
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 28 May 2024
Sec. Pediatric Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1315747
 M. E. Madeleine van der Perk1*
M. E. Madeleine van der Perk1* Anne-Lotte L. F. van der Kooi1,2
Anne-Lotte L. F. van der Kooi1,2 Simone L. Broer3
Simone L. Broer3 Maarten O. Mensink1
Maarten O. Mensink1 Annelies M. E. Bos1,3
Annelies M. E. Bos1,3 Marianne D. van de Wetering1
Marianne D. van de Wetering1 Alida F. W. van der Steeg1
Alida F. W. van der Steeg1 Marry M. van den Heuvel-Eibrink1,4
Marry M. van den Heuvel-Eibrink1,4Background: Infertility is an important late effect of childhood cancer treatment. Ovarian tissue cryopreservation (OTC) is established as a safe procedure to preserve gonadal tissue in (pre)pubertal girls with cancer at high risk for infertility. However, it is unclear whether elective laparoscopic OTC can also be performed safely in infants <1 year with cancer. This systematic review aims to evaluate the reported risks in infants undergoing elective laparoscopy regarding mortality, and/or critical events (including resuscitation, circulatory, respiratory, neurotoxic, other) during and shortly after surgery.
Methods: This systematic review followed the Preferred reporting Items for Systematic Review and Meta-Analyses (PRISMA) reporting guideline. A systematic literature search in the databases Pubmed and EMbase was performed and updated on February 15th, 2023. Search terms included ‘infants’, ‘intubation’, ‘laparoscopy’, ‘mortality’, ‘critical events’, ‘comorbidities’ and their synonyms. Papers published in English since 2000 and describing at least 50 patients under the age of 1 year undergoing laparoscopic surgery were included. Articles were excluded when the majority of patients had congenital abnormalities. Quality of the studies was assessed using the QUIPS risk of bias tool.
Results: The Pubmed and Embase databases yielded a total of 12,401 unique articles, which after screening on title and abstract resulted in 471 articles to be selected for full text screening. Ten articles met the inclusion criteria for this systematic review, which included 1778 infants <1 years undergoing elective laparoscopic surgery. Mortality occurred once (death not surgery-related), resuscitation in none and critical events in 53/1778 of the procedures.
Conclusion: The results from this review illustrate that morbidity and mortality in infants without extensive comorbidities during and just after elective laparoscopic procedures seem limited, indicating that the advantages of performing elective laparoscopic OTC for infants with cancer at high risk of gonadal damage may outweigh the anesthetic and surgical risks of laparoscopic surgery in this age group.
Survival rates of childhood cancer have increased up to 80% (1). However, up to 75% of childhood cancer survivors (CCS) develop one or more late effects such as cardiomyopathy, hypertension, as well as gonadal damage leading to premature ovarian insufficiency and consequently impaired fertility (2, 3). Infertility is rated one of the most important and impairing late effects according to patients, parents of children with cancer and survivors, and is highly associated with decreased quality of life (4, 5). Currently, ovarian tissue cryopreservation (OTC) is often pursued by laparoscopy as an established safe procedure to preserve gonadal tissue in (pre)pubertal girls with cancer at high risk for infertility (6–8). Due to the small size of the ovaries a unilateral oophorectomy or salpingo-oophorectomy is usually performed in prepubertal girls (6, 7, 9, 10). However, currently no international consensus has been published regarding best practice in infants (9, 10). For these girls, less invasive fertility preservation techniques available to adult women, such as oocyte or embryo cryopreservation, are not feasible. Even though no lower age limit for performing OTC is recommended in guidelines (8, 11), and the American Society for Reproductive Medicine reported in 2019 that OTC should no longer be considered experimental, it may be challenging to implement an OTC program due to the perceived risk associated with ovarian tissue harvest in children, typically performed by laparoscopy. Some countries are cautious to perform such an elective procedure in infants under the age of 12 months due to the reported increased risk of perioperative critical events and severe complications after surgery in general in infants based on large pediatric cohort studies (12, 13). As such cohort studies included all types of anesthesia including high risk surgery and all infants, including prematurely born infants or infants with congenital abnormalities, the results may not be representative of elective laparoscopic procedures for OTC in children with cancer (12, 13).
