- 1Department of Gynecology and Obstetrics, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
- 2Department of TCM Gynecology, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Pathology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
Background: Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multiple systems. Patients with SLE are prone to a variety of malignancies, especially neoplasms of the female reproductive tract. Synchronous tumors, considered to involve multiple sites, are rare in the female reproductive tract. There are hardly any reports of SLE with synchronous reproductive tract tumors.
Case presentation: We report the occurrence of two to three reproductive tract tumors in two women with SLE. A 52-year-old woman was diagnosed with vulvar cancer and cervical cancer. Another woman, aged 67, was diagnosed with concurrent vulvar cancer, vaginal cancer, and cervical cancer and also presented with a suspected lung cancer.
Conclusion: The presence of synchronous tumors of the reproductive tract in patients with SLE is uncommon and can be easily disregarded. It is crucial to highlight that SLE patients with multiple primary malignancies exhibit notable late-stage presentation at the time of diagnosis, inadequate disease-free survival, poor overall survival, rapid progression rates, and mortality. Consequently, greater awareness must be raised regarding synchronous reproductive tract tumors in patients with SLE. Regular comprehensive cancer screening and management should be implemented for individuals diagnosed with SLE.
1 Background
Systemic lupus erythematosus (SLE) is a prevalent chronic multisystem autoimmune disorder that impacts 3.41 million individuals globally, with a significantly higher prevalence among women (10:1 female-to-male ratio) (1). The standard treatment for SLE includes glucocorticoids and immunosuppressants. Studies have confirmed an association between SLE and various malignancies (2), such as breast cancer, lung cancer, and Hodgkin’s lymphoma (3–5). In addition, SLE is a risk factor for neoplasms of the female reproductive tract. It has been reported that cervical and vulvar cancers are more common in patients with SLE than in the general population (6, 7). Synchronous tumors, which encompass multiple locations, are infrequent in the female reproductive tract, only comprising a mere 0.6%–5.4% of all tumors of the female genital system (8). Vulvar and cervical cancers occur infrequently concurrently, whereas endometrial and ovarian cancers are the most prevalent synchronous tumors (8). There are almost no reports of SLE in conjunction with synchronous tumors of the reproductive tract, despite the fact that the association between SLE and malignancies is becoming increasingly acknowledged.
In this study, we report the concurrent development of malignancies in the reproductive tract of two patients with SLE. Since being diagnosed with SLE more than two decades ago, both individuals have been taking prednisone. Vulvar carcinoma and cervical carcinoma were identified in the first patient, a 52-year-old woman. The second patient, a 67-year-old woman, was diagnosed with vulvar, vaginal, and cervical cancers, along with a suspected case of lung cancer. This study aimed to demonstrate that women with SLE may have a higher risk of reproductive tract tumors, even synchronous ones. Therefore, appropriate screening and attention should be given to these patients.
2 Case presentation
2.1 Case no. 1
A 52-year-old postmenopausal married woman presented to our outpatient department in June 2022 with a “vulvar mass found for over 7 years, with pain for 3 years.” She had a history of SLE and was first diagnosed in 1989. In the first year, she took oral prednisone acetate (Prednisone) tablets (40 mg, qd), but later decreased (7.5 mg, qd), and continued to take them. From 2021, dispersible mycophenolate mofetil (CellCept®) tablets (0.5 g, qd) and hydroxychloroquine sulfate (Plaquenil®) tablets (0.2 g, bid) were added. She also had hypertension and was on 1# of irbesartan once a day.
Specialist examination revealed adhesions of the left labia majora, with a 3.0 cm × 3.0 cm × 1.5 cm mass. The skin over the mass had hypopigmentation and localized ulceration, with localized white lesions on both labia minora. The vagina was patent, with a 2.5 cm × 2.0 cm × 1.0 cm gray-white mass at the cervical labium posterius and blood was present on touch by medical cotton swab. The uterus was in retroversion, of normal size, with no abnormalities in the adnexa bilaterally. Laboratory tumor markers showed squamous cell carcinoma antigen (SCCA) at 2.6 ng/ml. Positron emission tomography-computed tomography indicated a cervical mass (1.8x2.8cm) and a vulvar mass (2.4x1.0cm) with significantly increased metabolism of 18F-fluorodeoxyglucose, suggestive of cervical cancer (Figures 1 A-D). Human papillomavirus (HPV)16 was positive, and the thinprep cytologic test (TCT) was negative for intraepithelial lesions or malignancy. Cytology DNA presented one or two aneuploid abnormal cells. Biopsy pathology of the cervical and vulvar masses indicated vulvar in situ squamous carcinoma and squamous cell carcinoma (SCC) of the cervix.
