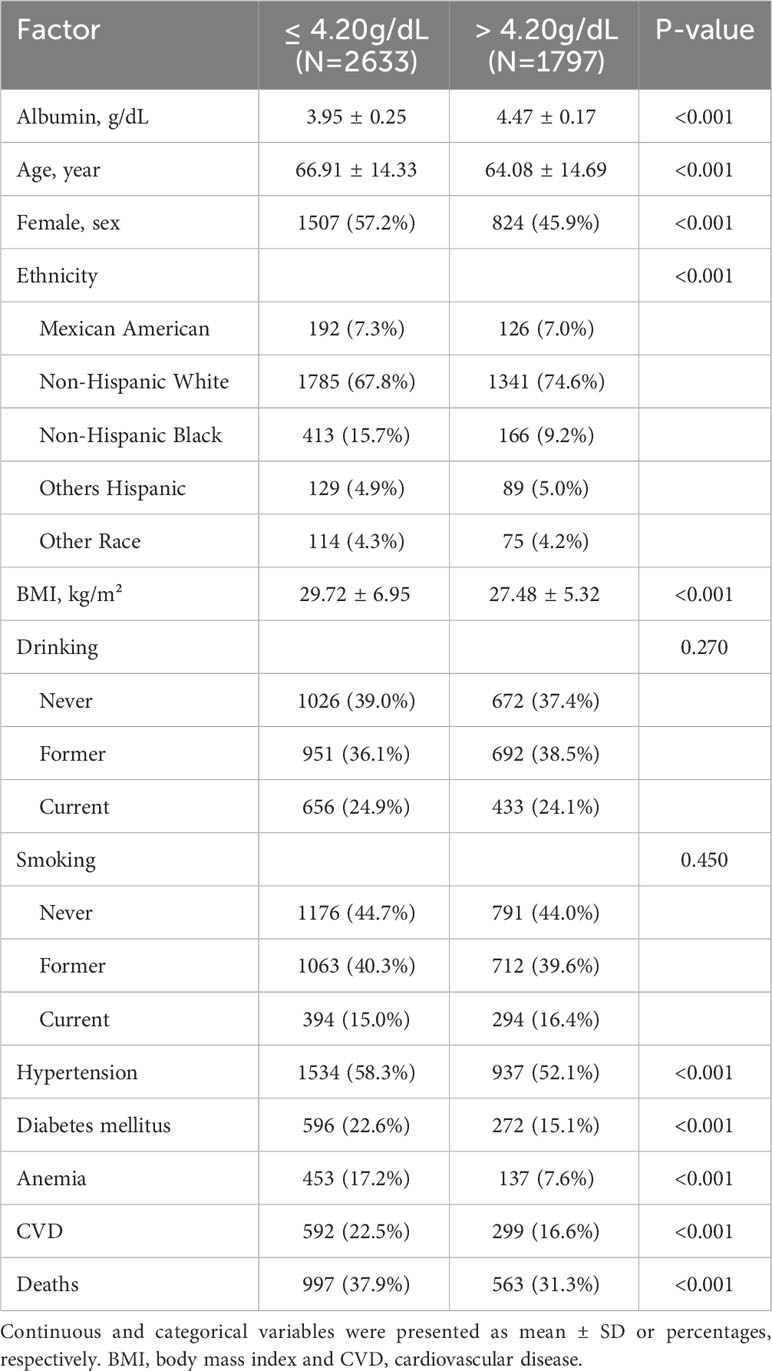
This study encompassed a total of 4,430 patients with cancer, with 1,560 deaths occurring throughout a median follow-up period of 85 months. The patient population consisted of 2,202 individuals who survived less than 85 months and 2,228 who survived beyond that timeframe. Serum albumin levels were found to be normally distributed, with a mean ± SD of 4.16 ± 0.34 and a median (IQR) of 4.2 (0.4). A diverse range of cancer diagnoses were made, encompassing over 30 different types, led by skin cancer (non-melanoma), breast cancer, and prostate cancer (Figure 1). Upon segmentation based the median albumin level, there were 2,633 cases with albumin levels ≤ 4.2 g/dL and 1,797 cases with albumin levels > 4.2 g/dL (Table 1). Notably, cancer patients with albumin levels ≤ 4.2 g/dL were characterized by older age, a higher likelihood of being female, a greater propensity for being overweight, and a statistically significant increase in the prevalence of diabetes mellitus, hypertension, cardiovascular disease, and anemia. Furthermore, these patients exhibited a higher mortality rate compared to those with albumin levels > 4.2 g/dL (Table 1).
Association between serum albumin levels and cancer mortality
The Kaplan-Meier survival curves revealed that patients with serum albumin levels ≤ 4.2 g/dL experienced a lower overall survival rate compared to those with levels > 4.2 g/dL, regardless of the extent of cancer malignancy (Figure 2).