Therefore, this review aimed to evaluate the available evidence of surgical, anesthetic and neurotoxic complication risk in patients undergoing elective laparoscopic surgery under the age of 1 year of only non-high risk surgeries. We aimed to specifically answer the question whether infants undergoing elective laparoscopy are at risk of mortality during and in the first week after surgery, and of critical events (including resuscitation, circulatory, respiratory, neurotoxic, other) during and within the first 24 hours after the surgery. By doing so, we aimed to offer evidence based arguments on the question whether an elective laparoscopic OTC in infants younger than 12 months would be safe.
A systematic electronic literature search was performed in December 2019 and updated on February 15th, 2023 in the databases Pubmed and EMbase including Medline. Medical Subject Heading (MeSH), Embase subject heading (Emtree) and Title/Abstract (TiAb) terms were applied in the research strategy to detect articles that mention outcome or complications after laparoscopic surgery under general anesthesia in children under the age of 1 year. The following search terms and their synonyms were included: infants (not limited to gender), laparoscopy (not limited to location), intubation, critical events, mortality, comorbidity. The complete search syntaxes are provided in Supplementary Texts 1, 2. Cross reference checks were performed to identify additional potentially relevant articles. This systematic review followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) reporting guideline (14).
We included papers published since 2000, as advances in perioperative safety and developments in pediatric anesthesia over the past 30 years make older studies unrepresentative for the current clinical setting. An example is monitoring by a pulse oximeter, which only became standard care in anesthesia since the late 1990s (15, 16). Articles in English including at least 50 well documented patients under the age of 1 year undergoing elective laparoscopic surgery were included. We decided to exclude articles when the cohort consisted of >75% prematurely born infants, or >50% infants with severe congenital abnormalities, including neurological, cardiac or pulmonary disease, or low- and middle-income countries (LMIC) settings. We excluded thoracoscopic procedures, since they are not our focus of interest as the OTC procedure is performed using abdominal laparoscopy and is hardly ever combined with a thoracoscopic procedure. Additionally, thoracoscopic procedures pose different challenges, e.g. deflation of a lung, compared to laparoscopic abdominal procedures and may therefore not be comparable regarding risks. Details on inclusion and exclusion criteria according to the PICOTS format can be found in Table 1 (17). Conference abstracts, systematic reviews, book chapters, articles without full text, and case reports were excluded.