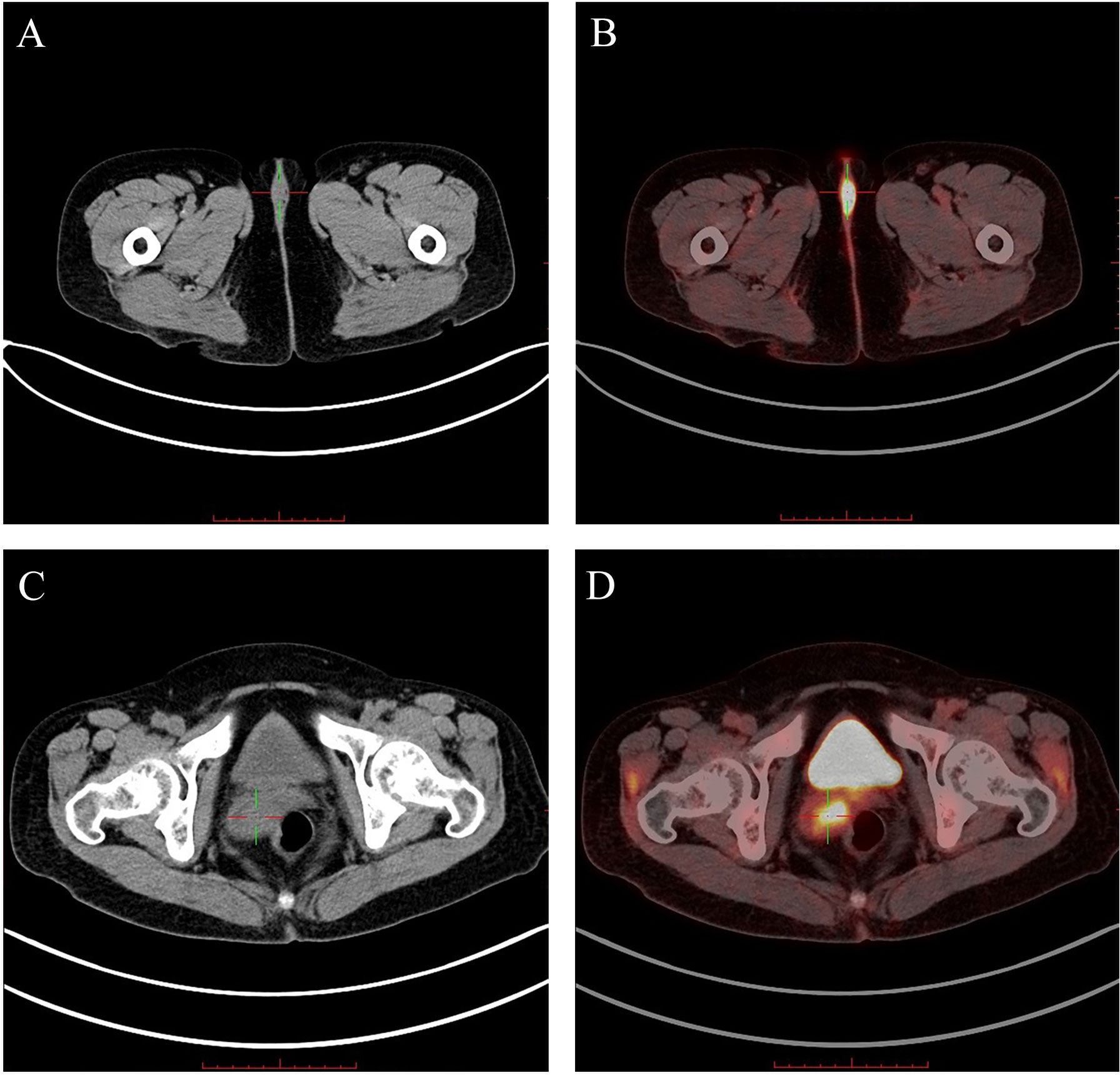
Figure 1 The PET-CT scans obtained from case 1 ("+" presents the vulvar/cervical lesion): (A) The CT showed a low-density nodular shadow in the vulva. (B) The PET-CT showed significantly increased 18F-FDG metabolism in the vulvar lesion. (C) The CT showed an uneven density mass shadow in the cervix. (D) The PET-CT showed significantly increased 18F-FDG metabolism in the cervical lesion.18F-FDG, 18F-fluorodeoxyglucose.
Ultimately, the patient underwent transabdominal radical hysterectomy, pelvic lymphadenectomy, pelvic adhesion release, para-abdominal aortic lymphadenectomy, bilateral salpingo-oophorectomy, local radical vulvectomy and bilateral inguinal lymph node dissection. Histologically, the cervix showed moderately differentiated SCC with nest-like arrangements of cells (Figures 2A, B), significant cellular atypia, numerous mitotic figures, and lymph-vascular space invasion. Cancer tissue invaded approximately half of the cervical wall but did not reach the internal os, with high-grade squamous epithelial lesions visible in the vaginal wall, negative surgical margins on the anterior and posterior vaginal walls, negative parametria bilaterally, and negative blood vessels in both ovaries. Vulvar mass showed high- to moderately differentiated SCC (Figures 2E, F): tumor with nest-like arrangements, marked cellular atypia, numerous mitotic figures, keratin pearl formation, invasive growth of the tumor, high-grade squamous intraepithelial lesion (HSIL) in the surrounding rough area, and negative surgical margins around the skin and at the base. Immunohistochemistry indicated that the tumor cells were positive for epithelial markers CK5/6 and P40 (Figure 2D). The ki-67 index of cervical tumors was 70%, and that of vulvar tumors was 90% (Figure 2G). The expression of P53 was less than 5%, indicating that the tumor was wild-type. The strong positive expression of P16 proved that the tumor was HPV-related (Figures 2C, H). The blood vessels and lymphatic vessels were labeled with CD34 and D2-40, which showed vascular invasion of the tumor.