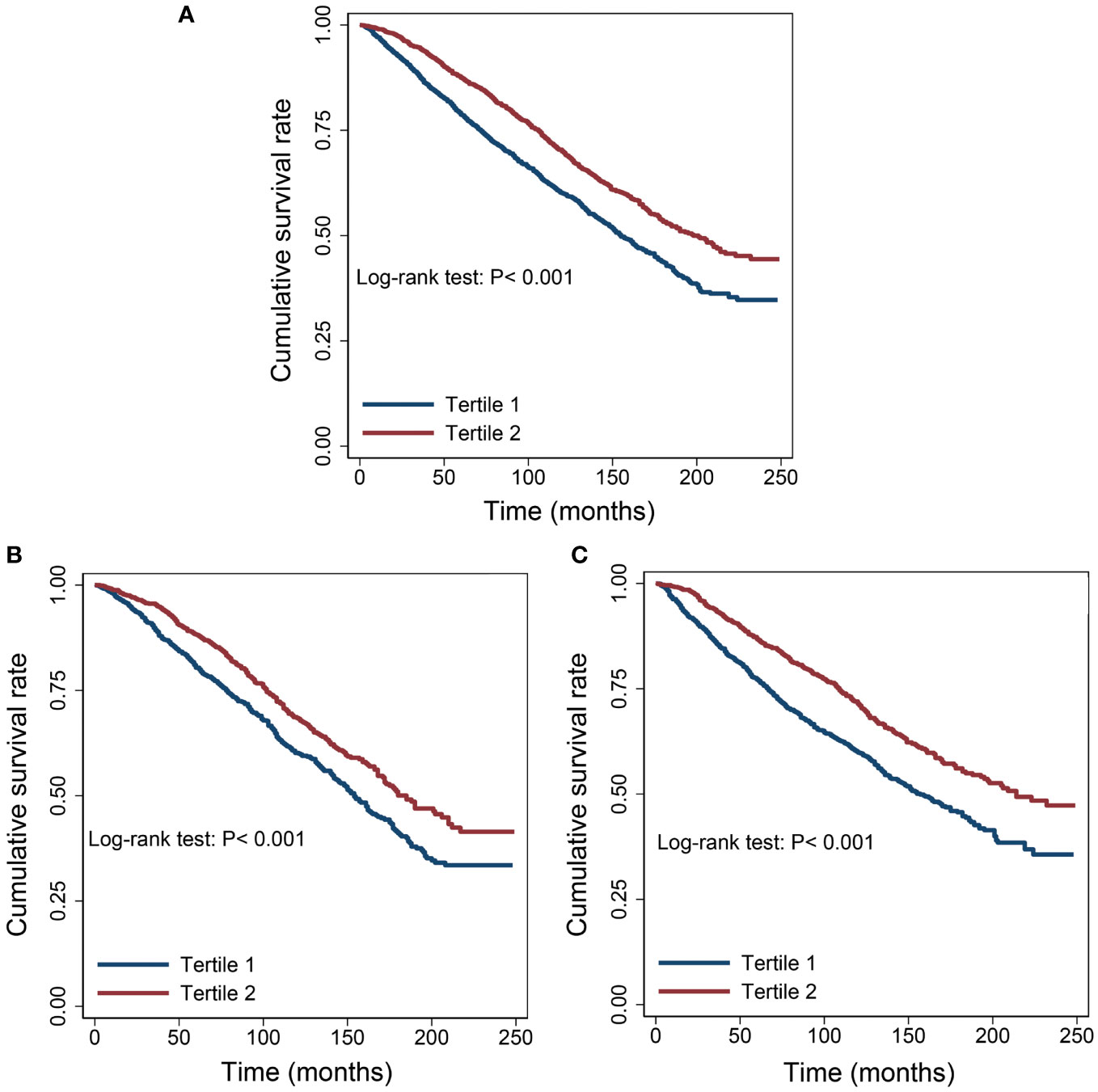
Figure 2 Survival differences between the low albumin group and the high albumin group. (A) All cancers. (B) Well survived cancers (Thyroid, Breast, Prostate and Non-melanoma Skin). (C) Poorly survived cancers (Other cancers except “B”).
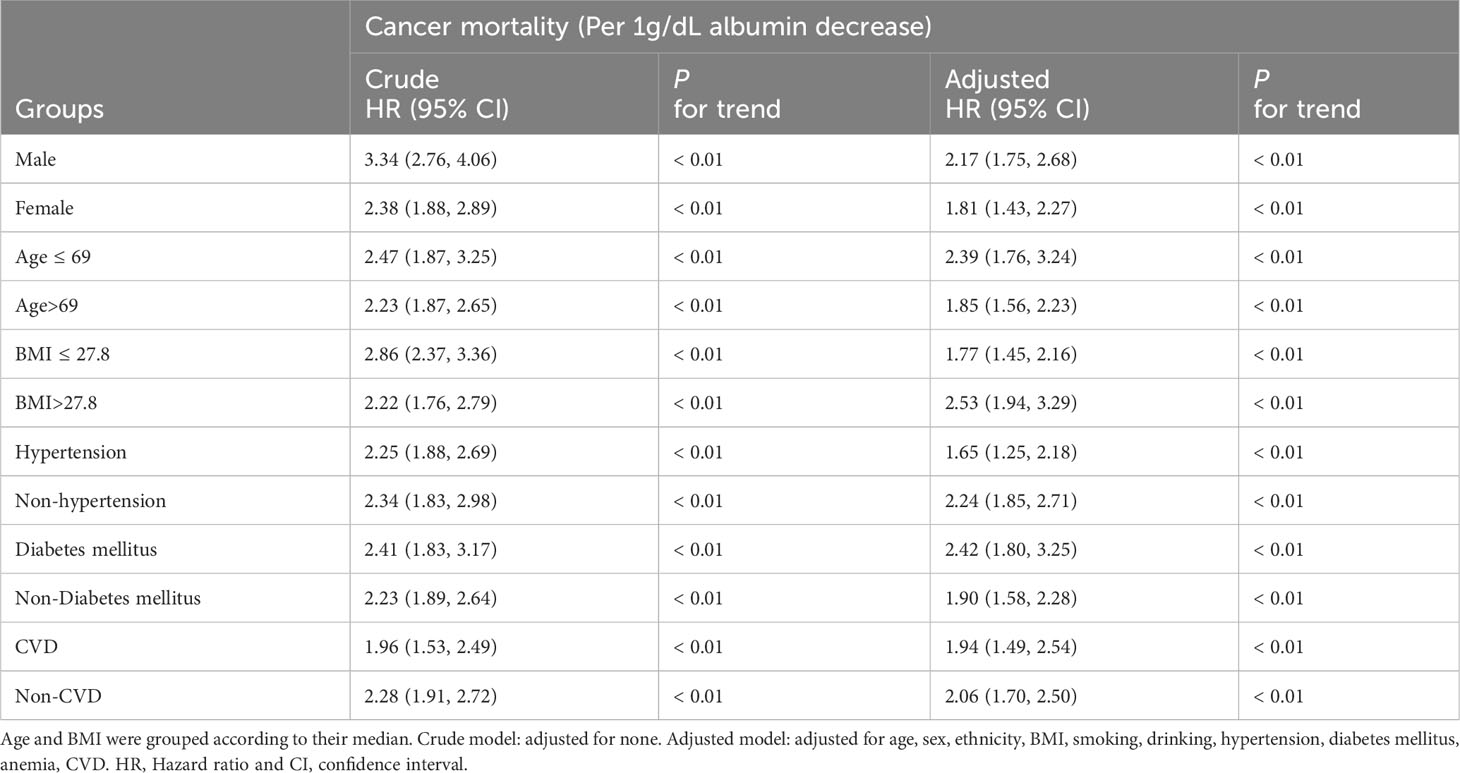
When adjusting for potential confounders, on a continuous scale, each decrease in albumin level was associated with an elevated risk of cancer-related mortality across all patient groups [all cancers, HR (95%CI) = 2.03(1.73, 2.37); well survived cancers, HR (95%CI) = 1.78(1.38, 2.32); and poorly survived cancers, HR (95%CI) = 1.99(1.64, 2.42)] (Table 2).
