- 1Department of Anesthesiology, Affiliated Sport Hospital of CDSU, Chengdu, Sichuan, China
- 2Department of Pain, Zibo Central Hospital, Zibo, Shandong, China
- 3Department of Geriatric Orthopaedics II, Sichuan Orthopaedic Hospital, Chengdu, Sichuan, China
With the increasing prevalence of tumors, effective symptom management has emerged as a cornerstone of patient care. While surgical interventions remain pivotal, non-surgical nursing methods have gained prominence in providing relief from pain, discomfort, and other tumor-related symptoms. This review delves into the various non-surgical approaches employed, emphasizing tumor sedation and analgesia. We discuss the array of non-pharmacological and pharmacological strategies, shedding light on their indications, contraindications, and potential side effects. Furthermore, the importance of addressing individual differences in pain perception and the ethical considerations in symptom management are highlighted. We conclude by providing insights into the recent innovations in the field, emphasizing the need for personalized and comprehensive care to enhance patients’ quality of life. Tumor sedation, Tumor analgesia, Non-surgical nursing care, Pain management, Non-pharmacological interventions, Palliative care, Recent innovations, Symptom management.
Highlights
● Non-Surgical Nursing Methods: Pivotal shift from surgical to non-surgical approaches in tumor symptom management, expanding care options for patients.
● Comprehensive Strategies: Balanced examination of non-pharmacological and pharmacological methods, offering a holistic view on pain and symptom management.
● Individual Pain Perception: Emphasis on the variability in pain perception, advocating for personalized treatment plans.
● Ethical Considerations: Integration of ethical perspectives in symptom management, addressing complex healthcare dilemmas.
● Innovations in the Field: Insights into recent advancements and future possibilities in tumor symptom management, guiding research and clinical practice.
1 Introduction
With the increasing prevalence of tumors, effective symptom management has emerged as a cornerstone of patient care. While surgical interventions remain pivotal, non-surgical nursing methods have gained prominence in providing relief from pain, discomfort, and other tumor-related symptoms. This review delves into the various non-surgical approaches employed, emphasizing tumor sedation and analgesia. We discuss the array of non-pharmacological and pharmacological strategies, shedding light on their indications, contraindications, and potential side effects. Furthermore, the importance of addressing individual differences in pain perception and the ethical considerations in symptom management are highlighted. We conclude by providing insights into the recent innovations in the field, emphasizing the need for personalized and comprehensive care to enhance patients’ quality of life (1).
In recent years, the medical community has witnessed a renewed emphasis on the significance of pain and discomfort management in patients with tumors (2). As the global incidence of tumors continues to rise, so does the urgency to address the multifaceted challenges these patients face (3). Pain, often a relentless companion of tumor growth, has profound implications not only on a patient’s physical well-being but also on their psychological health, impacting facets of daily life, from sleep quality to interpersonal relationships (4). Recent research underscores that unmanaged or poorly managed pain can lead to heightened levels of anxiety, depression, and even decreased survival rates in some cases (5). In this evolving landscape, non-surgical nursing care has emerged as a critical player (6). These approaches, ranging from pharmacological interventions to holistic care models, have showcased potential in not only alleviating pain but also in enhancing the overall quality of life for patients (7). Recent studies indicate that patients receiving comprehensive non-surgical care often report improved outcomes, reduced hospital stays, and a more positive prognosis (8). As the dynamics of tumor care shift towards a more patient-centric model, the pivotal role of non-surgical nursing care in bridging the gap between medical intervention and enhanced patient well-being cannot be overstated.
2 Tumor sedation
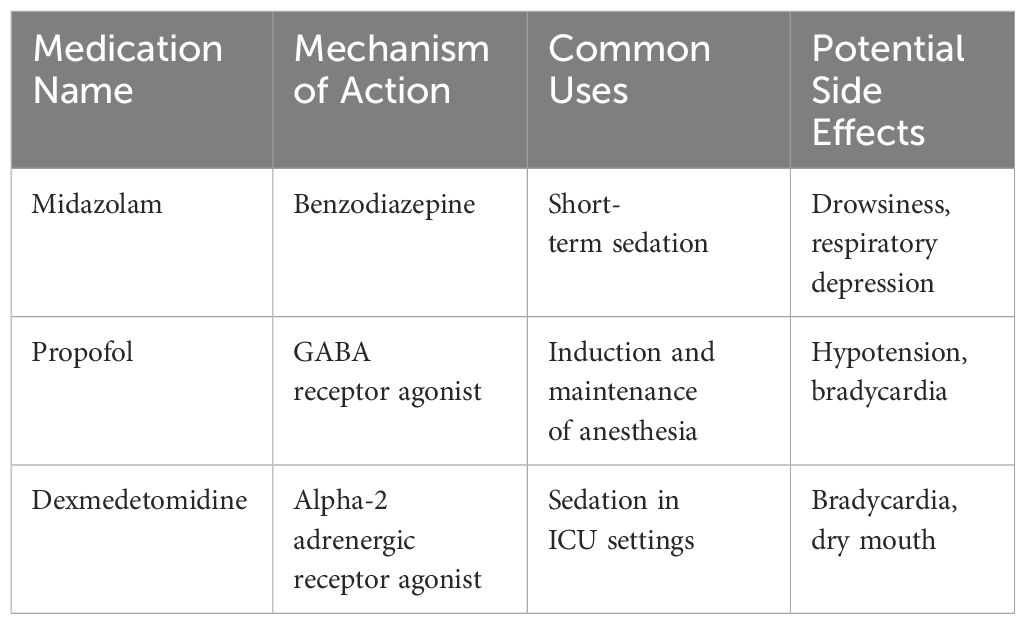
A study on high-quality care for postoperative inflammation and prognosis in advanced non-small cell lung cancer (NSCLC) patients suggests that high-quality care significantly reduces hospitalization duration, improves postoperative inflammation management, symptom control, and quality of life when compared to patients receiving standard care. In comparison to regular care, high-quality care reduces anxiety, depression levels, and psychological distress in postoperative advanced NSCLC patients. The results indicate that high-quality care prolongs the survival time and reduces the recurrence rate of postoperative advanced NSCLC patients (9). Tumor sedation has garnered considerable attention in contemporary oncological care, reflecting its significance in improving patient comfort and quality of life (10). Defined as the deliberate use of medications to relieve extreme symptoms, especially refractory pain and distress in advanced cancer patients (11), tumor sedation aims to achieve a state where the patient’s consciousness is reduced while ensuring their comfort and dignity (12). The core objective of this approach is to mitigate suffering, especially when other treatments fail to provide relief. In recent times, advancements in pharmacology have introduced new agents and refined protocols that offer more controlled and individualized sedation, minimizing potential side effects (13). Moreover, the development of precision monitoring tools aids healthcare professionals in ensuring that sedation is maintained at optimal levels (14), allowing the patients to interact with their loved ones and respond to their environment, even if minimally. These recent strides in tumor sedation techniques underline the medical community’s commitment to enhancing the end-of-life experience for patients, emphasizing comfort, autonomy, and humanity. Table 1 provides a comparison of medications used for tumor sedation, including Midazolam, Propofol, and Dexmedetomidine, along with their respective mechanisms of action, common uses, and potential side effects.