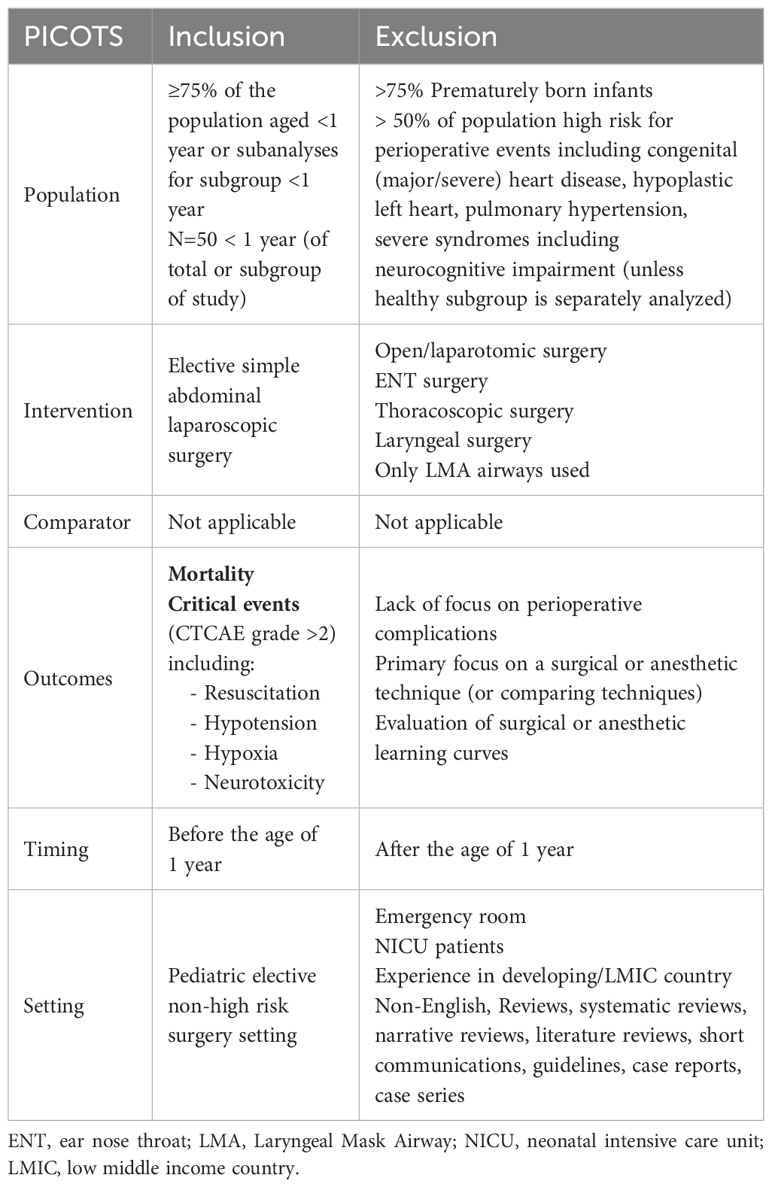
Table 1 Inclusion and exclusion criteria using the PICOTS criteria (17).
All articles were independently screened on title/abstract and full text and reviewed by at least two authors (M.E.M.v.d.P, A.L.F.v.d.K, A.M.E.B, S.L.B, A.W.F.v.d.S., M.O.M. and M.M.v.d.H.E) using the screening tool Rayyan (18). Disagreements between the reviewers were resolved by discussion and reaching consensus including a third author if necessary, and discussion with the full author group. Abstracts selected for full text screening were selected based on the inclusion criteria in Table 1. From the selected papers, the following data were collected: sample size, patient characteristics (age and weight at time of the surgery), diagnosis and type of surgery, airway and anesthesia details, confounding factors including American Society of Anesthesiologists (ASA) score and comorbidities. Complications occurring within the first 24 hours and the first week were recorded. Complications included perioperative mortality in the first week after start of anesthesia. Complications such as resuscitation/cardiac arrest and critical events during and within the first 24 hours were defined as respiratory, circulatory and/or neurological events needing serious intervention and/or with possible negative outcomes or consequences.
A risk of bias assessment was performed to define the quality of the included publications. We used the QUIPS tool as previously described by Hayden et al. (19). Six different domains were included: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting (19). The papers were graded for separate domains as having high, moderate or low level of bias. According to the recommendation of Hayden et al., the most relevant domains were defined in advance (study participation, prognostic factor measurement, outcome measurement, and study confounding) to judge the overall risk of bias in the included studies (19).
The initial and updated search identified a total of 15,535 articles by February 15th, 2023, 4817 from the Pubmed database and 10,718 from the Embase database. After removal of 3134 duplicates, 12,401 articles were screened on title and abstract and 471 articles were subsequently screened on full text. Ten articles fulfilled all inclusion criteria and were included in this systematic review (Figure 1) (20–29).