In terms of categorical analysis, multivariate Cox proportional hazards regression demonstrated that patients with albumin levels ≤ 4.2 g/dL carried a greater mortality risk than those with levels > 4.2 g/dL across all cancer groups. [all cancers, HR (95%CI) = 1.23(1.10, 1.37); well survived cancers, HR (95%CI) = 1.12(1.05, 1.30); and poorly survived cancers, HR (95%CI) = 1.30(1.12, 1.50)] (Table 3).
RCS analyses highlighted a consistent and negative nonlinear relationship between albumin levels and cancer mortality risk within all patient groups, with or without the adjustment for confounding factors (Figure 3).
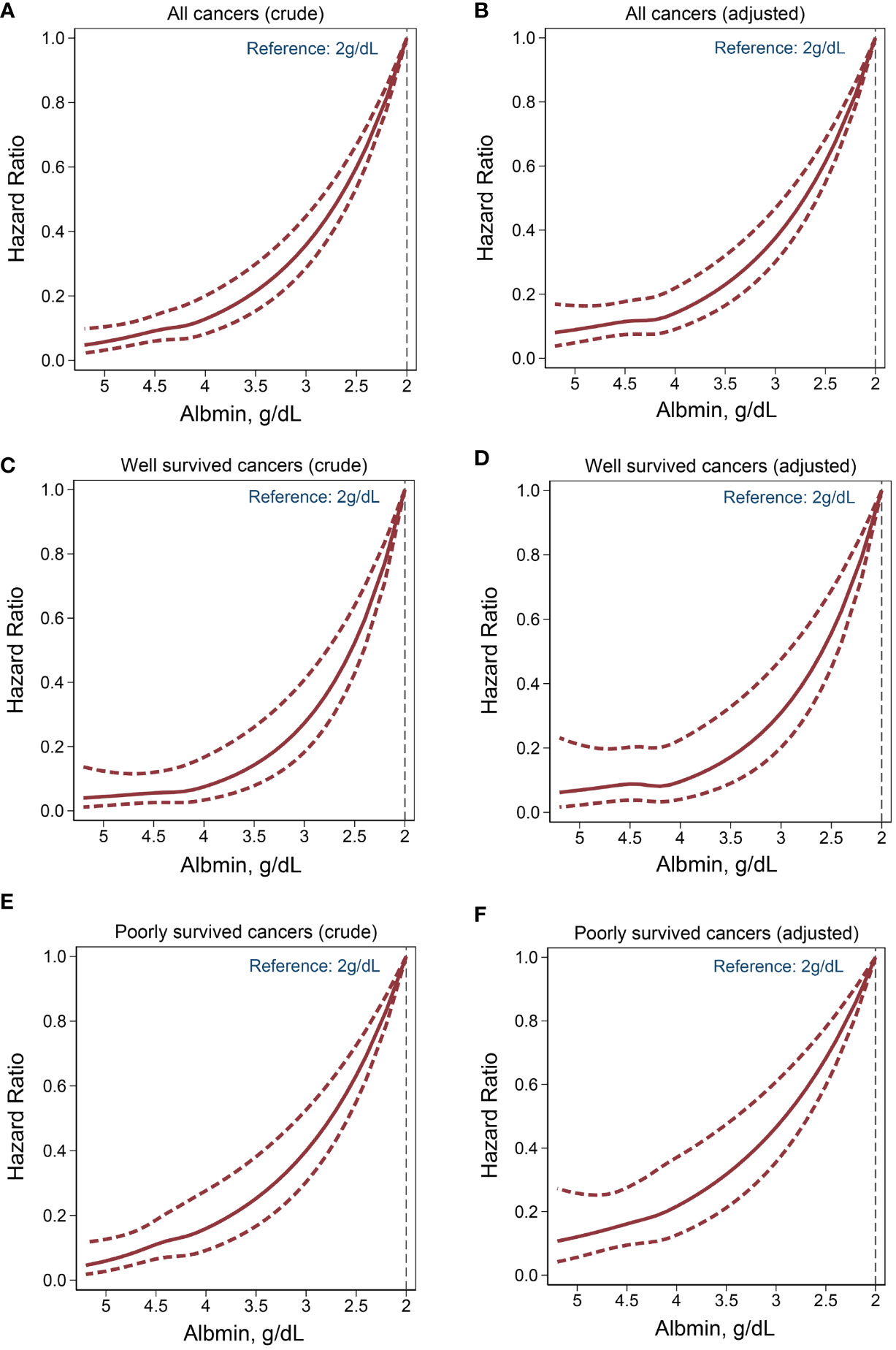
Figure 3 Nonlinear association between serum albumin levels and cancer mortality. Crude model: adjusted for none. Adjusted model: adjusted for age, sex, ethnicity, BMI, smoking, drinking, hypertension, diabetes mellitus, anemia, CVD. (A, B) All cancers. (C, D) Well survived cancers. (E, F) Poorly survived cancers.