The landscape of tumor sedation has evolved considerably in the past few years, enriched by a confluence of innovative methods and advanced medications tailored to meet the unique needs of tumor patients (15). Traditionally, benzodiazepines, such as midazolam, have been a mainstay for sedation due to their rapid onset and short duration of action (16). However, the introduction of newer agents, including propofol and dexmedetomidine, has expanded the therapeutic arsenal (17). Propofol, in particular, offers rapid sedation with a smooth recovery profile, while dexmedetomidine, an alpha-2 adrenergic receptor agonist, provides sedation without causing respiratory depression (18). Beyond pharmacological advances, non-pharmacological techniques, like progressive muscle relaxation and guided imagery, have gained traction as adjunctive therapies, enhancing the sedative experience while minimizing drug-related side effects (19). Furthermore, the integration of continuous monitoring systems allows for real-time adjustments of sedative doses, ensuring optimal sedation levels and patient safety (20). These advancements underscore a holistic and patient-centered approach, where the choice of method and medication is intricately aligned with the patient’s clinical status, preferences, and the intended depth of sedation.
In the ever-evolving realm of oncology, clear guidelines for when and when not to employ tumor sedation are pivotal to ensuring patient safety and optimal outcomes (21). Recent consensus and evidence-based guidelines have delineated the indications for tumor sedation more explicitly (22). Primarily, it is reserved for patients with refractory symptoms—those distressing symptoms unresponsive to standard medical interventions, such as uncontrolled pain, agitated delirium, or severe dyspnea (11). The goal is to alleviate suffering when other treatments fall short. In certain end-of-life scenarios, sedation may also be employed to ensure a peaceful transition for terminal patients (23). On the flip side, contraindications have been more rigorously defined (24). Tumor sedation is generally avoided in patients with reversible causes of distress, those who might benefit from other interventions, or where the intent might be misunderstood or misconstrued by the patient’s family (25).
A systematic review and meta-analysis studying the impact of enhanced care in liver cancer patients indicate that enhanced care significantly improves patient anxiety, depression, and quality of life. Most liver cancer patients receiving enhanced care are highly satisfied with their quality of life. Furthermore, the analysis also demonstrates significant improvements in patients’ physical functioning and overall activity scores due to enhanced care (26).
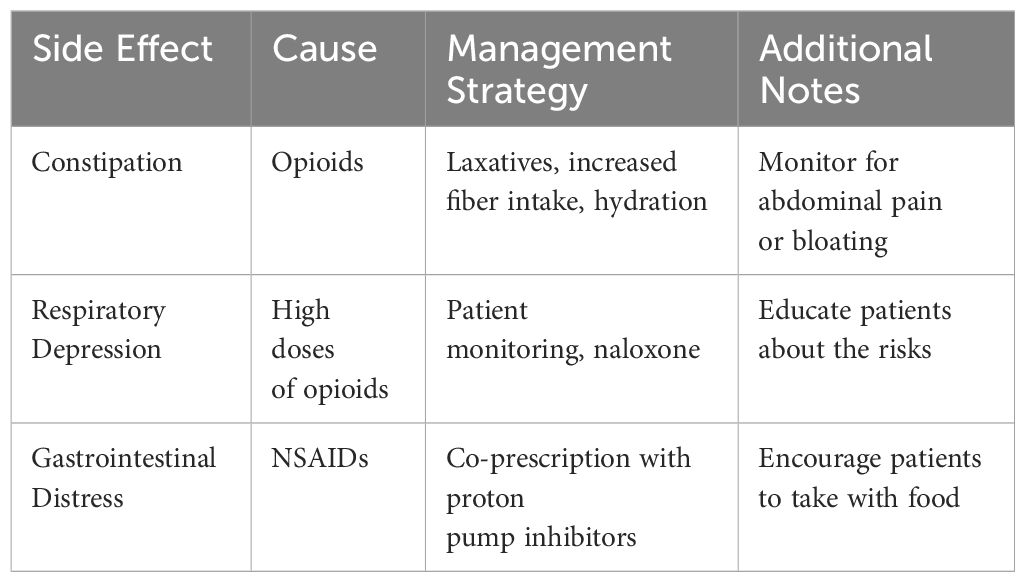
A study on the effectiveness of patient-reported personalized symptom management in liver cancer intervention therapy found that patients who received personalized management experienced significantly milder symptoms such as pain, nausea, anxiety, and fatigue compared to those receiving standard care. Moreover, patients in the intervention group showed a significant improvement in Karnofsky performance scores and satisfaction with care (27) Table 2 outlines various alternative therapies and their potential benefits, including Acupuncture, Aromatherapy, and Music Therapy. Additionally, certain medications used for sedation may be contraindicated in patients with specific allergies or organ dysfunctions. With advancements in diagnostic tools and a better understanding of patient physiology, the decision-making process around tumor sedation has become more refined, ensuring that it is applied judiciously and benefits those truly in need. Table 3 offers strategies to manage common side effects of pain medications, such as constipation, respiratory depression, and gastrointestinal distress. These strategies are particularly useful when dealing with opioids, high doses of opioids, and NSAIDs, respectively.
Lastly, a cluster randomized clinical trial explored the effectiveness of primary palliative care interventions provided by oncology nurses, which did not improve patients’ quality of life, symptom burden, or mood symptoms within 3 months. However, the study highlighted that higher-dose interventions may be beneficial for most advanced cancer patients who lack access to palliative care specialists (28).
3 Tumor analgesia (pain management)
In the contemporary landscape of oncology, the emphasis on pain management in tumor patients has never been more pronounced (29). Pain, often chronic and debilitating, is an all-too-common companion for many tumor patients, profoundly impacting their physical and psychological well-being (30). Recent studies underscore that inadequately managed pain not only diminishes the quality of life but also can exacerbate tumor progression, potentially influencing metastasis and immune suppression (31). The physiological stress induced by persistent pain can lead to elevated cortisol levels, which, in turn, can have detrimental effects on the body’s ability to fight off tumor cells (32). Moreover, effective pain management is intricately linked to improved patient outcomes, including better treatment adherence, reduced hospitalization durations, and enhanced overall survival rates (33). The realm of tumor analgesia has also witnessed a paradigm shift towards a holistic model, where pain is viewed not merely as a physiological symptom but as a complex interplay of emotional, social, and psychological factors (34). This comprehensive approach underscores the critical need for personalized pain management strategies, ensuring that patients can lead a life with dignity, comfort, and hope.