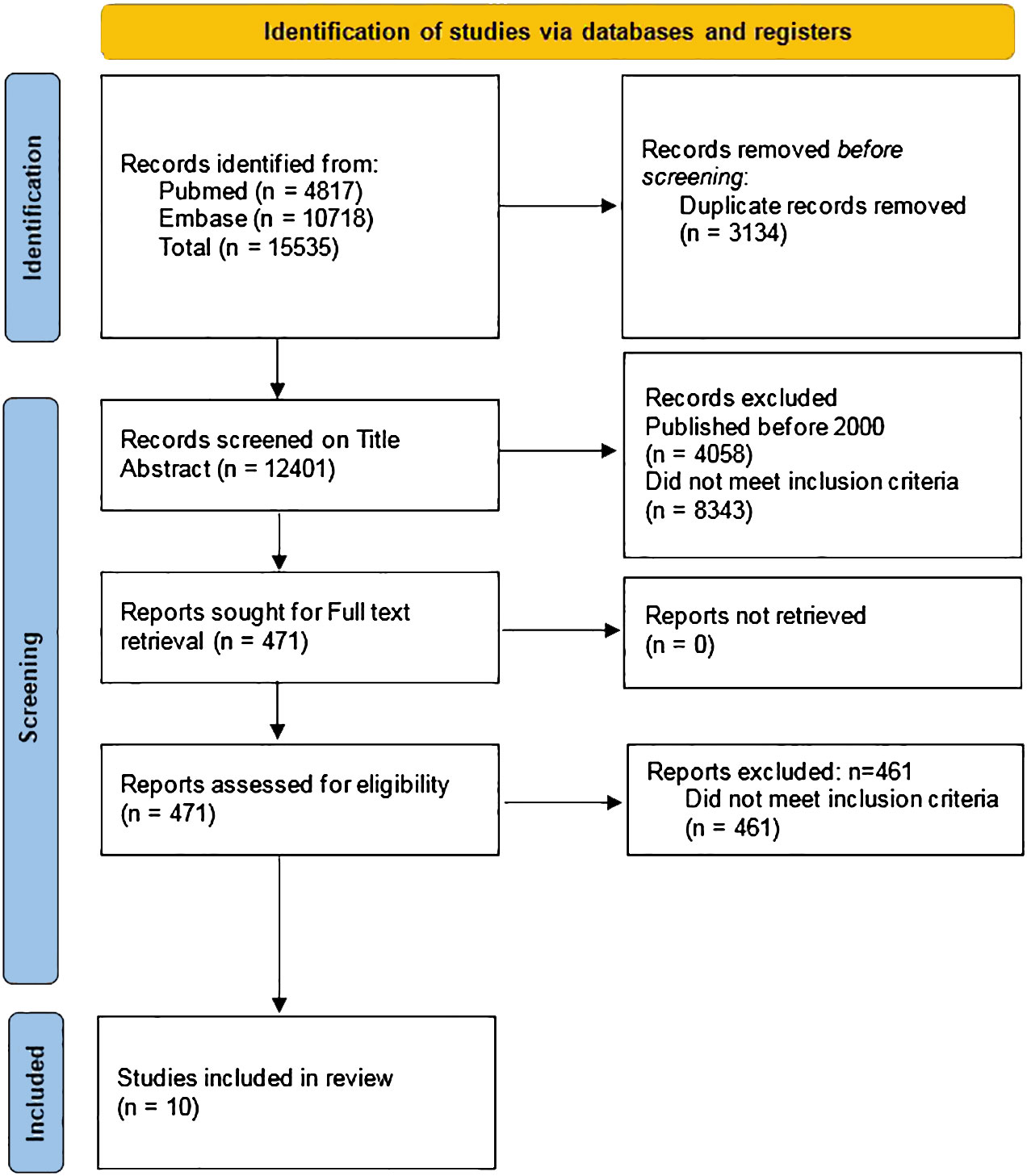
Figure 1 PRISMA 2020 flow diagram of the search strategy (12).
The included studies discussed surgical and anesthetic risks of various abdominal laparoscopic surgeries in 1778 children aged <1 years. The results are presented in Table 2; Supplementary Tables 1–10 (20–29). The reported laparoscopic surgeries included transanal endorectal pull-through (TERPT) for Hirschsprung disease, pyloric stenosis repair/pyloromyotomy, gastrostomy, congenital megacolon, diaphragmatic hernia, malrotation and inguinal hernia repair. Described methods of anesthesia included general anesthesia only or general anesthesia combined with regional anesthesia. Methods to secure the airway were only reported by 2 studies and both used endotracheal intubation (21, 22). Confounders were inconsistently reported and when reported, results had rarely been corrected for these confounding factors (20, 22, 24, 26–28). Most studies scored a moderate risk of bias (22–26, 28), three a high risk of bias (20, 21, 29) and only one study a low risk of bias (27) (Table 3). Insufficiently detailed reporting of prognostic factors, and not reporting confounders were the most common causes for high risk of bias.