Sensitivity and subgroup analysis
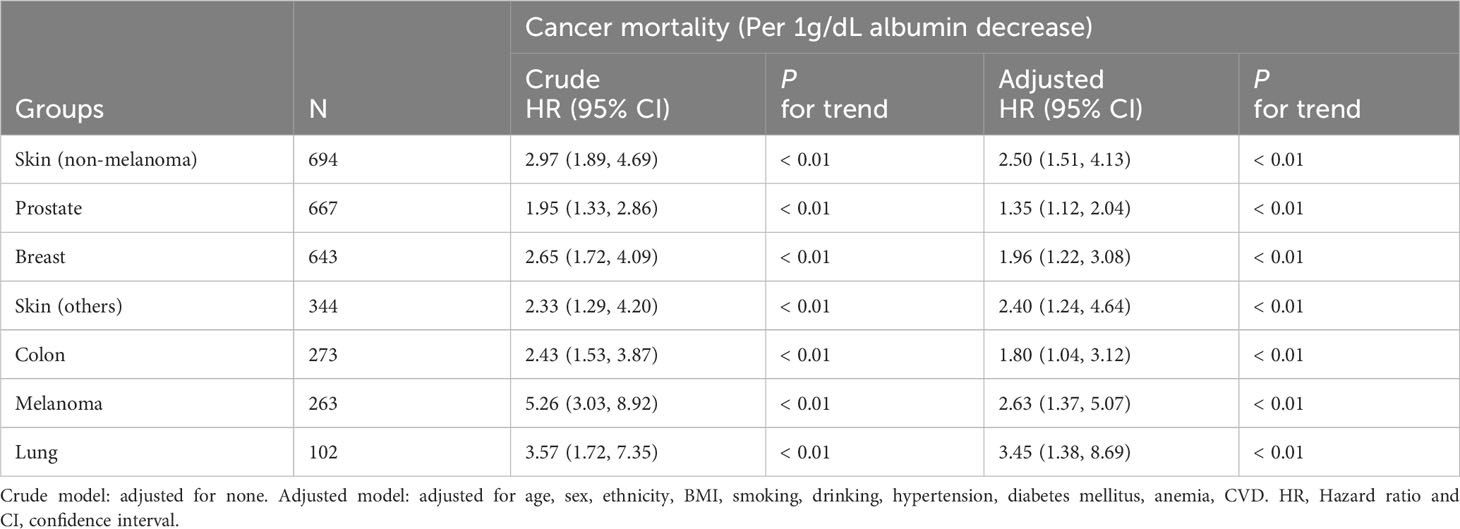
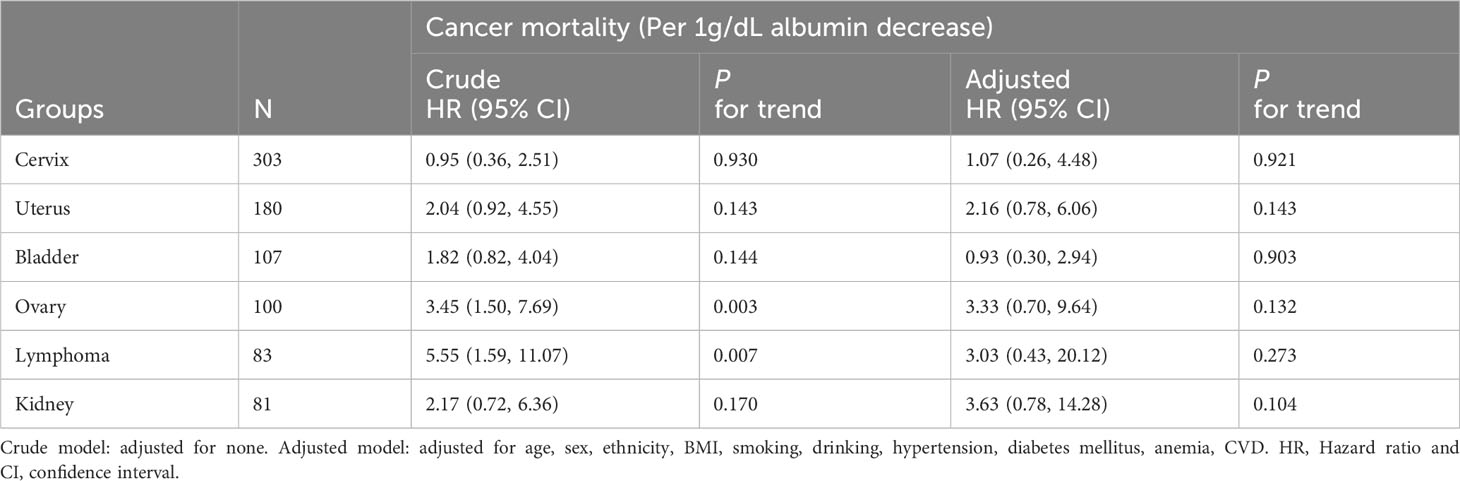
We examined the relationship between albumin levels and mortality in various cancer types, including nonmelanoma skin cancer, prostate cancer, breast cancer, colorectal cancer, and melanoma, respectively, corroborating the overarching findings of this investigation (Table 4). Subgroup analyses further validated the associations between albumin levels and cancer mortality, as observed in the current study, across a wide range of risk factors (Table 5). Additionally, we conducted analyses on several rare cancer types and found no significant association between their albumin levels and cancer mortality (Figure 4; Table 6).
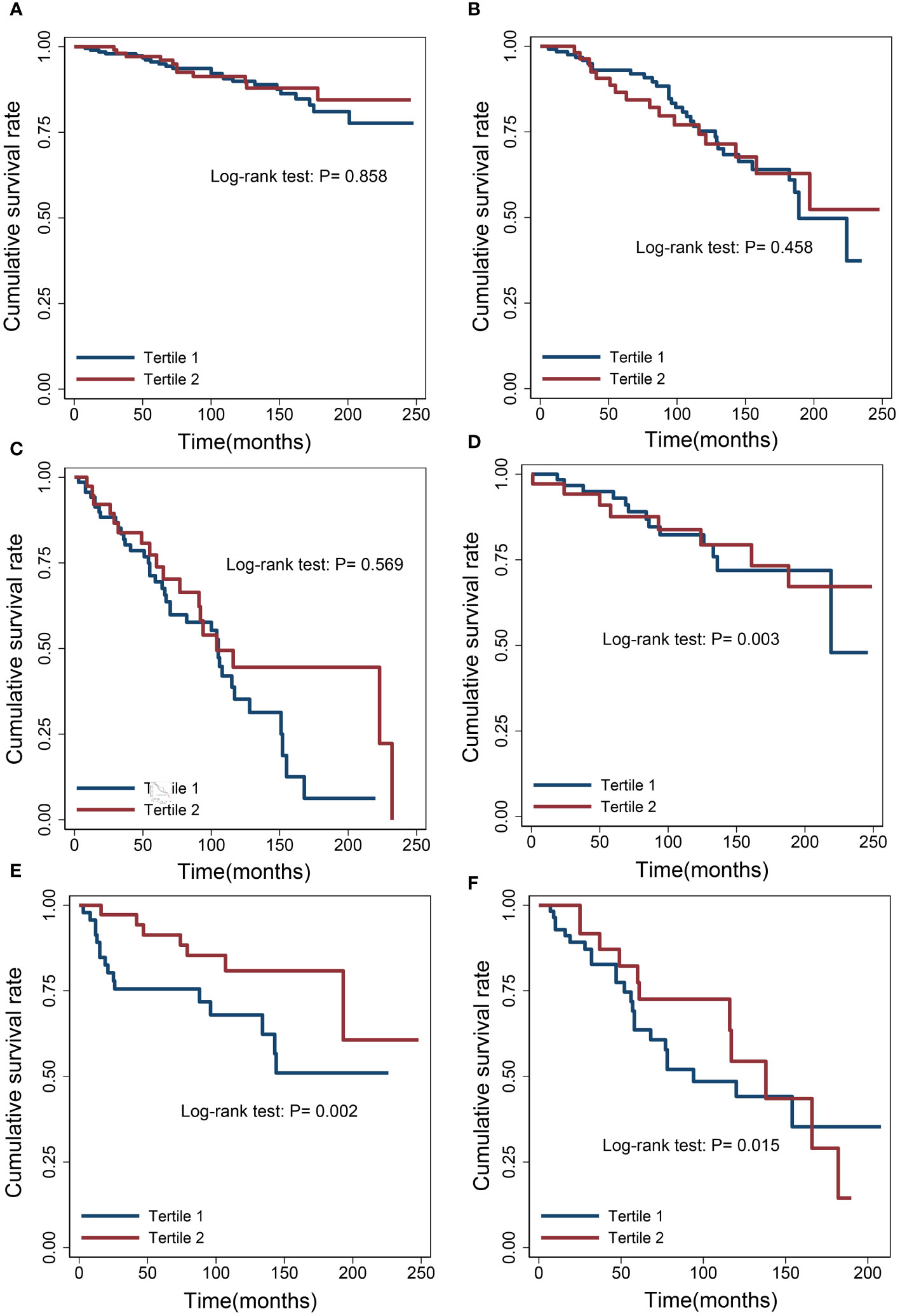
Figure 4 Survival differences between the low albumin group and the high albumin group. (A) Cervix. (B) Uterus. (C) Bladder. (D) Ovary. (E) Lymphoma. (F) Kidney.