The pharmacological landscape of tumor analgesia has witnessed remarkable advancements in recent years, offering patients a broader and more tailored spectrum of pain relief options (35). Opioids, such as morphine, oxycodone, and fentanyl, remain at the forefront of managing moderate to severe cancer pain (36). Their effectiveness is, however, often counterbalanced by concerns of tolerance, addiction, and side effects like constipation and respiratory depression (37). To address these challenges, extended-release formulations and targeted delivery systems have been developed, optimizing pain control while minimizing adverse effects (38). Parallelly, non-opioids like acetaminophen and NSAIDs provide relief for milder pain and can synergistically enhance opioid efficacy (39). Adjuvant analgesics, including anticonvulsants and antidepressants, have emerged as pivotal players, especially for neuropathic pain, a type of pain frequently associated with tumors and cancer treatments (40). The recent emphasis on personalized medicine has also fostered innovations in drug delivery (33). While oral administration remains common, the rise of transdermal patches, intravenous infusions, and even implantable drug delivery systems cater to specific patient needs (41), ensuring consistent pain relief while reducing systemic side effects (42). Collectively, these advancements underscore a commitment to a multifaceted and patient-centric approach to tumor analgesia, aiming for optimal pain relief with the least possible inconvenience or discomfort to the patient.
The management of pain in tumor patients, while imperative, is often accompanied by a range of medication-induced side effects that can pose substantial challenges to both patients and healthcare providers (43). Recognizing this, recent advances have been directed towards not just enhancing analgesic efficacy but also mitigating these adverse effects (44). For opioids, constipation remains a predominant concern; the introduction of peripherally-acting mu-opioid receptor antagonists, such as naloxegol, has been transformative in managing opioid-induced constipation without affecting central pain relief (45). Respiratory depression, another critical opioid-related side effect, is now better managed with the advent of naloxone nasal sprays and injectables, offering rapid reversal in emergent situations (46). To combat the gastrointestinal side effects of NSAIDs, co-prescription with proton pump inhibitors or the use of selective COX-2 inhibitors has gained traction (47). Furthermore, the practice of rotating opioids, a technique where one opioid is substituted for another, has shown promise in reducing tolerance and side effects (48). Patient education, regular monitoring, and the use of digital health platforms for real-time symptom tracking and reporting have also become integral to a proactive management approach (49). These strategies, born out of a blend of technological innovation and refined pharmacological understanding, represent a concerted effort to ensure that pain management is both effective and tolerable for tumor patients.
4 Other non-surgical nursing care methods
In the realm of tumor care, the surge of interest in alternative therapies has redefined the boundaries of non-surgical nursing interventions (50). While conventional treatments remain foundational, a growing body of evidence suggests that alternative therapies can play a significant role in enhancing patient well-being and potentially alleviating tumor-related symptoms. Acupuncture, a traditional Chinese medical practice, has made notable inroads in the oncological setting (51). Recent studies indicate its efficacy in managing chemotherapy-induced nausea, postoperative pain, and even cancer-related fatigue (52). Aromatherapy, the therapeutic use of essential oils, has been spotlighted for its potential in reducing anxiety, improving sleep, and enhancing overall mood in tumor patients (53). Lavender, chamomile, and frankincense are among the oils frequently utilized (54). Meanwhile, music therapy, a confluence of art and science, has emerged as a potent tool (55). Tailored musical interventions, whether passive listening or active participation, have been linked to reduced pain perception, decreased levels of stress hormones, and improved emotional well-being (56). While these therapies might not replace conventional treatments, their integration into the holistic care model underscores a broader understanding of patient needs, emphasizing not just physical health but also psychological and emotional harmony.
As the complexities of tumor care continue to unfold, addressing multifaceted tumor-related symptoms has become paramount. Beyond pain, symptoms like fatigue, nausea, and cognitive disturbances can severely impede a patient’s quality of life (57). In recent years, targeted interventions have been developed to combat these challenges (58). Fatigue, often cited as one of the most debilitating symptoms by tumor patients, is now being addressed through a combination of pharmacological agents, like modafinil, and non-pharmacological strategies (59), such as graded exercise therapy and cognitive behavioral therapy. Nausea, particularly post-chemotherapy, has seen significant advancements in management (60). The introduction of newer antiemetic drugs, like the NK1 receptor antagonists and olanzapine, has improved control rates, especially in patients undergoing highly emetogenic treatments (61). Additionally, techniques like progressive muscle relaxation and guided imagery have shown promise in alleviating nausea (62). For cognitive disturbances or “chemo brain,” interventions ranging from cognitive rehabilitation programs to mindfulness meditation have been explored (57). The integration of digital health tools, offering real-time symptom tracking and personalized interventions, is also revolutionizing the approach to symptom management (63). These innovations underscore the evolving nature of tumor care, where a nuanced understanding of symptoms and a multi-pronged approach to their management ensure that patients lead a life not just free of pain, but also enriched in well-being (64).
Palliative care, once perceived as a last-resort intervention for terminal patients, has undergone a paradigm shift in recent years (65). Today, it’s recognized as an integral component of comprehensive tumor care, introduced early in the disease trajectory and woven seamlessly alongside curative treatments (66). The primary objective of modern palliative care is to enhance the quality of life, addressing physical symptoms, emotional distress, spiritual concerns, and social challenges (67). Recent studies have illuminated its profound impact: patients receiving early palliative care interventions report better symptom control, improved mood, and even, in some cases, extended survival (68). This holistic approach emphasizes person-centered care, focusing on the patient’s goals, values, and preferences (69). Technological advancements, such as telemedicine platforms, have further broadened the reach of palliative care, ensuring that even those in remote areas or with limited mobility can access these crucial services (70). Interdisciplinary collaboration, encompassing doctors, nurses, therapists, and counselors, ensures that every facet of a patient’s well-being is addressed (71). As the medical community continues to understand the complexities of tumor care, the role of palliative care as a beacon of comfort, dignity, and hope in the patient’s journey becomes ever more central.
5 Challenges in non-surgical nursing care
Navigating the intricacies of non-surgical nursing care for tumor patients has brought to light the profound individual variability in pain perception and tolerance (72). Recent research underscores that pain, far from being a uniform experience, is deeply personal, shaped by a mosaic of genetic, physiological, psychological, and cultural factors (73). Genetic polymorphisms can influence the metabolism of analgesic drugs, leading some patients to require higher or lower doses for effective relief (74). Additionally, psychological states, such as anxiety or depression, can modulate pain perception, often amplifying the experience of pain (75). Cultural beliefs and past experiences can also play pivotal roles in shaping how pain is expressed and tolerated (76). Addressing these individual nuances has posed significant challenges in standardizing care (77). However, the emergence of precision medicine and pharmacogenomics offers promise (33). Tailored pain management strategies, based on an individual’s genetic makeup and holistic assessment, are being explored (78). Furthermore, interdisciplinary approaches, combining medical, psychological, and socio-cultural insights, are being employed to ensure a more comprehensive understanding and management of pain (79). While the path is fraught with challenges, the commitment to individualized, patient-centric care remains unwavering in the face of these complexities (80).