Figure 2 Characteristics of the cervical squamous cell carcinoma and vulvar squamous cell carcinoma for case 1. (A) The cervical tumor cells were arranged in a nest-like pattern, with obvious cell abnormalities. (B) The cervical tumor cells were polygonal and the nuclei were round or oval. The mitotic appearance was easy to see, and no keratinized beads were observed. (C) P16 was diffuse positive in the cervical tumor cells. (D) P40 was diffuse positive in cervical tumor cells. (E) The vulvar tumor cells were arranged in a nested pattern, invading the stroma. (F) Keratinocytes were visible in vulvar tumor cells. (G) Ki-67 was approximately 90% positive in vulvar tumor cells. (H) P16 was diffusely positive in vulvar tumor cells.
2.2 Case no. 2
A 67-year-old postmenopausal woman with a history of vulvar ulcers and pain for 3 years presented for consultation in July 2022. She was diagnosed with SLE in 1997. At the beginning of 3 months, she took prednisone (10 mg, bid), hydroxychloroquine (0.2 g, po bid), and cyclophosphamide injection (the dose and period were not known). Afterward, she felt that the medical treatment was troublesome; therefore, she continued the use of prednisone but stopped taking the other medications. Prednisone gradually decreased in dosage to 7.5 mg (qd) for the long term. Due to leukopenia, she had been prescribed leucogen tablets (40 mg, qd). In addition, she suffered from hypertension and took an antihypertensive medication intermittently.
The gynecological examination revealed a mass at the posterior commissure of the labia, with a keratinized and ulcerated surface showing hypopigmentation, measuring approximately 3.0 cm × 2.0 cm on the left and 1.5 cm ×2.0 cm on the right, adjacent to the vaginal orifice. With the aid of a speculum placed vaginally, the cervix was exposed, showing cervical atrophy and ulcerative changes at the 10 o’clock position, with punctate congestion at the 2–4 o’clock positions on the vaginal wall, but without obvious tumorous protrusions. The uterus was atrophic, with no abnormalities in the adnexa bilaterally. HPV testing was positive for HPV33/53/82, while cervical cytology DNA showed more than three aneuploid abnormal cells. TCT was ASC-H. Further biopsies revealed HSIL/VIN III in the vaginal mucosa. The cervical mucosa showed HSIL/CIN III, suspicious for microinvasion. The right labia minora mucosa showed HSIL/VIN III with microinvasion. The results of her CT target reconstruction evaluation indicated a solid ground-glass nodule in the upper lobe of the right lung. Compared to the results from January 18, 2020, it can be seen that the ground-glass portion was slightly larger and the solid portion was similar. A specialized consultation in the pulmonary nodule outpatient department was needed. Specialist physicians thought that the possibility of a malignancy could not be ruled out, and surgical resection was recommended.
Under laparoscopy, inguinal lymph node dissection, pelvic lymphadenectomy, pelvic adhesion lysis, bilateral salpingo-oophorectomy, transvaginal modified radical hysterectomy, vaginectomy, and local radical vulvectomy were performed. Histology suggested the cervix was HSIL/CIN III with microinvasion, invading the stroma. (Figure 3A). Atypical cell polarity was disordered, reaching the upper one-third of the squamous epithelial layer. HSIL with focal invasion was noted in the vulva and the vagina (Figures 3B, F), with no lymph node metastasis detected. The immunohistochemistry results showed tumor cells positive for P16, Ki-67, EGFR, MLH1, PMS2, MSH2, and MSH6. P16 showed diffuse strong positivity (Figure 3D), and the Ki-67 positive rate was 90% (Figures 3C, H). However, negative staining for D2-40 was observed in the partial area of the basal lamina (Figures 3E, G).