Discussion
This study is the first to explore the relationship between serum albumin levels and cancer-related mortality across a diverse array of cancer types in a substantial sample size. Key insights from the current investigation include: i) Reduced serum albumin levels are linked to an increased risk of cancer mortality. When accounting for confounding factors, each 1 g/dL drop in albumin levels among patients with favorable survival was associated with a 1.78-fold elevated risk, while among patients with poor survival, the risk increased by 1.99-fold; ii) A consistent and negative nonlinear association between albumin levels and cancer mortality was observed, irrespective of cancer malignancy.
Serum albumin level serves as a critical indicator of nutritional status in cancer patients and is deeply intertwined with their cancer prognosis. Previous research has consistently identified an association between albumin levels and the risk of mortality across various cancer types. For instance, Chen-Yi Wu and colleagues investigated the link between serum albumin levels and cancer mortality in community-dwelling older adults, revealing that albumin levels below 4.2 g/dL were significantly associated with increased cancer mortality compared to levels at or above 4. g/dL (15). Shuai-Shuai Xu et al. suggested that low albumin levels could be a significant risk factor for patients undergoing pancreatic cancer resection (20). Additionally, a study demonstrated that pretreatment albumin levels predicted survival outcomes in head and neck squamous cell carcinoma (17). Tilman Kühn and colleagues found that lower serum albumin levels were significantly associated with increased mortality from breast, prostate, colorectal, and lung cancer (21). A retrospective study also indicated that lower serum albumin levels predicted a higher 60-day mortality rate from acute myeloid leukemia (22). Ali Ayhan et al. identified that preoperative albumin level was an independent prognostic factor for epithelial ovarian cancer patients following optimal debulking (23). Furthermore, early postoperative serum albumin level has been shown to predict survival following nephrectomy for curative renal cancer (24). These studies corroborate the relationship between serum albumin levels and cancer mortality in cancer types, aligning with the trends observed in the current investigation.
Serum albumin is a pivotal component in certain cancer scoring systems, serving as a stabilizing factor in cancer prognosis. The Fibrinogen-to-Albumin Ratio has emerged as a potential risk predictor for patients with bladder cancer, oral cancer, gastrointestinal mesenchymal tumors, gastric cancer undergoing chemotherapy, IB-IIA cervical cancer, hepatocellular carcinoma, gallbladder cancer, and pancreatic cancer (25–32). The C-reactive Protein to Albumin Ratio has been demonstrated to predict unfavorable outcomes in colorectal cancer, oral squamous cell carcinoma, gallbladder cancer, lung cancer, and thoracic esophageal cancer (33–37). A study has highlighted the prognostic significance of the Blood Urea Nitrogen -to-Albumin Ratio in lung cancer patients receiving intensive care (38). The Albumin-Bilirubin Grade has gained importance as a tool for evaluating liver function and prognosis in patients with hepatocellular carcinoma (39, 40).
Several basic science studies have elucidated why lower albumin levels can exacerbate the risk of cancer mortality. The primary reasons include: i) The essential physiological functions of albumin; and ii) Its critical role in the context of other diseases.
Serum albumin is responsible for 80% of plasma oncotic pressure and is also the main protein in the interstitium, helping to maintain interstitial colloid osmotic pressure (41, 42). The decreased oncotic pressure due to serum albumin loss can trigger or exacerbate interstitial fluid accumulation (ascites, pleural effusion, etc.), which affects circulatory function, catabolism and anabolism, substance and drug transport, and increases the chance of infection (43–45). Therefore, oncotic pressure is a reliable predictor for measuring survival, especially in critically ill patients. The unique structure of serum albumin allows it to bind and transport various molecules, including metabolites (e.g., cholesterol, fatty acids and ions), gases (e.g., NO), and exogenous substances (e.g., drugs and dietary-derived compounds). It is well known that hypercholesterolemia increases the risk of cardiovascular disease as well as predicts their poor prognosis. In recent years, a positive correlation has also been found between serum cholesterol levels and the risk and extent of cancer development (46). Recently, targeting cholesterol metabolism for cancer treatment has been proposed (47). Animal studies showed that the release of unsaturated fatty acids is pro-inflammatory (48). Reducing unsaturated fatty acids in mouse serum can prevent lung and kidney injury, systemic inflammation, and reduces mortality (49). Thus, albumin may improve cancer survival by lowering free cholesterol and fatty acids in the blood through its binding effect. Serum albumin is also a short-term storehouse of free NO. In the presence of tissue hypoxia, albumin releases NO to maintain vascular tone (50), which may be beneficial for cancer prognosis. In addition, albumin can be used as a carrier to transport cancer-targeting drugs and food components. Decreased albumin levels directly affect the outcome and prognosis of cancer treatment. Currently, the role of recombinant albumin and albumin nanocarriers in drug delivery and cancer therapy is being extensively studied (51, 52).
Another crucial aspect of albumin’s function is its antioxidant activity in plasma. The reduced sulfur group of albumin is a major scavenger of reactive oxygen species (ROS) (53) and, in addition, albumin can limit the production of ROS by binding free copper (54). ROS have been shown to activate pro-tumor signaling in various tumors, promote cancer cell survival and proliferation, and lead to DNA damage and genetic instability (55). ROS can also modulate the tumor microenvironment, affecting various stromal cells that provide metabolic and immune support to the tumor (56). These evidences suggest that albumin may inhibit tumor growth and metastasis through antioxidant pathways, thereby improving prognosis.