The multifaceted pharmacological landscape of tumor care, while pivotal in managing symptoms, has brought to the fore the intricate challenge of potential drug interactions and side effects (81). As patients often receive a plethora of medications, ranging from chemotherapeutic agents to adjuvant analgesics, the risk of unforeseen interactions escalates (82). These interactions can potentiate drug toxicity, diminish therapeutic efficacy, or introduce new, unanticipated side effects (83). To address this, recent advancements have prioritized comprehensive drug interaction databases and real-time monitoring systems (84). Advanced algorithms, informed by vast pharmacological data, now provide clinicians with immediate alerts if a proposed medication regimen risks harmful interactions (85). Alongside this, there’s a growing emphasis on pharmacovigilance, where systematic post-market monitoring of drugs ensures timely detection and mitigation of side effects (86). Patient education has also emerged as a cornerstone; empowering patients with knowledge about potential side effects and fostering open communication channels allows for early detection and intervention (7). Additionally, the rise of personalized medicine, where treatment regimens are tailored based on genetic and metabolic profiles, offers hope in minimizing adverse reactions (87). Through a blend of technology, vigilant monitoring, and patient engagement, the medical community is steering towards safer and more effective pharmacological management in tumor care (88).
Amidst the complex dynamics of pain and symptom management in tumor care, ethical considerations have risen to the forefront (89), demanding a delicate balance between alleviating suffering and ensuring patient autonomy and dignity (90). The recent discourse has intensified around issues like over-prescription of opioids, where the intent to relieve pain may inadvertently lead to dependence or misuse (91). Consequently, there’s a pressing call for clear guidelines, informed consent, and regular monitoring to ensure opioids are used judiciously (92). Similarly, the decision to initiate, withhold, or withdraw treatments, especially in end-of-life scenarios, is fraught with ethical dilemmas (93). Shared decision-making models, emphasizing transparent communication between patients, families, and healthcare providers, are gaining prominence (94). These models prioritize the patient’s values, beliefs, and preferences, ensuring that interventions align with their overall well-being and life goals (95). Furthermore, the potential disparities in access to pain and symptom management resources, especially in underserved populations, have raised ethical concerns about equitable care (96). Efforts are underway to bridge these gaps through policy reforms and community outreach (97). As the medical community navigates these ethical waters, the commitment remains clear: to offer compassionate, respectful, and individualized care, always placing the patient’s holistic well-being at the heart of every decision.
6 Recent advances and innovations
The last few years have ushered in a renaissance of innovation in non-surgical tumor care, driven by groundbreaking research and technological advancements (98).
In support of the viewpoint advocating early specialized palliative care, the results of this study provide compelling evidence. While the CONNECT program did not significantly improve patients’ quality of life and symptom burden within 3 months, this finding underscores the challenges faced by current oncology patients. Many patients lack access to early specialized palliative care, which may impact their quality of life and symptom management. However, it’s worth noting that the CONNECT program showed a greater effect in patients who completed the full course, suggesting that high-dose primary palliative care may be particularly beneficial for certain patients. Therefore, we recommend that despite the current results not fully endorsing the effectiveness of early specialized palliative care, future research should focus more on high-dose primary palliative care interventions to meet specific patient needs and enhance their quality of life (99).
At the nexus of this evolution lies the burgeoning field of digital health, with wearable sensors and telemedicine platforms offering real-time symptom monitoring and personalized interventions (100). These tools, harnessing the power of artificial intelligence and big data analytics, enable clinicians to preemptively address symptoms, enhancing patient comfort and reducing hospitalizations (101). Another significant leap has been in the realm of pharmacogenomics, where individualized drug regimens, tailored to a patient’s genetic makeup, promise optimized therapeutic effects with minimized side effects (102).
The research on depression and anxiety among people living with and beyond cancer emphasizes the psychological well-being of cancer patients, particularly highlighting significant variations in anxiety and depression levels across different stages of cancer treatment (103). These differences profoundly impact the quality of life for patients. Throughout the process of cancer treatment, considering psychological health factors becomes crucial, especially in pain management and enhancing patient comfort. The paper on “Stress and Cancer” delves into the roles of psychological therapy and medication in managing cancer-related psychological stress, depression, and anxiety (104–106). These studies also mention emerging psychological treatment methods, such as tailored psychological interventions for advanced cancer patients. This research supports your perspective of emphasizing personalized treatment and comprehensive care in managing cancer symptoms. Therefore, it is imperative to focus not only on patients’ physical health but also on their psychological well-being.
Moreover, Wang et al.’s meta-analysis provides evidence of the relationship between depression, anxiety, and cancer incidence and mortality rates among various cancer types (107). The study results indicate a significant correlation between depression and anxiety with cancer incidence, cancer-specific mortality rates, and overall mortality rates among cancer patients. These findings once again underscore the pivotal role of psychological states in cancer treatment and prognosis, especially in pain management and improving the quality of life for patients. Other discussion explores how psychological stress affects tumor development through biobehavioral pathways (108). This review article emphasizes the close connection between psychological well-being and the management of tumor-related symptoms and patient care. While clinical evidence regarding the relationship between psychological stress and cancer may be inconsistent, animal studies suggest that prolonged psychological stress can significantly promote tumor progression. This discovery underscores the need to prioritize psychological health management alongside physical treatment in cancer care to provide holistic care and enhance the overall quality of life for patients.
Additionally, the exploration of neuromodulation techniques, such as transcranial magnetic stimulation, offers novel avenues for managing refractory pain and other tumor-related symptoms (109). Non-invasive brain stimulation methods are being researched for their potential in modulating pain pathways, offering relief without drugs (110). On the holistic front, integrative therapies combining Western medicine with traditional practices, such as yoga and mindfulness meditation, are gaining empirical validation (111), showcasing their efficacy in enhancing overall well-being. As the tapestry of non-surgical tumor care continues to expand and diversify, it’s clear that the future holds a multidimensional approach, seamlessly blending technology, pharmacology, and holistic care to revolutionize the patient experience (112). The horizon of non-surgical tumor care is brimming with possibilities, shaped by an interplay of technological innovation, scientific discovery, and evolving patient needs (113). One of the most anticipated advancements is the fusion of precision medicine with artificial intelligence, enabling predictive modeling of individual patient responses to various treatments, thereby optimizing therapeutic outcomes (114). This synergy promises to tailor interventions not just based on genetic profiles, but also by analyzing real-time physiological, behavioral, and environmental data (115). Another promising avenue is the exploration of bioelectronic medicine, harnessing the potential of electrical signals within the body to modulate pain and other symptoms without the use of drugs (116). As our understanding of the human microbiome deepens, there’s growing optimism about leveraging its potential to modulate pain and inflammation, offering novel therapeutic interventions (117). On the holistic front, there’s a palpable momentum towards integrating traditional healing practices from various cultures into mainstream care, backed by rigorous scientific validation. Additionally, the role of virtual and augmented reality in pain management and patient education is an emerging area of interest. As these innovations coalesce, the future of non-surgical tumor care envisions a holistic, integrated, and patient-centric model that transcends boundaries, ensuring the best possible quality of life for patients.