When combining the results of all 10 studies, only 1 death occurred in 1778 (0.056%) reported elective laparoscopic surgeries, which was reported to be not related to the surgery (death due to severe pulmonary hypertension in a child with pre-existent severe pulmonary disease) (Table 2; Supplementary Tables 1–10) (20–29). The deceased infant (death not surgery-related), described in the study of Ponsky et al. (n=649 infants <5kg), had multiple comorbidities and severe pulmonary hypertension after Nissen fundoplication (29). In the other studies no mortality was observed, including the only study with a low risk of bias, a randomized trial including 63 infants with a mean age of approximately 50 days (27).
Walsh et al. reported no anesthetic complications in a cohort of 80 infants who underwent laparoscopic inguinal hernia repair (24).
Resuscitation was only specifically reported in two studies (n=82 and n=204) and both studies reported that resuscitation did not occur (21, 25).
In total, critical events occurred in 53/1778 (3.2%) patients (20, 23, 25–27). Notably, some patients had a combination of multiple critical events. Since one study reporting 20 adverse events after laparoscopy and 6 after thoracoscopy, did not specify which specific event occurred in which group, for some adverse events ranges are presented (25). Circulatory events consisted of tachycardia (n=1) (20), bradycardia (n=2-3) (23, 25), and hypotension (n=3-9) (25), respiratory events consisted of apnea and stridor (n=1) (20), hypoxia/desaturation (n=8-14) (25, 27), postoperative respiratory failure (n=11) (26) and pneumonia (n=7) (25–27).
Surgical events included bowel injury upon entry for inguinal hernia repair (n=1) (23), bladder perforation from trocar insertion (n=NR) and trocar site bleed (n=NR), but also multiple other complications related to the surgery (n=NR) (29), critical events related to the pneumoperitoneum included hypercapnia (n=0-5) (25) and metabolic acidosis (n=0-2) (25). In some series no details are described (25).
None of the studies specifically included neurotoxicity after laparoscopic surgery in infants.
Other events included hypothermia (n=4-8) (25, 27) (of which 1 led to bradycardia).
Summarizing, mortality was reported in 1 in 1778 (0 to 0.15%) well-described elective non-high risk laparoscopic procedures in infants without congenital abnormalities, and this death was not surgery-related. The range of incidence of perioperative serious events ranged from 0% to 12.12% (desaturation) (Table 2).
Since no studies have been published comparing the perioperative risk of critical events in children <1 year and >1 year in elective non-high risk laparoscopic surgery, we described the results in this review in the scope of previously published reports on perioperative risks of critical events of elective laparoscopy in children and infants, excluding high risk surgeries. This is challenging as these previously published risks of anesthesia-related mortality (0.1%-3.2%) and severe critical events (2–8%) (12, 30–32) in large cohorts, often did not correct for the invasiveness of the surgery or (congenital or acquired) comorbidities (12, 13, 16, 33–42). Nevertheless, the results from our review illustrate that the incidence of mortality of elective non-high risk laparoscopic procedures in infants without congenital abnormalities is relatively low, 1/1778 cases (0%-0.15%) as is the risk of perioperative events of 3.2% (0% to 12.12% (desaturation)) (Table 2). One of the two largest pediatric studies the Anesthesia Practice in Children Observational Trial (APRICOT) study reported a 30 day mortality rate of 0.1%, and perioperative severe critical events rate of 5.2% in children aged 0-15 years during and after diagnostic and surgical procedures, elective, urgent or emergency, under sedation or general anesthesia (results not specified for infants) (12). The NEonate and Children audiT of Anaesthesia pRactice IN Europe (NECTARINE) study included infants under the age of 60 weeks post menstrual age undergoing anesthesia for surgical, non-surgical or diagnostic procedures (including procedures in the ICU) and reported a 90 day mortality rate of 3.2%, and a perioperative serious critical event rate of 35.2%, and 16.3% experienced 1 or more other complications 30 days after the procedure (13). Notably, these two large cohort studies included patients with (congenital) comorbidities, at high risk for severe events, and since they did not report detailed results on complication risk separately for infants without severe (congenital) comorbidities undergoing elective abdominal laparoscopic surgery, the studies could not be included in our review.