Serum albumin can also interact with other pathways to exert anti-inflammatory effects (5). Studies have demonstrated that serum albumin levels predict the prognosis of acute inflammatory conditions such as primary trauma, burns, or acute infections (57–59). In addition, studies have found that albumin has anticoagulant and antiplatelet properties, but evidence for this is scarce (3, 4). Albumin may improve the long-term survival of cancer patients by reducing disease complications and unexpected deaths through anti-inflammatory and anti-thrombotic effects.
Serum albumin is a valuable biomarker for a variety of pathological conditions, including inflammation, ischemia, autoimmunity, and metabolic disorders (42). As already mentioned above, lower albumin levels lead to a decrease in the body’s anti-inflammatory, antioxidant, and antithrombotic capacity, which further contributes to the poor prognosis of the disease. Pre-existing hypoproteinemia is a commonly used prognostic indicator indicating a worse prognosis for a wide range of diseases from medical conditions to surgery (60, 61). A very large body of evidence supports the prognostic role of serum albumin levels in cardiovascular disease (62, 63), abdominal surgical disease (64, 65), orthopedic disease (66, 67), gynecological disease (68, 69), and infectious disease (70). Therefore, reduced albumin levels may indirectly affect the prognosis of cancer by promoting the development of other diseases.
This study uncovers a nonlinear relationship between serum albumin levels and cancer mortality risk, suggesting that the risk of mortality triggered by a decrease in albumin levels is ampl at lower levels of albumin, thus exhibiting threshold effects. Albumin infusion is primarily used for plasma replacement and rehydration in critically ill patients, as well as treating hepatic diseases such as ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and post-perforation syndrome (71). However, many conditions with reduced albumin levels are considered unsuitable for albumin infusion due to the lack of significant benefits in terms of prognosis and reduced mortality in several previous basic studies and clinical trials (72–76). The threshold effect identified in this study may help explain the ineffectiveness of albumin infusion under certain circumstances.
This study demonstrates that albumin is a significant predictor of cancer mortality in both aggressive and less aggressive cancers, supporting its potential as a stable risk predictor for the most common cancers. Furthermore, the nonlinear relationship suggests the importance of choosing the appropriate timing for albumin infusion in cancer patients. In terms of clinical research, future studies should refine the inclusion criteria, expand the sample size, focus on exploring the relationship between albumin and the prognosis of specific cancer types, particularly the nonlinear relationship. Moreover, efforts should be made to fully develop and utilize various scoring models related to albumin to better serve cancer patients. In terms of basic research, future studies should further investigate the links between albumin and the pathological mechanisms of cancer to better understand its role in cancer prognosis.
Limitations
Firstly, the primary findings of this study focus on the relationship between albumin levels and overall cancer mortality, which can be applied to various aggressive and less aggressive cancers, but not to specific cancer types. Although we examined some specific cancer types, the limited sample size may result in findings that need to be further validated. Secondly, inconsistencies in lifestyle and indicator measurements among patients in different cycles may lead to measurement bias. Some covariates selected for this study, such as smoking, alcohol consumption, hypertension, and diabetes mellitus, were obtained through subjective questionnaires, which may have resulted in unavoidable information recall bias. Lastly, due to the limitations of the database, several confounding factors that may influence cancer mortality, such as treatment modality, drug use, and compliance, were not adjusted for.
Conclusions
Low serum albumin levels predict higher cancer mortality. Furthermore, a nonlinear negative association was observed between serum albumin levels and the risk of cancer mortality.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Institute of Health Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QT: Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing. CS: Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. The work was done with no specific funding.
Acknowledgments
We would like to thank the NHANES administration for providing publicly available data and reports that enabled us to prepare this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; CVD, cardiovascular disease; RCS, restricted cubic spline; BMI, body mass index; ADA, American Diabetes Association; SD, Standard deviation; HR, Hazard ratio; CI, confidence interval.
References
1. Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int J Biol Macromol. (2019) 123:979–90. doi: 10.1016/j.ijbiomac.2018.11.053
2. Zoanni B, Brioschi M, Mallia A, Gianazza E, Eligini S, Carini M, et al. Novel insights about albumin in cardiovascular diseases: Focus on heart failure. Mass Spectrom Rev. (2023) 42(4):1113–28. doi: 10.1002/mas.21743
3. Mikhailidis DP, Mikhailidis AM, Dandona P. Effect of human plasma proteins on stabilisation of platelet anti-aggregatory activity of prostacyclin. Ann Clin Biochem. (1982) 19:241–4. doi: 10.1177/000456328201900408
4. Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. (2013) 132:69–76. doi: 10.1016/j.thromres.2013.04.018
5. Manolis AA, Manolis TA, Melita H, Mikhailidis DP, Manolis AS. Low serum albumin: A neglected predictor in patients with cardiovascular disease. Eur J Intern Med. (2022) 102:24–39. doi: 10.1016/j.ejim.2022.05.004
6. Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. (2016) 9:229–55. doi: 10.2147/ijgm.S102819
7. Chien SC, Chen CY, Lin CF, Yeh HI. Critical appraisal of the role of serum albumin in cardiovascular disease. biomark Res. (2017) 5:31. doi: 10.1186/s40364-017-0111-x
8. Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Corrigendum to ‘Low albumin levels are associated with mortality risk in hospitalized patients’. Am J Med 2017. (2020) 130:1465. doi: 10.1016/j.amjmed.2017.07.020
9. Rabbani G, Ahn SN. Review: Roles of human serum albumin in prediction, diagnoses and treatment of COVID-19. Int J Biol Macromol. (2021) 193:948–55. doi: 10.1016/j.ijbiomac.2021.10.095
10. Caraceni P, Domenicali M, Tovoli A, Napoli L, Ricci CS, Tufoni M, et al. Clinical indications for the albumin use: still a controversial issue. Eur J Intern Med. (2013) 24:721–8. doi: 10.1016/j.ejim.2013.05.015
11. Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. (2004) 8:1041–8. doi: 10.1016/j.gassur.2004.09.033
12. Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. (2006) 40:592–5. doi: 10.1097/00004836-200608000-00006
13. Suh B, Park S, Shin DW, Yun JM, Keam B, Yang HK, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. (2014) 25:2260–6. doi: 10.1093/annonc/mdu274
14. Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. (1989) 2:1434–6. doi: 10.1016/s0140-6736(89)92042-4
15. Wu CY, Hu HY, Huang N, Chou YC, Li CP, Chou YJ. Albumin levels and cause-specific mortality in community-dwelling older adults. Prev Med. (2018) 112:145–51. doi: 10.1016/j.ypmed.2018.04.015
16. D’Erasmo E, Pisani D, Ragno A, Romagnoli S, Spagna G, Acca M. Serum albumin level at admission: mortality and clinical outcome in geriatric patients. Am J Med Sci. (1997) 314:17–20. doi: 10.1097/00000441-199707000-00004