7 Conclusion
In reflection, the evolving landscape of tumor care underscores the undeniable significance of non-surgical nursing interventions in managing the multifaceted challenges faced by patients. Beyond the immediate relief from pain and discomfort, these interventions play a pivotal role in enhancing the overall quality of life, influencing physical well-being, psychological health, and social interactions. Recent advancements, whether technological, pharmacological, or holistic, have all converged towards one central theme: the importance of personalization. Recognizing that each patient’s journey with a tumor is unique, the emphasis has shifted towards tailored interventions that account for individual genetic, physiological, and emotional nuances. Furthermore, the move towards a more integrated and comprehensive care model, which seamlessly blends traditional and innovative practices, is a testament to the medical community’s commitment to ensuring that every patient receives the best possible care. As we navigate the complexities of tumor care, the value of a patient-centric, compassionate, and holistic approach remains at the heart of all endeavors, guiding the future trajectory of non-surgical interventions.
Author contributions
WW: Data curation, Methodology, Supervision, Conceptualization, Validation, Writing – original draft, Writing – review & editing. PW: Data curation, Supervision, Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. PQ: Data curation, Supervision, Conceptualization, Validation, Writing – original draft, Writing – review & editing. ZL: Data curation, Methodology, Supervision, Conceptualization, Formal analysis, Visualization, Writing – review & editing. QH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Clinical Innovation Project of Sports Hospital Affiliated to Chengdu Sports Institute, No.:LCCX22C02.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schenker Y, Currow DC, Abernethy AP, Smith TJ, Kvale PA, Coyne PJ. Effect of an oncology nurse-led primary palliative care intervention on patients with advanced cancer: the CONNECT cluster randomized clinical trial. JAMA Internal Med (2021) 181(11):1451–60. doi: 10.1001/jamainternmed.2021.5185
2. Bergqvist C, Dugast E, Kalamar A, Wolkenstein P, Vidaud M, Le Merrer M. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. (2020) 15(1):1–23. doi: 10.1186/s13023-020-1310-3
3. Chintapally N, Parikh PM, Bapat B, Chaturvedi P, DeSouza GA, Gore M. State of cancer care in India and opportunities for innovation. Future Oncol. (2023) 19(5):541–53. doi: 10.2217/fon-2023-0047
4. Ying W, Ling TM. Understanding the psychosocial impact of oral squamous cell carcinoma (OSCC) on patients. J Adv Anal Healthc Manage (2023) 7:132–51. doi: 10.1002/jaan.202301165
5. Kondaguli11 SV, Rai A, Choudhari SK, Malik S. Apprehension and anxiety in patients prior to angioplasty: A comprehensive review. (2023).
6. Smith K. Advanced imaging techniques for emergency radiology and surgery. (2023). doi: 10.31219/osf.io/532td
7. Kumari Y, Kumari P, Kumari S, Kumari R, Kumari A, Kumari M. Advancements in the management of endocrine system disorders and arrhythmias: A comprehensive narrative review. Cureus. (2023) 15(3):e14648. doi: 10.7759/cureus.46484
8. Mu X, Wang Y, Li J, Zhang H, Liu X, Zhao J. Non-surgical therapy for the treatment of chronic low back pain in patients with Modic changes: A systematic review and meta-analysis. BMJ Open. (2023) 12(2):e12764. doi: 10.1136/bmjopen-2022-064485
9. Wang XB, Sun LF, Niu ME, Cai JZ, Wang HF. The effect of nursing management of patients undergoing interventional therapy for liver cancer compared with standard care on patient-reported outcomes. Clin Nurs Res (2022) 31:1100–6. doi: 10.1177/10547738221090556
10. Gerlach C, Baus M, Gianicolo E, Bayer O, Haugen DF, Weber M, et al. What do bereaved relatives of cancer patients dying in hospital want to tell us? Analysis of free-text comments from the International Care of the Dying Evaluation (i-CODE) survey: a mixed methods approach. Supportive Care in Cancer (2023) 31:81. doi: 10.1007/s00520-022-07490-9
11. Patel C, Kleinig P, Bakker M, Tait P. Palliative sedation:'A safety net for the relief of refractory and intolerable symptoms at the end of life'. Aust J Gen Pract (2019) 48:838–45. doi: 10.31128/AJGP-05-19-4938
12. Dos Santos CG, De Faria ME, Sant'Ana RSE, Bastos RA, de Leon Rodrigues JRP, Gomes Campos CJ, et al. Psychological fantasies about the administration of palliative sedation to terminal cancer patients: A qualitative study of reports from Brazilian nurses in a specialized hospital. (2023). doi: 10.20944/preprints202306.1336.v1
13. Goff J, Hina M, Malik N, McLardy H, Reilly F, Robertson M, et al. Can opioid-free anaesthesia be personalised? A narrative review. J Pers Med. (2023) 13:500. doi: 10.3390/jpm13030500
14. Meeusen V, Barach P, van Zundert A. Designing safe procedural sedation: adopting a resilient culture. In: Handbook of Perioperative and Procedural Patient Safety. Elsevier (2024). p. 115–63.
15. Zohuri B, Behgounia F. Application of artificial intelligence driving nano-based drug delivery system. In: A Handbook of Artificial Intelligence in Drug Delivery. Elsevier (2023). p. 145–212.
16. Edinoff AN, Nix CA, Hollier J, Sagrera CE, Delacroix BM, Abubakar T, et al. Benzodiazepines: uses, dangers, and clinical considerations. Neurol Int. (2021) 13:594–607. doi: 10.3390/neurolint13040059
17. Lepeltier H, Lepetit A, Gauberti M, Escalard C, Salaun J-P, Bénard C, et al. Dexmedetomidine sedation vs. inhaled general anesthesia for pediatric MRI: A retrospective cohort study: Dexmedetomidine sedation vs. inhaled general anesthesia for MRI. Archives de Pédiatrie: Organe Officiel de la Sociéte Française de Pédiatrie (2022) 29:213–8. doi: 10.1002/ppul.2104722
18. Tasbihgou SR, Barends C, Absalom AJBP, Anaesthesiology, R.C. The role of dexmedetomidine in neurosurgery. Best Pract Res Clin Anaesthesiol. (2021) 35:221–9. doi: 10.1016/j.bpa.2020.10.002
19. Patterson M, Hudson SL, Falk KM. Pain: Assessment And Management. McGraw-Hill Education, New York, NY, USA. (2020).
20. Briki M, et al. Precision oncology by point-of-care therapeutic drug monitoring and dosage adjustment of conventional cytotoxic chemotherapies: A perspective. Pharmaceutics. (2023) 15:1283. doi: 10.3390/pharmaceutics15041283
21. Alves JL, Barbosa M. New surgical approaches in glioblastoma. In: New Insights Into Glioblastoma. Elsevier. p. 167–86. p. 2023.
22. Kremeike K, Pralong A, Boström K, Bausewein C, Simon ST, Lindner R, et al. Desire to Die’in palliative care patients—legal framework and recommendations of the national evidence-based guideline on palliative care in Germany. Front Psychiatry. (2021) 10:3594610–3593610. doi: 10.3233/WOR-20403835
23. Jimenez O-JB, Trajera SM, Ching GS. Providing end-of-life care to COVID-19 patients: the lived experiences of ICU nurses in the Philippines. Int J Environ Res Public Health (2022) 19:12953. doi: 10.3390/ijerph191912953
24. Eddie D, Hoffman L, Vilsaint C, Abry A, Bergman B, Hoeppner B, et al. Lived experience in new models of care for substance use disorder: a systematic review of peer recovery support services and recovery coaching. Front Psychol. (2019) 10:1052. doi: 10.3389/fpsyg.2019.01052
25. Cascella M. Introductory Chapter. In: Delirium in Palliative Care. Springer Nature Switzerland, Cham, Switzerland., vol. 3. (2021). J.S.f.A.C. & Care, N.-C.I.i.P.