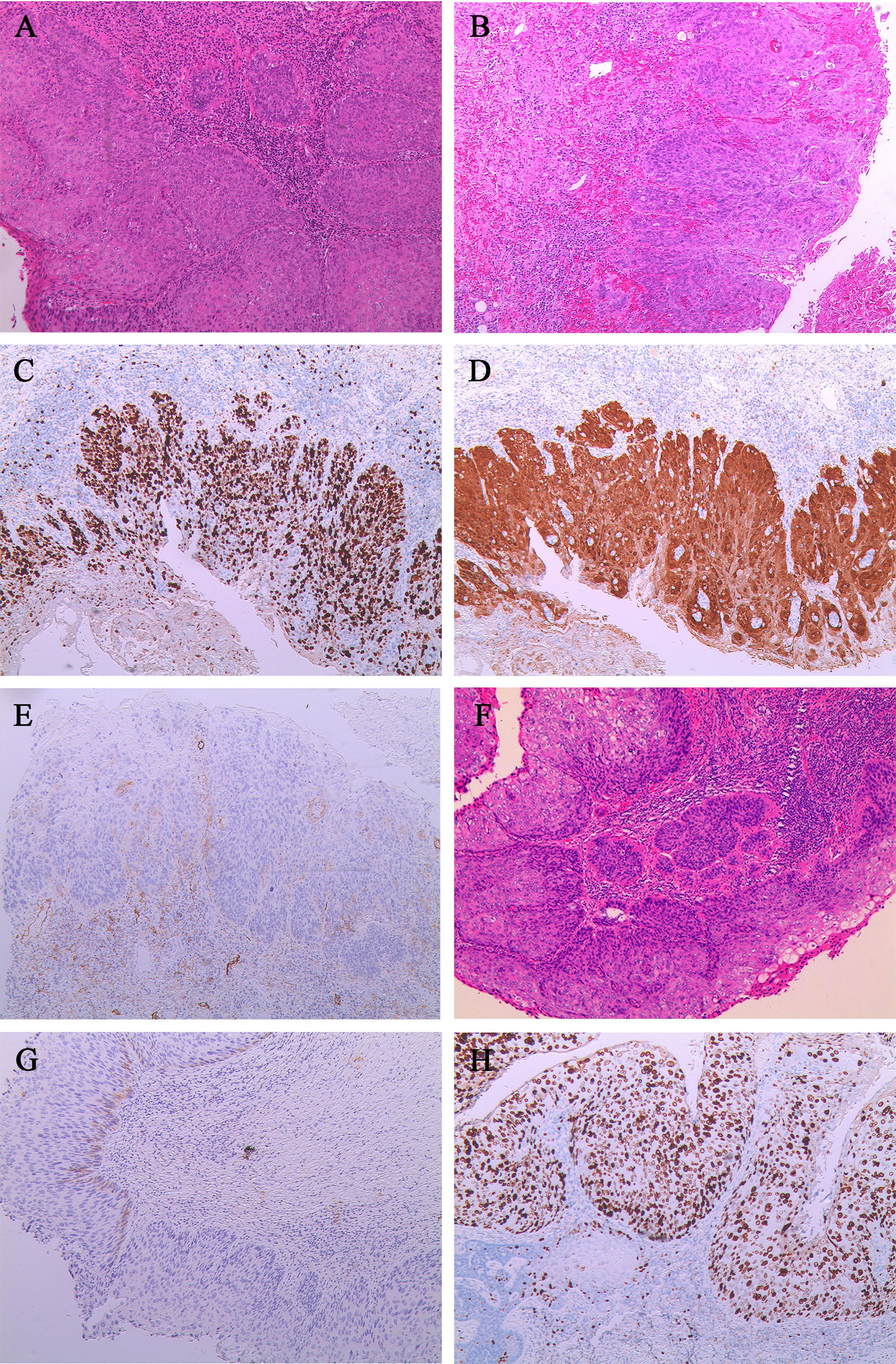
Figure 3 Characteristics of the cervical squamous cell carcinoma, vulvar squamous cell carcinoma, and vaginal squamous cell carcinoma for case 2. (A) The cervix was HSIL/CIN III with microinvasion (stroma invasion depth, <0.3cm).(B) HSIL in the vulva with focal invasion (stroma invasion depth, <0.1cm). (C) The Ki-67 was about 90% positive in vulvar tumor cells.(D) The P16 was diffusely positive in vulvar tumor cells. (E) The D2-40 is negative in the partial basal layer of the vulvar squamous epithelium at the microinvasion.(F) HSIL in the vaginal wall with focal invasion (stroma invasion depth, <0.1cm).(G) The D2-40 was negative in the partial basal layer of the vaginal squamous epithelium at the microinvasion. (H) The Ki-67 epithelial was 90% positive in vaginal tumor cells.
2.3 Outcome and follow-up
Based on the clinical and pathological results, both patients were considered to have synchronous tumors of the reproductive tract. The first patient was diagnosed with the following: 1) vulvar malignancy (stage IB); 2) cervical malignancy (stage IB2); and 3) high-grade squamous lesion of the vaginal wall. The second was diagnosed with 1) vulvar malignancy (stage IB); 2) vaginal malignancy (stage I); and 3) cervical malignancy (stage IA1). The patient in case 1 received intensity-modulated radiation therapy using 6-MV X-rays, with a planned dose of 45 Gy delivered in 25 fractions. However, due to acute appendicitis, only one fraction was administered. Case 2 did not undergo postoperative radiotherapy and chemotherapy. Moreover, she was diagnosed with bipolar disorder caused by SLE, with intermittent use of Depakin and psychotherapy. Therefore, she and her family did not seek surgical treatment for pulmonary nodules. At the end of a 1-year follow-up period, neither of the two patients showed any signs of tumor recurrence or metastasis.