17. Lim WS, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope. (2017) 127:E437–e442. doi: 10.1002/lary.26691
18. Definitions of smoking and drinking status. Available online at: https://www.health.govt.nz/.
19. NHANES Questionnaires, Datasets, and Related Documentation (2011-2014). Available online at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
20. Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. (2020) 26:828–38. doi: 10.3748/wjg.v26.i8.828
21. Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. (2017) 117:1572–9. doi: 10.1038/bjc.2017.313
22. Xiao Z, Li H, Xiao D, Liu Y, Chen X, Luo S, Ji Y. Association between serum albumin and 60-day mortality in Chinese Hakka patients with non-APL acute myeloid leukemia: a retrospective cohort study. BMC Cancer. (2022) 22:1127. doi: 10.1186/s12885-022-10231-0
23. Ayhan A, Günakan E, Alyazıcı İ, Haberal N, Altundağ Ö, Dursun P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. (2017) 296:989–95. doi: 10.1007/s00404-017-4511-9
24. Tang Y, Liu Z, Liang J, Zhang R, Wu K, Zou Z, et al. Early post-operative serum albumin level predicts survival after curative nephrectomy for kidney cancer: a retrospective study. BMC Urol. (2018) 18:111. doi: 10.1186/s12894-018-0427-3
25. An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer. (2020) 20:691. doi: 10.1186/s12885-020-07191-8
26. Barone B, Napolitano L, Reccia P, De Luca L, Morra S, Turco C, et al. Preoperative fibrinogen-to-albumin ratio as potential predictor of bladder cancer: A monocentric retrospective study. Medicina (Kaunas). (2022) 58(10):1490. doi: 10.3390/medicina58101490
27. Li R, Song S, He X, Shi X, Sun Z, Li Z, et al. Relationship between fibrinogen to albumin ratio and prognosis of gastrointestinal stromal tumors: A retrospective cohort study. Cancer Manag Res. (2020) 12:8643–51. doi: 10.2147/cmar.S271171
28. Zhu H, Zhang W, Ye P, Wu M. Preoperative circulating fibrinogen to albumin ratio in predicting 5-year prognosis of oral cancer radical surgery. Neoplasma. (2022) 69:1246–52. doi: 10.4149/neo_2022_220422N441
29. Zhang L, Wang Z, Xiao J, Zhang Z, Li H, Wang Y, et al. Prognostic value of fibrinogen-to-albumin ratio in patients with gastric cancer receiving first-line chemotherapy. Oncol Lett. (2020) 20:10. doi: 10.3892/ol.2020.11871
30. Sun H, Ma J, Lu J, Yao ZH, Ran HL, Zhou H, et al. Fibrinogen-to-albumin ratio predicts overall survival of hepatocellular carcinoma. World J Gastrointest Oncol. (2023) 15:1662–72. doi: 10.4251/wjgo.v15.i9.1662
31. Xu WY, Zhang HH, Xiong JP, Yang XB, Bai Y, Lin JZ, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. (2018) 24:3281–92. doi: 10.3748/wjg.v24.i29.3281
32. Fang L, Yan FH, Liu C, Chen J, Wang D, Zhang CH, et al. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat. (2021) 53:131–9. doi: 10.4143/crt.2020.330
33. Keinänen A, Uittamo J, Marinescu-Gava M, Kainulainen S, Snäll J. Preoperative C-reactive protein to albumin ratio and oral health in oral squamous cell carcinoma patients. BMC Oral Health. (2021) 21:132. doi: 10.1186/s12903-021-01516-0
34. Sugimoto A, Toyokawa T, Miki Y, Yoshii M, Tamura T, Sakurai K, et al. Preoperative C-reactive protein to albumin ratio predicts anastomotic leakage after esophagectomy for thoracic esophageal cancer: a single-center retrospective cohort study. BMC Surg. (2021) 21:348. doi: 10.1186/s12893-021-01344-7
35. Yue L, Lu Y, Li Y, Wang Y. Prognostic value of C-reactive protein to albumin ratio in gastric cancer: A meta-analysis. Nutr Cancer. (2021) 73:1864–71. doi: 10.1080/01635581.2020.1817510
36. Deng TB, Zhang J, Zhou YZ, Li WM. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Med (Baltimore). (2018) 97:e13505. doi: 10.1097/md.0000000000013505
37. Liao CK, Yu YL, Lin YC, Hsu YJ, Chern YJ, Chiang JM, et al. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J Surg Oncol. (2021) 19:139. doi: 10.37766/inplasy2021.4.0103
38. Peng X, Huang Y, Fu H, Zhang Z, He A, Luo R. Prognostic value of blood urea nitrogen to serum albumin ratio in intensive care unit patients with lung cancer. Int J Gen Med. (2021) 14:7349–59. doi: 10.2147/ijgm.S337822
39. Hiraoka A, Kumada T, Michitaka K, Kudo M. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. (2019) 8:312–25. doi: 10.1159/000494844
40. Kudo M. Newly developed modified ALBI grade shows better prognostic and predictive value for hepatocellular carcinoma. Liver Cancer. (2022) 11:1–8. doi: 10.1159/000521374
41. Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. (1998) 53:789–803. doi: 10.1046/j.1365-2044.1998.00438.x
42. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. (2012) 33:209–90. doi: 10.1016/j.mam.2011.12.002