26. Zhang Q, Wan R, Liu C. The impact of intense nursing care in improving anxiety, depression, and quality of life in patients with liver cancer: A systematic review and meta-analysis. Medicine (2020) 99:e21677. doi: 10.1097/MD.0000000000021677
27. Gao Z, Fang L, Yin P, Deng Y, Pei M, Zhou T, et al. Effects of nursing care for the treatment of patients with bladder cancer: A systematic review and meta-analysis. Comput Math Methods Med (2022) 2022:9554223. doi: 10.1155/2022/9554223
28. Chung V, Sun V, Ruel N, Smith TJ, Ferrell BR. Improving palliative care and quality of life in pancreatic cancer patients. J Palliative Med (2022) 25:720–7. doi: 10.1089/jpm.2021.0187
29. Vallath N, Rahul RR, Mahanta T, Dakua D, Gogoi PP, Venkataramanan R, et al. Oncology-based palliative care development: The approach, challenges, and solutions from North-East Region of India, a model for low-and middle-income countries. JCO Global Oncol. (2021) 7:223–32. doi: 10.1200/GO.20.00487
30. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin (2013) 63:295–317. doi: 10.3322/caac.21186
31. Candido KD, Kusper TM, Knezevic NN. New cancer pain treatment options. Curr Pain Headache Rep (2017) 21:1–12. doi: 10.1007/s11916-017-0613-0
32. Spiegel DJ. Mind matters in cancer survival. Psychooncology (2012) 21:588–93. doi: 10.1002/pon.3067
33. Sugandh F, Chandio M, Raveena F, Kumar L, Karishma F, Khuwaja S, et al. Advances in the management of diabetes mellitus: a focus on personalized medicine. (2023) 15:. doi: 10.7759/cureus.43697
34. Rivi V, Rigillo G, Toscano Y, Benatti C, Blom JM. Narrative review of the complex interaction between pain and trauma in children: A focus on biological memory, preclinical data, and epigenetic processes. Children (Basel) (2023) 10:1217. doi: 10.3390/children10071217
35. Jamal A. Antibiotics in contemporary medicine: advances, obstacles, and the future. Antibiotics (2023) 2:548–57. doi: 10.3390/antibiotics202004137
36. Chwistek M, Sherry D, Kinczewski L, Silveira MJ, Davis M. Should Buprenorphine be considered a first-line opioid for the treatment of moderate to severe cancer pain? (2023). doi: 10.1016/j.jpainsymman.2023.06.022
37. López-Cano M, Font J, Aso E, Sahlholm K, Cabré G, Giraldo J, et al. Remote local photoactivation of morphine produces analgesia without opioid-related adverse effects. Pain (2023) 180:958–74. doi: 10.1016/j.bpa.2020.10.002
38. Singh S, Kumar A, Mittal G. Ketamine-polymer based drug delivery system for prolonged analgesia: recent advances, challenges and future prospects. Expert Opin Drug Deliv. (2021) 18:1117–30. doi: 10.1080/17425247.2021.1887134
39. Virgen CG, Kelkar N, Tran A, Rosa CM, Cruz-Topete D, Amatya S, et al. Pharmacological management of cancer pain: novel therapeutics. Biomed Pharmacother. (2022) 10(6):113871. doi: 10.1016/j.biopha.2022.113871
40. Shkodra M, Caraceni AJC. Treatment of neuropathic pain directly due to cancer: an update. Cancers (2022) 14:1992. doi: 10.3390/cancers14081992
41. Lee K, Ng K W, Li J, Wang Y, Zhang H, Liu X, et al. Non-transdermal microneedles for advanced drug delivery. Adv Drug Deliv Rev. (2020) 165:41–59. doi: 10.1016/j.addr.2019.11.010
42. Grubb T, Lobprise H. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet Med Sci (2020) 6:209–17. doi: 10.1002/vms3.219
43. Winn P. Integrating Palliative Care into Long-Term Care. In: Post-Acute and Long-Term Care Medicine: A Guide for Practitioners. Springer, Germany. (2023). p. 197–228.
44. Babaie S, Mohammadi-Samani S, Farhadi S, Jahromi SF, Rahimi-Nasrabadi M, Hamblin M, Mansourian M, Mohammadi MR, Salehi H, Ebrahimi M, Rafiei H, et al. Recent advances in pain management based on nanoparticle technologies. J Nanobiotechnology (2022) 20:1–25. doi: 10.1186/s12951-022-01473-y
45. Nightingale JM, Paine P. Chronic Small Bowel Dysfunction. In: Intestinal Failure. Springer, Germany. (2023). p. 243–68.
48. Harris J-D. Management of expected and unexpected opioid-related side effects. Clin J Pain (2008) 24:S8–S13. doi: 10.1097/AJP.0b013e31816b58eb
49. Anthony JB. Integrating telemedicine to support digital health care for the management of COVID-19 pandemic. Int J Healthc Manage (2021) 14:280–9. doi: 10.1080/20479700.2020.1870354
50. Street J. Chiropractors see it differently: A surgeon’s observations. Handb Spine Technol (2021), 67–91. doi: 10.1007/978-3-319-44424-6_134
51. Jansen C, Oskam L, van der Greef J, Boersma T, Voortman T, Cnubben N, Van den Berg W, Vreugdenhil G, De Vries J, Van der Lee J, Van den Heuvel W, et al. Medicine in motion: Opportunities, challenges and data analytics-based solutions for traditional medicine integration into western medical practice. J Ethnopharmacol. (2021) 267:113477. doi: 10.1016/j.jep.2020.113477
52. Wang K-L, Chang Y-C, Chen Y-J, Huang C-C, Lin C-H, Chen Y-C, et al. Recent advances in Glycyrrhiza glabra (Licorice)-Containing herbs alleviating radiotherapy-and chemotherapy-induced adverse reactions in cancer treatment. Metabolites. (2022) 12(6):535. doi: 10.3390/metabo12060535
53. Zuzarte M, Vitorino C, Salgueiro L, Girão HJP. Plant nanovesicles for essential oil delivery. Pharmaceutics. (2022) 14:2581. doi: 10.3390/pharmaceutics14122581
54. Tabatabaeichehr M, Mortazavi H. The effectiveness of aromatherapy in the management of labor pain and anxiety: A systematic review. Ethiop J Health Sci (2020) 30. doi: 10.4314/ejhs.v30i3.16
55. Wosch T, Wigram T. Microanalysis in music therapy: Methods, techniques and applications for clinicians, researchers, educators and students. Jessica Kingsley Publishers, London, United Kingdom. (2007).