3 Literature review
Only one English-language case of synchronous lesions in the reproductive tract among women with SLE was found through a systematic PubMed search. A summary of the key characteristics of this report, in addition to our two cases, is provided in Table 1.
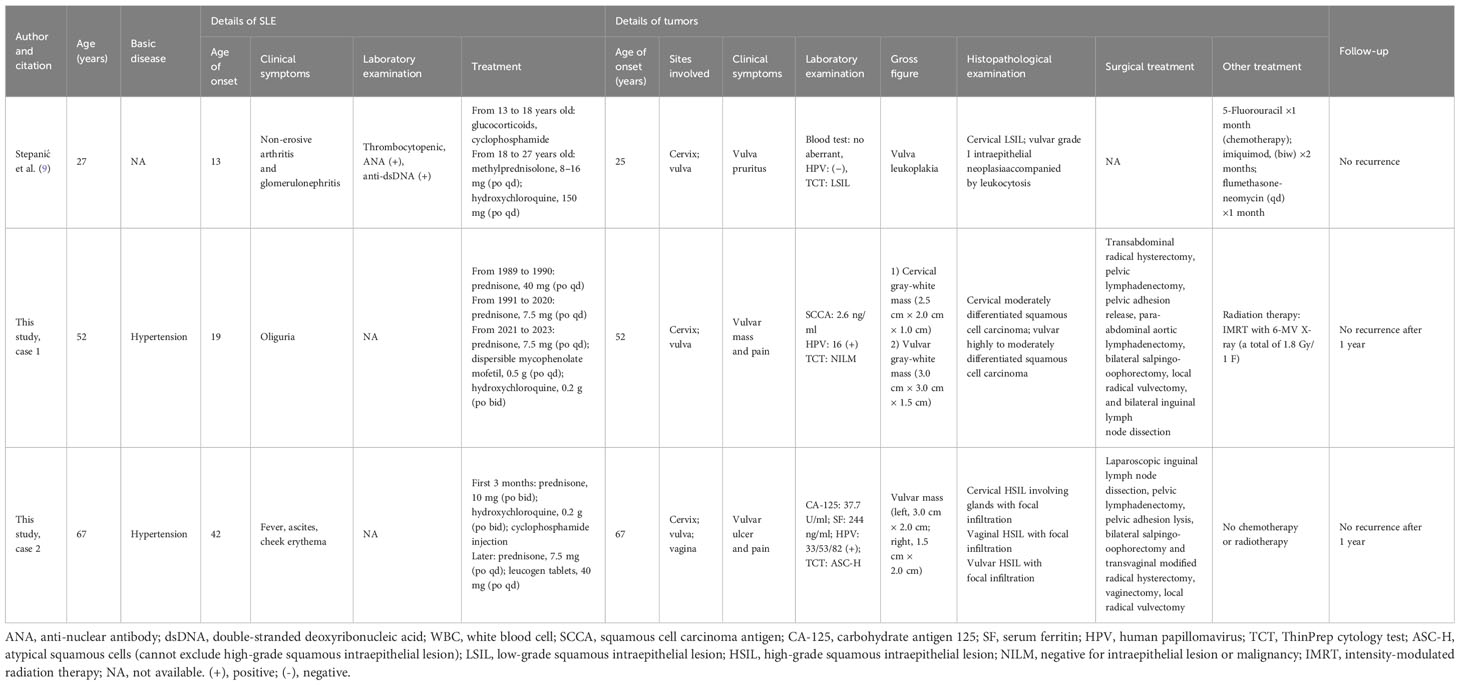
Table 1 Features of synchronous reproductive tract tumors in female systemic lupus erythematosus (SLE) patients.
A 13-year-old girl was diagnosed with SLE and had been undergoing prolonged methylprednisolone and hydroxychloroquine treatment. At the age of 27, she sought medical attention for a year-long episode of vulvar irritation. A routine Pap smear for cervical cytology revealed the presence of low-grade squamous intraepithelial lesions. Histopathological analysis of the vulvar biopsy and colposcopy confirmed the presence of grade I intraepithelial neoplasia accompanied by leukocytosis. A month after receiving 5-fluorouracil chemotherapy, the patient discontinued treatment due to an intolerance to the drug.
Therefore, it is indisputable that the co-occurrence of SLE and reproductive tract malignancy is exceedingly uncommon. Patients with SLE may present with malignancies affecting the reproductive tract and other locations. In such circumstances, failure to detect these malignancies early may result in an unfavorable prognosis.