The current review shows that the risk of mortality in infants undergoing elective laparoscopy is evidently low (0.056%). The mortality rates in infants <1 year reported for all anesthetic or surgical procedures in other studies varies from 53 to 59.7/10,000 <24h (36, 41, 43, 44) and 5.91-367.4/10,000 after 30 days (36, 37, 41, 43, 45–48). Again, here we excluded high risk surgeries and patients, since in some studies patients <1 year with an ASA score of 3-5 or extensive comorbidities showed highest incidence of perioperative problems and mortality (12, 34–41, 43, 49), while other reports show that surgical procedures can be safely performed in very young children (12, 44). For this subgroup no comparative studies have been published and due to the heterogeneity of the studies no meta-analysis could be performed. Future large scale international studies based on a prospective registry would be of value to evaluate mortality in infants after elective non-high risk laparoscopy.
Remarkably, even in infants (n=45-6325) with comorbidities, the risk of mortality after laparoscopy is low (0-0.6%) (61-70% comorbidities (cardiac risk factors, neurological impairment, hypoplastic left heart (HLHS), prematurity, infants <3-5kg)) (50–57). When including only open or both laparoscopic and open surgery, mortality rates range from 0 in small cohorts (58, 59) to 0.4-4.4% in larger studies (n=151-2967) even in cohorts with many patients with ASA≥3 and minor to severe cardiac risk factors (60–62). Thus, it may be concluded that the risk of surgery-related mortality in infants after laparoscopy is low, even in infants with comorbidities placing them at increased risk for adverse events (53, 60, 63–66). Nonetheless, mortality may occur due to the underlying disease (52).
Resuscitation or cardiac arrest after laparoscopic surgery in infants was specifically reported by two of our included studies, which reassuringly reported 0 resuscitations in 286 patients (21, 25). The event of resuscitation was not reported in the remaining 1492 infants in the other 8 studies (20, 22–24, 26, 27, 29). In the latter studies, it is possible that cardiac arrests did or did not occur but were not structurally reported, or did not occur due to the small size of this sample. Perioperative cardiac arrest related to anesthesia in infants has been reported to occur in 38.6/10,000, ranges being reported from 8.7 to 87.1/10,000 in a recent systematic review (n= 122,196) (44). But again, these cohorts differ from the included population of interest (34, 36, 37, 43, 58, 67). In cohorts including >40% infants <1 year with cardiac risk factors cardiac arrests occurred in 0.9% (n=2967) when undergoing major abdominal and thoracic surgery (60). This may indicate that the risk of resuscitation is reassuringly low in infants undergoing elective non-high risk laparoscopic surgery.