56. Mofredj A, Alaya S, Tassaioust K, Bahloul H, Mrabet A. Music therapy, a review of the potential therapeutic benefits for the critically ill. J Crit Care (2016) 35:195–9. doi: 10.1016/j.jcrc.2016.05.021
57. Biegler KA, Alejandro Chaoul M, Cohen L. Cancer, cognitive impairment, and meditation. J Acta Oncol (2009) 48:18–26. doi: 10.1080/02841860802415535
58. Botvin GJ, Griffin KW. School-based programmes to prevent alcohol, tobacco and other drug use. J Int Rev Psychiatry (2007) 19:607–15. doi: 10.1080/09540260701797753
59. Tur C. Fatigue management in multiple sclerosis. Curr Treat Options Neurol (2016) 18:1–12. doi: 10.1007/s11940-016-0411-8
60. Cangemi DJ, Kuo B. Practical perspectives in the treatment of nausea and vomiting. J Clin Gastroenterol (2019) 53:170–8. doi: 10.1097/MCG.0000000000001164
61. Ranganath P, Einhorn L, Albany C. Management of chemotherapy induced nausea and vomiting in patients on multiday cisplatin based combination chemotherapy. BioMed Res Int (2015) 2015. doi: 10.1155/2015/943618
62. Figueroa-Moseley C, Jean-Pierre P, Roscoe JA, Ryan JL, Kohli S, Palesh OG, et al. Behavioral interventions in treating anticipatory nausea and vomiting. J Natl Compr Canc Netw. (2007) 5:44–50. doi: 10.6004/jnccn.2007.0006
63. Ullah M, Hamayun S, Wahab A, Ullah Khan S, Ur Rehman M, Ul Haq Z, et al. Smart Technologies used as Smart Tools in the Management of Cardiovascular Disease and their Future Perspective. Cardiovasc Diagn Ther. (2023) 48:101922. doi: 10.1016/j.cpcardiol.2023.101922
64. Saceleanu VM, Toader C, Ples H, Covache-Busuioc R-A, Costin HP, Bratu B-G, et al. Integrative approaches in acute ischemic stroke: from symptom recognition to future innovations. Biomedicines (2023) 11:2617. doi: 10.3390/biomedicines11102617
65. Quill TE, Lo B, Brock DW, Meisel A. Last-resort options for palliative sedation. Ann Intern Med (2009) 151:421–4. doi: 10.7326/0003-4819-151-6-200909150-00007
66. Kaye EC, Friebert S, Baker JN. Early integration of palliative care for children with high-risk cancer and their families. Pediatr Blood Cancer (2016) 63:593–7. doi: 10.1002/pbc.25848
67. Jocham HR, Dassen T, Widdershoven G, Halfens R. Quality of life in palliative care cancer patients: a literature review. J Clin Nurs (2006) 15:1188–95. doi: 10.1111/j.1365-2702.2006.01274.x
68. Greer JA, Jackson VA, Meier DE, Temel JS. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin (2013) 63:349–63. doi: 10.3322/caac.21192
69. Lines LM, Lepore M, Wiener JM. Patient-centered, person-centered, and person-directed care: they are not the same. J Med Care (2015) 53:561–3. doi: 10.1097/MLR.0000000000000387
70. Doolittle GC, Sun XC, Hinds PS, Manske M, Chen X, Fulton L. TeleHospice: A community-engaged model for utilizing mobile tablets to enhance rural hospice care. Am J Hosp Palliat Care. (2019) 36(5):795–800. doi: 10.1177/1049909119829458
71. Bokhour BG. Communication in interdisciplinary team meetings: What are we talking about? J Interprof Care (2006) 20:349–63. doi: 10.1080/13561820600727205
73. Boddice R. Knowing Pain: A History of Sensation, Emotion, and Experience. John Wiley & Sons, New Jersey, United States. (2023).
74. Farley BJ, Li X, Johansson LE, Zhang H, Zhang Z, Sun L. Opioid-related genetic polymorphisms of cytochrome P450 enzymes after total joint arthroplasty: A focus on drug–drug–gene interaction with commonly coprescribed medications. Cytochrome P450. (2022) 53:361–75. doi: 10.1016/j.bja.2021.11.022
75. Cioffi IJO, Research C. Biological and psychological factors affecting the sensory and jaw motor responses to orthodontic tooth movement. (2023). doi: 10.1111/ocr.12688
76. Özel Y, Özkan B. Psychosocial approach to loss and mourning. Psychiatria Danubina (2020) 12:352–67.
77. Benrimoh D, Chheda FD, Margolese HC. The best predictor of the future—the metaverse, mental health, and lessons learned from current technologies. JMIR Preprints (2022) 9:. doi: 10.2196/preprints.40410
78. Mohamad T, Wu JS, Tseng HH, Chen YC, Lin YS, Chen CC. Individualizing medicinal therapy post heart stent implantation: tailoring for patient factors. Cureus. (2023) 15(3). doi: 10.7759/cureus.43977
79. Ceprnja D, Chipchase L, Liamputtong P, Gupta A. We are not there yet”: perceptions, beliefs and experiences of healthcare professionals caring for women with pregnancy-related pelvic girdle pain in Australia. Medicine (2023) 23:682. doi: 10.1007/s00520-023-07042-z
80. Clim AJA, Standards O. The future of digital healthcare: integrating rights, AI and open standards.
82. Miles R, Wanklyn S, Ross J. Principles of drug therapy MPS Limited, Cambridge, UK., Vol. 364. (2021).
83. Shruthi S, Mumbrekar KD, Rao BS, Shenoy BK. Gallic acid: a polyphenolic compound potentiates the therapeutic efficacy of cisplatin in human breast cancer cells. Toxicology Research (2023), tfad041. doi: 10.1093/toxres/tfad041
84. Ilyas A, Omar H, Salim SU, Jamil N, Khan M, Hussain S. Software architecture for pervasive critical health monitoring system using fog computing. J Ambient Intell Humaniz Comput. (2022) 160:84. doi: 10.1186/s13677-022-00371-w
85. Padhi A, Agarwal A, Saxena SK, Katoch CJV. Transforming clinical virology with AI, machine learning and deep learning: a comprehensive review and outlook. Perspective Review (2023) 34, 345–355. doi: 10.1007/s13337-023-00841-y
86. Munsaka M, Liu M, Xing Y, Yang H. Leveraging Machine Learning, Natural Language Processing, and Deep Learning in Drug Safety and Pharmacovigilance. In: Data Science, AI, and Machine Learning in Drug Development. Chapman and Hall/CRC, Boca Raton, Florida, United States. (2022). p. 193–229.