4 Discussion
SLE affects multiple systems and is classified as an autoimmune inflammatory connective tissue disease. Although early detection and advanced treatment have greatly improved the survival rate of patients with SLE, malignancy remains a major cause of mortality in these patients, and its incidence is still increasing (10). Many studies have indicated a certain correlation between patients with SLE and reproductive tract tumors (11), which results in a lower survival rate (12). As co-cancer of the reproductive tract is exceedingly uncommon, no reports have indicated an increased risk in patients with SLE. However, a multitude of studies have highlighted the substantial elevation in the incidence and risk of cervical cancer among individuals with SLE compared to the general populace. A retrospective study involving 21,016 patients with SLE showed that the incidence rate of cervical cancer was 0.729, with an odds ratio (OR) of 3.229 [95% confidence interval (CI) = 2.43–4.267] (13). Furthermore, medical evidence supported the notion that SLE is associated with increased susceptibility to malignancies of the female reproductive tract. A total of 302 cases of cervical cancer and 72 cases of vaginal cancer/vulvar cancer were identified among 247,575 patients with SLE (14). In addition, women with SLE have a significantly greater risk of developing precancerous lesions than those without SLE (15).
It is unknown why patients with SLE have a higher incidence of malignancies of the reproductive tract, but this may be attributable to the following: overlap with Sjogren’s syndrome, lupus-associated medications, viral infections, conventional cancer risk factors, and innate immune system abnormalities (11). SLE is characterized by the formation of numerous immune complexes through the binding of autoantibodies (anti-DNA and anti-histone antibodies) to their corresponding autoantigens (16, 17), which are deposited throughout the body, activating the complement system and leading to tissue damage. Patients with SLE have an increased risk of developing malignancies due to the accumulation of DNA damage caused by autoantibodies that interfere with DNA repair (18). In addition, SLE is a chronic, difficult-to-treat disease that can compromise normal organ function and impact multiple organ systems. Moreover, the compromised capacity to combat oncogenic viruses and inherent immune dysfunction may contribute to the increased cancer risk among patients with SLE. Furthermore, age, duration of SLE, and the risk of developing malignancies of the reproductive tract are positively correlated (2).
Patients with SLE frequently require the use of immunosuppressants or corticosteroids, which may promote the development of tumors, lower immunity, and increase the risk of infection (19). When the disease manifests refractory symptoms or impacts major organs, systemic glucocorticoids, cyclophosphamide, methotrexate, or azathioprine are suggested as treatment options (20). These medications may increase the risk of infection, decrease immunity, and promote the development of malignancies. Hsu et al. conducted a study that identified a potential dose-dependent association between cyclophosphamide treatment and the overall elevated risk of malignancy among patients with SLE (21). In addition, the use of hydroxychloroquine may reduce the overall risk of cancer (21, 22), indicating that the impact of drug treatment on the cancer risk in patients with SLE could be complex. Furthermore, an investigation conducted by Wadstrom et al. examined the correlation between exposure to immunosuppressive drugs and the likelihood of developing cervical neoplasia and pre-neoplastic lesions (23). In contrast to patients with SLE who received antimalarial therapy, those who underwent immunosuppressant treatment exhibited a 1.83-fold elevated risk of developing cervical neoplasia [hazard ratio (HR) = 1.83, 95% CI = 1.15–2.91)]. This indicates that the use of immunosuppressants may increase the incidence of cervical cancer among patients with SLE.
Women with SLE may exhibit an increased vulnerability to infection caused by viruses associated with reproductive tract tumors, such as HPV, which has been attributed to the progression of these tumors. Studies have indicated that female SLE patients have a higher rate of infection with high-risk HPV strains and are more likely to be infected with multiple HPV subtypes (24, 25), thereby increasing their risk of HSIL, especially those exposed to immunosuppressants (26). Immunosuppressants may reduce the ability of SLE patients to clear HPV infections (27). Studies have found that the incidence of guideline-recommended cervical cancer screening (CCS) among patients with SLE, even in tertiary hospitals (28, 29), is extremely low; this underscores the need for greater CCS awareness.
SLE-associated synchronous malignant tumors of the reproductive tract have not been documented in the past. However, there is evidence that patients with SLE have an increased risk of developing tumors of the reproductive tract; thus, early detection and consistent monitoring are vital. These tests, however, are easily disregarded, including HPV and TCT. Physicians must increase their focus on CCS to prevent the omission of precancerous lesions. The HPV vaccine is effective and well tolerated in patients with SLE. A recent study has found that immunogenicity is maintained in the majority of patients after 5 years (30). Patients with SLE who also have concurrent tumors are more effectively treated surgically. Treatment can be resumed post-chemotherapy completion without impacting the prognosis of the autoimmune disease. During subsequent chemotherapy, immunosuppressants or steroids should be discontinued. During cancer treatment, assessment of the activity of SLE is also extremely important (31); however, no reports have indicated that the activity of SLE is related to tumor progression. Patients with reproductive tract tumor prognoses are influenced by lymph node metastasis, tumor volume, stage, and vascular stromal infiltration. However, there is still a lack of studies regarding the effects of SLE on survival rates. During a 1-year follow-up period, neither of the two cases of genital tract double cancer in this report exhibited signs of recurrence; therefore, ongoing long-term surveillance is necessary. Subsequent validation using a more extensive sample size is also required.