43. Morissette MP. Colloid osmotic pressure: its measurement and clinical value. Can Med Assoc J. (1977) 116:897–900.
44. Bevan DR. Colloid osmotic pressure. Anaesthesia. (1980) 35:263–70. doi: 10.1111/j.1365-2044.1980.tb05094.x
46. Patel KK, Kashfi K. Lipoproteins and cancer: The role of HDL-C, LDL-C, and cholesterol-lowering drugs. Biochem Pharmacol. (2022) 196:114654. doi: 10.1016/j.bcp.2021.114654
47. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. (2020) 2:132–41. doi: 10.1038/s42255-020-0174-0
48. Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Trans Med. (2011) 3:107ra110–107ra110. doi: 10.1126/scitranslmed.3002573
49. de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest. (2020) 130:1931–47. doi: 10.1172/JCI132767
50. Minamiyama Y, Takemura S, Inoue M. Albumin is an important vascular tonus regulator as a reservoir of nitric oxide. Biochem Biophys Res Commun. (1996) 225:112–5. doi: 10.1006/bbrc.1996.1138
51. Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Delivery. (2015) 12:793–812. doi: 10.1517/17425247.2015.993313
52. Parodi A, Miao J, Soond SM, Rudzińska M, Zamyatnin AA Jr. Albumin nanovectors in cancer therapy and imaging. Biomolecules. (2019) 9(6):218. doi: 10.3390/biom9060218
53. Ascenzi P, Bocedi A, Antonini G, Bolognesi M, Fasano M. Reductive nitrosylation and peroxynitrite-mediated oxidation of heme-hemopexin. FEBS J. (2007) 274:551–62. doi: 10.1111/j.1742-4658.2006.05609.x
54. Duff MR Jr., Kumar CV. The metallomics approach: use of Fe(II) and Cu(II) footprinting to examine metal binding sites on serum albumins. Metallomics. (2009) 1:518–23. doi: 10.1039/b910253a
55. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. (2018) 80:50–64. doi: 10.1016/j.semcdb.2017.05.023
56. Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. (2022) 22:280–97. doi: 10.1038/s41568-021-00435-0
57. Egbert RC, Bouck TT, Gupte NN, Pena MM, Dang KH, Ornell SS, et al. Hypoalbuminemia and obesity in orthopaedic trauma patients: body mass index a significant predictor of surgical site complications. Sci Rep. (2020) 10:1953. doi: 10.1038/s41598-020-58987-4
58. de Tymowski C, Pallado S, Anstey J, Depret F, Moreno N, Benyamina M, et al. Early hypoalbuminemia is associated with 28-day mortality in severely burned patients: A retrospective cohort study. Burns. (2020) 46:630–8. doi: 10.1016/j.burns.2019.09.013
59. Yin M, Si L, Qin W, Li C, Zhang J, Yang H, et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: A prospective cohort study. J Intensive Care Med. (2016) 33:687–94. doi: 10.1177/0885066616685300
60. Pavliša G, Labor M, Puretić H, Hećimović A, Jakopović M, Samaržija M. Anemia, hypoalbuminemia, and elevated troponin levels as risk factors for respiratory failure in patients with severe exacerbations of chronic obstructive pulmonary disease requiring invasive mechanical ventilation. Croat Med J. (2017) 58:395–405. doi: 10.3325/cmj.2017.58.395
61. Jia L, Zhang H. Hypoalbuminemia in guillain-barré syndrome. J Clin Neurosci. (2020) 77:249–50. doi: 10.1016/j.jocn.2020.04.105
62. Inagaki E, Farber A, Eslami MH, Kalish J, Rybin DV, Doros G, et al. Preoperative hypoalbuminemia is associated with poor clinical outcomes after open and endovascular abdominal aortic aneurysm repair. J Vasc Surg. (2017) 66:53–63.e51. doi: 10.1016/j.jvs.2016.10.110
63. Berbel-Franco D, Lopez-Delgado JC, Putzu A, Esteve F, Torrado H, Farrero E, et al. The influence of postoperative albumin levels on the outcome of cardiac surgery. J Cardiothoracic Surg. (2020) 15:78. doi: 10.1186/s13019-020-01133-y
64. Rungsakulkij N, Tangtawee P, Suragul W, Muangkaew P, Mingphruedhi S, Aeesoa S. Correlation of serum albumin and prognostic nutritional index with outcomes following pancreaticoduodenectomy. World J Clin cases. (2019) 7:28–38. doi: 10.12998/wjcc.v7.i1.28
65. Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Colorectal Dis. (2017) 32:1439–46. doi: 10.1007/s00384-017-2884-7
66. Rynecki ND, Congiusta DV, Fields M, Patel R, Vosbikian MM, Ahmed IH. Increased risk of complications in patients with hypoalbuminemia undergoing revision total hip arthroplasty. J Orthop. (2020) 21:253–7. doi: 10.1016/j.jor.2020.03.006
67. Kishawi D, Schwarzman G, Mejia A, Hussain AK, Gonzalez MH. Low preoperative albumin levels predict adverse outcomes after total joint arthroplasty. J Bone Joint Surg Am. (2020) 102:889–95. doi: 10.2106/jbjs.19.00511
68. Bekos C, Polterauer S, Seebacher V, Bartl T, Joura E, Reinthaller A, et al. Pre-operative hypoalbuminemia is associated with complication rate and overall survival in patients with vulvar cancer undergoing surgery. Arch Gynecol Obstet. (2019) 300:1015–22. doi: 10.1007/s00404-019-05278-7
69. Palavalli Parsons LH, Roane B, Manders DB, Richardson DL, Kehoe SM, Carlson M, et al. Hypoalbuminemia is a predictive factor for fistula formation in recurrent cervical cancer. Am J Clin Oncol. (2018) 41(10):933–7. doi: 10.1097/COC.0000000000000403
70. Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. (2021) 22(9):4496. doi: 10.3390/ijms22094496
71. Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, et al. The Therapeutic use of human albumin in cancer patients’ management. Crit Rev Oncol Hematol. (2017) 120:203–9. doi: 10.1016/j.critrevonc.2017.11.008
72. Kaminski MV Jr., Williams SD. Review of the rapid normalization of serum albumin with modified total parenteral nutrition solutions. Crit Care Med. (1990) 18:327–35. doi: 10.1097/00003246-199003000-00018
73. Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Bmj. (1998) 317:235–40. doi: 10.1136/bmj.317.7153.235
74. Wilkes MM, Navickis RJ. Patient survival after human albumin administration. Ann Internal Med. (2001) 135:149–64. doi: 10.7326/0003-4819-135-3-200108070-00007
75. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. (2004) 350:2247–56. doi: 10.1056/NEJMoa040232
Keywords: albumin levels, cancer mortality, National Health and Nutrition Examination Survey (NHANES), cardiovascular disease (CVD), restricted cubic spline (RCS)
Citation: Tang Q, Li X and Sun C-R (2024) Predictive value of serum albumin levels on cancer survival: a prospective cohort study. Front. Oncol. 14:1323192. doi: 10.3389/fonc.2024.1323192
Received: 17 October 2023; Accepted: 22 February 2024;
Published: 04 March 2024.
Edited by:
Philip Rosenberg, National Cancer Institute (NIH), United StatesReviewed by:
Qiang Tang, Zhejiang University, ChinaSanjima Pal, McGill University Health Center, Canada
Biagio Barone, Azienda Ospedaliera di Caserta, Italy
Copyright © 2024 Tang, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Rong Sun, amlueXV5YW5nZ29uZ0AxMjYuY29t
†These authors have contributed equally to this work
 Quan Tang1†
Quan Tang1† Xu Li
Xu Li