87. Senthil J, Meena M, Ignacy A, Ramanathan T. Advances in precision medicine: current landscape and future directions. Front Med. (2023) 42:1204–11. doi: 10.1007/s00520-023-07052-9
88. Newman B, Donnelly P, Doherty A, Légaré F, Montori VM, Kho ME. Do patient engagement interventions work for all patients? A systematic review and realist synthesis of interventions to enhance patient safety. Health Expectations. (2021) 24:1905–23. doi: 10.1111/hex.13343
89. Williams J, Johnson R, Davis A, Miller K, Anderson P, Thomas S. Recommendations to leverage the palliative nursing role during COVID-19 and future public health crises. J Hosp Palliat Nurs.. (2020) 22:260. doi: 10.1097/NJH.0000000000000665
90. Manderius C, Clintståhl K, Sjöström K, Örmon K. The psychiatric mental health nurse’s ethical considerations regarding the use of coercive measures–a qualitative interview study. J Psychiatr Ment Health Nurs. (2023) 22:23. doi: 10.1007/s00520-023-07053-8
91. Quintero G, Nichter M. Revisiting generation Rx: emerging trends in pharmaceutical enhancement, lifestyle regulation, self-medication, and recreational drug use. Drugs and Society: Exploring the Social and Political Context of Medicine (2022), 295–314. doi: 10.1002/9781119718963.ch17
92. Jones CM, Dieleman JP, Skene D, Choy E, Gamble C, Grafton J, et al. Opioid analgesia for acute low back pain and neck pain (the OPAL trial): a randomised placebo-controlled trial. The Lancet. (2023) 402:304–12. doi: 10.1016/S0140-6736(23)00404-X
93. Tanaka Gutiez M, Efstathiou N, Innes R, Metaxa V. End-of-life care in the intensive care unit. Intensive Care Med. (2023) 78:636–43. doi: 10.1007/s00520-023-07054-7
94. Gerritse K. Shared decision-making in transgender healthcare: ethical and conceptual challenges and the co-creation of an ethics support tool. (2023). doi: 10.1016/j.bpa.2022.10.021
95. Thomas TH, Koenig T, Breese JD, Moinpour N, Thompson D, Basch EM, et al. Priorities to improve cancer caregiving: report of a caregiver stakeholder workshop. Support Care Cancer. (2021) 29:2423–34. doi: 10.1007/s00520-020-05760-y
96. Ahmad A, Tariq A, Hussain HK, Gill AY. Equity and artificial intelligence in surgical care: A comprehensive review of current challenges and promising solutions. Front Surg. (2023) 2:443–55. doi: 10.1136/bmjopen-2022-063124
97. Golden RL, Emery-Tiburcio EE, Post S, Ewald B, Newman M. Connecting social, clinical, and home care services for persons with serious illness in the community. J Am Geriatr Soc. (2019) 67:S412–8. doi: 10.1111/jgs.15900
98. Treacy P. The Evolution of Aesthetic Medicine: The Evolution of a New Field of Medicine by a Pioneer Voted the Top Aesthetic Doctor in the World. Austin Macauley Publishers (2022).
99. Wang M, Sun Y, Zhang M, Yu R, Fu J. Effects of high-quality nursing care on quality of life, survival, and recurrence in patients with advanced nonsmall cell lung cancer. Medicine (2022) 101:e30569. doi: 10.1097/MD.0000000000030569
100. Aungst TD. Reevaluating medication adherence in the era of digital health. Expert Rev Med Devices (2021) 18:25–35. doi: 10.1080/17434440.2021.2019012
101. Jeddi Z, Bohr A. Remote patient monitoring using artificial intelligence. In: Artificial Intelligence in Healthcare. Elsevier (2020). p. 203–34.
103. Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer (2019) 19:943. doi: 10.1186/s12885-019-6181-4
104. Blanc-Lapierre A, Rousseau MC, Parent ME. Perceived workplace stress is associated with an increased risk of prostate cancer before age 65. Front Oncol (2017) 7:269. doi: 10.3389/fonc.2017.00269
105. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Practice. Oncol (2008) 5:466–75.
106. Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol (London England) (2010) 6:1863–81. doi: 10.2217/fon.10.142
107. Wang Y-H, Wang L, Lv J, Liu Z, Sun W, Hu Y, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry (2020) 25:1487–99. doi: 10.1038/s41380-019-0595-x
108. Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer (2021) 21:767–85. doi: 10.1038/s41568-021-00395-5
109. Boerger TF, Isnard JL, Kleinberg LR, Duffau H. Large-scale brain networks and intra-axial tumor surgery: a narrative review of functional mapping techniques, critical needs, and scientific opportunities. Front Hum Neurosci. (2023) 17:1170419. doi: 10.3389/fnhum.2023.1170419
110. Young JR, Carney SE, Krystal JH, Leuchter AF, Nestler EJ, Rubin DT, et al. Non-invasive brain stimulation modalities for the treatment and prevention of opioid use disorder: a systematic review of the literature. J Subst Use Drug Alcohol Depend. (2020) 209:186–99. doi: 10.1080/10550887.2020.1736756
111. Dai C-L, Sharma M, Chen C-C, Yesilyurt E, Godbey S. Yoga as an alternative therapy for weight management in child and adolescent obesity: A systematic review and implications for research. Childhood Obesity (2021) 27:48–55.
112. Yeung K. The health care sector’s experience of blockchain: A cross-disciplinary investigation of its real transformative potential. JMIR Research Protocols (2021) 23:. doi: 10.2196/24109
113. Meyer-Szary J, Schreiner M, Kummer FJ, Gebauer M, Günal SY, Vogl TJ, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals—cross-sectional multispecialty review. Sensors. (2022) 36(12):3331–43. doi: 10.1007/s12032-023-01624-6
114. Singh AV, Kumar A, Malodia P, Somashekar SK, Jaiswal N. Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomed Pharmacother (2023) 163:114784. doi: 10.1016/j.biopha.2023.114784
115. Singh S, Jaiswal N, Kumar A, Malodia P, Somashekar SK. Unveiling the future of metabolic medicine: omics technologies driving personalized solutions for precision treatment of metabolic disorders. Biochem Biophys Res Commun. (2023) 580:24–32. doi: 10.1016/j.bbrc.2023.09.064
116. Qayyum I, Munawar MA, Uddin N, Zahoor M, Khan S. Progressive innovations in advanced functional materials for emerging bio-electronics, drugs sensing and healthcare. Emerg Mater Sci Eng. (2023). doi: 10.1007/s12032-023-01625-5
Keywords: tumor sedation, tumor analgesia, non-surgical nursing care, pain management, nonpharmacological interventions, palliative care, recent innovations, symptom management
Citation: Wei W, Wang P, Qing P, Li Z and He Q (2024) Non-surgical nursing care for tumor patients: an overview of sedation, analgesia, and recent innovations. Front. Oncol. 14:1322196. doi: 10.3389/fonc.2024.1322196
Received: 15 October 2023; Accepted: 12 January 2024;
Published: 17 September 2024.
Edited by:
Marco Pellicciaro, University of Rome Tor Vergata, ItalyReviewed by:
Giada Iafrate, Università di Roma Tor Vergata, ItalyAndrea Campisi, Agostino Gemelli University Policlinic, Italy
Copyright © 2024 Wei, Wang, Qing, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi He, aGVxaWFmZmlsaWF0ZWRzcG9ydHNob3NwaXRhbEBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Wei Wei1†
Wei Wei1† Qi He
Qi He