We present cases of SLE co-occurring with synchronous genital tract malignant tumors for the first time. Physicians should prioritize the reproductive health of female SLE patients and ensure that they receive comprehensive follow-up, as demonstrated by these cases. Routine monitoring and assessment are imperative to promptly identify any potential tumors and concurrent malignancies should elicit apprehension. This uncommon case report offers more comprehensive insights into the correlation between SLE and tumors of the reproductive tract, thereby suggesting possible preventive and therapeutic approaches.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Formal Analysis, Methodology, Writing – original draft. NS: Visualization, Writing – original draft. JW: Data curation, Formal analysis, Writing – original draft. SS: Formal Analysis, Writing – original draft. HY: Writing – original draft. QZ: Supervision, Writing – review & editing. XC: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the funds from the Project of Medicine Science and Technology Program of Zhejiang Province (2024KY135).
Acknowledgments
We thank all staff and patients who devoted their time and efforts to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SLE, systemic lupus erythematosus; HPV, human papillomavirus; TCT, ThinPrep cytology test; HSIL, high-grade squamous intraepithelial lesion; CCS, cervical cancer screening; SCCA, squamous cell carcinoma antigen; SCC, squamous cell carcinoma; OR, odds ratio; CI, confidence interval; HR, hazard ratio.
References
1. Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis (2023) 82(3):351–6. doi: 10.1136/ard-2022-223035
2. Mao S, Shen H, Zhang J. Systemic lupus erythematosus and Malignancies risk. J Cancer Res Clin Oncol (2016) 142(1):253–262. doi: 10.1007/s00432-015-2032-0
3. Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther (2018) 20(1):270. doi: 10.1186/s13075-018-1760-3
4. Clarke AE, Pooley N, Marjenberg Z, Langham J, Nicholson L, Langham S, et al. Risk of Malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis. Semin Arthritis Rheumatol (2021) 51(6):1230–41. doi: 10.1016/j.semarthrit.2021.09.009
5. Treppo E, Toffolutti F, Manfrè V, Taborelli M, De Marchi G, De Vita S, et al. Risk of cancer in connective tissue diseases in northeastern Italy over 15 years. J Clin Med (2022) 11(15):4272. doi: 10.3390/jcm11154272
6. Kang KY, Kim HO, Yoon HS, Lee J, Lee WC, Ko HJ, et al. Incidence of cancer among female patients with systemic lupus erythematosus in Korea. Clin Rheumatol (2010) 29(4):381–8. doi: 10.1007/s10067-009-1332-7
7. Zhou Z, Liu H, Yang Y, Zhou J, Zhao L, Chen H, et al. The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large-scale cohort study in China. Cancer Commun (Lond). (2022) 42(5):435–46. doi: 10.1002/cac2.12283
8. Adhya AK, Mohanty R. Triple synchronous tumour of female genital tract: cervical squamous cell carcinoma, right ovarian dermoid cyst and left ovarian benign Brenner tumour. BMJ Case Rep (2019) 12(7):e230695. doi: 10.1136/bcr-2019-230695
9. Stepanić V, Corusić A, Matković V, Sentić M, Bosnić D, Mahovlić V. Vulvar intraepithelial neoplasia in a young woman with systemic lupus erythematosus: a case report. Lupus (2010) 19(1):96–9. doi: 10.1177/0961203309345742
10. Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheumatol (2012) 41(6):830–9. doi: 10.1016/j.semarthrit.2011.11.002
11. Ladouceur A, Tessier-Cloutier B, Clarke AE, Ramsey-Goldman R, Gordon C, Hansen JE, et al. Cancer and systemic lupus erythematosus. Rheum Dis Clin North Am (2020) 46(3):533–50. doi: 10.1016/j.rdc.2020.05.005
12. Kariniemi S, Rantalaiho V, Virta LJ, Kautiainen H, Puolakka K, Elfving P. Malignancies among newly diagnosed systemic lupus erythematosus patients and their survival. Lupus (2022) 31(14):1750–8. doi: 10.1177/09612033221131501
13. Bae EH, Lim SY, Han KD, Jung JH, Choi HS, Kim CS, et al. Systemic lupus erythematosus is a risk factor for cancer: a nationwide population-based study in Korea. Lupus (2019) 28(3):317–23. doi: 10.1177/0961203319826672
14. Zhang M, Wang Y, Wang Y, Bai Y, Gu D. Association between systemic lupus erythematosus and cancer morbidity and mortality: findings from cohort studies. Front Oncol (2022) 12:860794. doi: 10.3389/fonc.2022.860794
15. Chung SH, Oshima K, Singleton M, Thomason J, Currier C, McCartney S, et al. Determinants of cervical cancer screening patterns among women with systemic lupus erythematosus. J Rheumatol (2022) 49(11):1236–41. doi: 10.3899/jrheum.220105
16. Zucchi D, Silvagni E, Elefante E, Signorini V, Cardelli C, Trentin F, et al. Systemic lupus erythematosus: one year in review 2023. Clin Exp Rheumatol (2023) 41(5):997–1008. doi: 10.55563/clinexprheumatol/4uc7e8
17. Klein A, Polliack A, Gafter-Gvili A. Systemic lupus erythematosus and lymphoma: Incidence, pathogenesis and biology. Leuk Res (2018) 75:45–9. doi: 10.1016/j.leukres.2018.11.004
18. Noble PW, Bernatsky S, Clarke AE, Isenberg DA, Ramsey-Goldman R, Hansen JE. DNA-damaging autoantibodies and cancer: the lupus butterfly theory. Nat Rev Rheumatol (2016) 12(7):429–34. doi: 10.1038/nrrheum.2016.23
19. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis (2021) 80(1):14–25. doi: 10.1136/annrheumdis-2020-218272
20. Tseng HW, Huang WC, Lu LY. The influence of immunosuppressants on the non-melanoma skin cancer among patients with systemic lupus erythematosus and primary Sjögren’s syndrome: a nationwide retrospective case-control study in Taiwan. Clin Exp Rheumatol (2019) 37(6):946–52.