Critical events occurred in 53/1778 (3.2%) patients in the included studies, which seems to be lower than reported in other studies describing also open surgeries and anesthetic procedures in infants, in which the rates vary between 4.6% and 30.8% (34, 35, 44–46, 48, 68, 69). The APRICOT study reported a higher rate of cardiovascular and respiratory critical events in neonates (0–1 month) and infants (1 month to 1 year) (12). In neonates cardiovascular complications occurred in 12.1% (12). The NECTARINE study reported perioperative serious critical event in 35.2% of cases and 16.3% experienced 1 or more other complications 30 days after the procedure (13). Notably, endpoint definitions were not consistent between the included studies, which is in line with other literature and critical events included respiratory, cardiovascular and neurological events such as hypoxemia, hypotension, hypo- or hyperthermia, anaphylaxis, intubation problems, vomiting, coma/seizure (34, 35, 45, 46, 48, 68, 69). Some smaller studies (n<50) report a severe perioperative event rate of 0 (51, 59) or 1 (severe hypercapnia) (58) and that laparoscopic surgery or minimal access surgery is safe even in very small infants (56, 57). Currently published risk factors seem to be related to cardiovascular and respiratory complications, which is in line with infant anatomy and physiology (49, 70–72), and in contrast to the risk factors associated with anesthetic use (halothane) or anesthetic procedures in the past (42, 44). None of the included studies reported laryngospasm, despite the fact that in general infants <1 year have an increased risk of laryngospasm (2.7%) compared to older children (35). In general, in children laryngospasms are among the most common critical events occurring during/after general anesthesia (73–75). So overall, also regarding critical events, laparoscopic surgery appears safe for infants.
Notably, none of the included studies reported neurotoxicity after laparoscopy, despite the fact that neurotoxicity after anesthesia has received attention in the past (33, 76–83). In 2012 a consensus was published to state that necessary procedures or surgeries in infants and children of preschool age should not be postponed due to fear of neurotoxicity (84, 85). This was supported by the encouraging studies on the long term impact on infants of anesthesia (<1 hour) during inguinal hernia repair (86, 87). The large ongoing international randomized controlled trial (General Anesthesia compared to Spinal anesthesia (GAS) study) and the ongoing Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) study showed that psychomotor development (at age 5 years) was unremarkable (86, 87).
This is the first systematic review evaluating the surgical and anesthetic risks of elective non-high risk laparoscopic surgery in infants <12 months. It seems that laparoscopic surgery in infants is safe, yet no adequate registry or study has been published to specifically evaluate this. For this review, confounders were not always sufficiently described (26, 28), leading to higher risk of bias in some studies and not all studies systematically reported neurotoxicity, ASA status or resuscitations (12, 34–38, 49, 88–93). Available systematic reviews on critical events and complications in pediatric anesthesia in general revealed already that there is a great variability within and between studies regarding definitions of events and diagnostic criteria for complications (44, 94). This variability between studies may limit generalizability to the pediatric oncology population. The large differences between the studies prohibited a clinically relevant meta-analysis.
The currently available recommendations for fertility preservation in children with cancer do not include any recommendation regarding a safe lower age limit for laparoscopic OTC (8, 11) and anesthetic and surgical risk of laparoscopic OTC specifically in infants <1 year have not been published (95–97). The fact that our review only identified one deceased case (among n=1778 well documented cases) and that only low percentages of circulatory and respiratory events were observed, suggests that elective laparoscopic surgeries can safely be performed in infants <12 months. The available evidence on the risk of anesthesia and abdominal laparoscopic surgery <1 year may suggest that these risks do not outweigh the advantages of performing fertility preservation for those at high risk of gonadal damage and premature ovarian failure. However, since none of the studies studied OTC specifically, it may be argued that the included procedures such as inguinal hernia repair in infants may not be comparable to OTC regarding surgical complexity and complication risk, as children are also suffering from childhood cancer.
Specifically, some infants may not tolerate the pneumoperitoneum (51, 52, 56, 98), and other challenges include the presence of a large abdominal tumor, limiting the operative space and/or impairing pulmonary capacity, or disrupted blood counts in patients with leukemia or after intensive chemotherapy. On the other hand, the offer of OTC may not be withheld from girls with a clear high risk of infertility, such as those needing allogenic stem cell transplantation as primary salvage treatment for juvenile myelomonocytic leukemia (JMML) for instance or whole abdominal radiotherapy for a massive rupture of a nephroblastoma at presentation. Obviously, these risks need to be weighed against the benefit of OTC, especially now that the first live births have been reported after autotransplanation of ovarian tissue, harvested in children (6, 99–103) and the first results of auto-transplantation in prepubertally harvested ovarian tissue look promising (6, 104, 105).