21. Hsu CY, Lin MS, Su YJ, Cheng TT, Lin YS, Chen YC, et al. Cumulative immunosuppressant exposure is associated with diversified cancer risk among 14 832 patients with systemic lupus erythematosus: a nested case-control study. Rheumatol (Oxford). (2017) 56(4):620–8. doi: 10.1093/rheumatology/kew457
22. Guo J, Ren Z, Li J, Li T, Liu S, Yu Z. The relationship between cancer and medication exposure in patients with systemic lupus erythematosus: a nested case-control study. Arthritis Res Ther (2020) 22(1):159. doi: 10.1186/s13075-020-02228-6
23. Wadström H, Arkema EV, Sjöwall C, Askling J, Simard JF. Cervical neoplasia in systemic lupus erythematosus: a nationwide study. Rheumatol (Oxford). (2017) 56(4):613–9. doi: 10.1093/rheumatology/kew459
24. He N, Leng X, Zeng X. Systemic lupus erythematosus following human papillomavirus vaccination: A case-based review. Int J Rheum Dis (2022) 25(10):1208–12. doi: 10.1111/1756-185X.14404
25. García-Carrasco M, Mendoza-Pinto C, Rojas-Villarraga A, Molano-González N, Vallejo-Ruiz V, Munguía-Realpozo P, et al. Prevalence of cervical HPV infection in women with systemic lupus erythematosus: A systematic review and meta-analysis. Autoimmun Rev (2019) 18(2):184–91. doi: 10.1016/j.autrev.2018.09.001
26. Goulenok T, Mendes C, Dayan L, Ferre VM, Bucau M, Farhi F, et al. Improving human papillomavirus-related cervical cancer screening in patients with systemic lupus erythematosus. J Rheumatol (2023) 50(12):1642–4. doi: 10.3899/jrheum.2022-1335
27. Tam LS, Chan PK, Ho SC, Yu MY, Yim SF, Cheung TH, et al. Risk factors for squamous intraepithelial lesions in systemic lupus erythematosus: a prospective cohort study. Arthritis Care Res (Hoboken). (2011) 63(2):269–76. doi: 10.1002/acr.20367
28. Bruera S, Lei X, Zogala R, Pundole X, Zhao H, Giordano SH, et al. Cervical cancer screening in women with systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2021) 73(12):1796–803. doi: 10.1002/acr.24414
29. Murakawa Y, Dobashi H, Kondo M, Nishiyama S, Okazaki R, Hasegawa Y, et al. Questionnaire survey on the prevention and development of cervical cancer in patients with systemic lupus erythematosus in Japan. Mod Rheumatol (2023) road028. doi: 10.1093/mr/road028
30. Mok CC, Ho LY, To CH. Long-term immunogenicity of a quadrivalent human papillomavirus vaccine in systemic lupus erythematosus. Vaccine (2018) 36(23):3301–7. doi: 10.1016/j.vaccine.2018.04.056
Keywords: synchronous tumors, female reproductive tract tumors, systemic lupus erythematosus, vulvar cancer, vaginal cancer, cervical cancer, case report
Citation: Wang L, Zhang Q, Shi N, Wang J, Song S, Yang H and Chen X (2024) Case report: Synchronous tumors of the female reproductive tract in systemic lupus erythematosus: report of two cases and review of the literature. Front. Oncol. 14:1322598. doi: 10.3389/fonc.2024.1322598
Received: 16 October 2023; Accepted: 08 January 2024;
Published: 21 February 2024.
Edited by:
Paolo Scollo, Kore University of Enna, ItalyReviewed by:
Mattia Tarascio, Cannizzaro Hospital, ItalyFrancinne Machado Ribeiro, Rio de Janeiro State University, Brazil
Copyright © 2024 Wang, Zhang, Shi, Wang, Song, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingbei Chen, Y2hlbnhiMTk5MEAxMjYuY29t
†These authors have contributed equally to this work
 Ling Wang
Ling Wang Qin Zhang
Qin Zhang Nan Shi2
Nan Shi2 Xingbei Chen
Xingbei Chen