Specific risks in the highly selected patients who are eligible for OTC need to be discussed during counseling for OTC. In addition, risks may be reduced by careful selection of the patients, pursuing these procedures in expert pediatric oncology centers, where an oncofertility team, including pediatric surgeons, gynecologists and pediatric anesthesiologists, estimates the risk and anticipate the best controlled setting. This can include postponing OTC until after the first rounds of chemotherapy, in order to reduce the risk of complications including organ damage or tumor spill, but also to reduce circulating tumor cells in the ovarian tissue in leukemia patients. Furthermore, clear criteria should be in place to ensure laparoscopic OTC can be performed safely, including but not limited to platelet parameters to decrease bleeding risk. Hence, a multidisciplinary and personalized approach and a controlled oncological-surgery-fertility approach is important for all infants (and older girls) in which laparoscopic OTC is considered (60, 106).
The original contributions presented in the manuscript are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
MvdP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. AvdK: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. SB: Investigation, Methodology, Writing – review & editing. MM: Investigation, Methodology, Writing – review & editing. AB: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. MvdW: Investigation, Writing – review & editing. AvdS: Investigation, Methodology, Writing – review & editing. MvdHE: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. MvdP is supported by funding from the Princess Máxima Center Foundation, Stichting Kinderoncologisch Centrum Rotterdam (sKOCR) and the Twinning in Research and Education to Improve Survival in Childhood Solid Tumors in Lithuania (TREL) project (funded by European Union’s Horizon 2020 research and innovation programme H2020-EU.4.b). Funders/sponsors were not involved in the set-up of the study, the analyses, the interpretation of results or the writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1315747/full#supplementary-material
1. Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. (2009) 27:2308–18. doi: 10.1200/JCO.2009.22.3339
2. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. (2006) 355:1572–82. doi: 10.1056/NEJMsa060185
3. Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. (2007) 297:2705–15. doi: 10.1001/jama.297.24.2705
4. Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. (2019) 25:159–79. doi: 10.1093/humupd/dmy038
5. Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. (2012) 118:1710–7. doi: 10.1002/cncr.26459
6. van der Perk MEM, van der Kooi ALF, Bos AME, Broer SL, Veening MA, van Leeuwen J, et al. Oncofertility perspectives for girls with cancer. J Pediatr Adolesc Gynecol. (2022) 35:523–6. doi: 10.1016/j.jpag.2022.03.005
7. van der Perk MEM, van der Kooi ALF, van de Wetering MD, IM IJ, van Dulmen-den Broeder E, Broer SL, et al. Oncofertility care for newly diagnosed girls with cancer in a national pediatric oncology setting, the first full year experience from the Princess Maxima Center, the PEARL study. PloS One. (2021) 16:e0246344. doi: 10.1371/journal.pone.0246344
8. Practice Committee of the American Society for Reproductive Medicine. Electronic address aao. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. (2019) 112:1022–33. doi: 10.1016/j.fertnstert.2019.09.013
9. Corkum KS, Laronda MM, Rowell EE. A review of reported surgical techniques in fertility preservation for prepubertal and adolescent females facing a fertility threatening diagnosis or treatment. Am J Surg. (2017) 214:695–700. doi: 10.1016/j.amjsurg.2017.06.013
10. Rowell EE, Corkum KS, Lautz TB, Laronda MM, Walz AL, Madonna MB, et al. Laparoscopic unilateral oophorectomy for ovarian tissue cryopreservation in children. J Pediatr Surg. (2019) 54:543–9. doi: 10.1016/j.jpedsurg.2018.06.005
11. Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. (2021) 22:e45–56. doi: 10.1016/S1470-2045(20)30594-5
12. Habre W, Disma N, Virag K, Becke K, Hansen TG, Johr M, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. (2017) 5:412–25. doi: 10.1016/S2213-2600(17)30116-9
13. Disma N, Veyckemans F, Virag K, Hansen TG, Becke K, Harlet P, et al. Morbidity and mortality after anaesthesia in early life: results of the European prospective multicentre observational study, neonate and children audit of anaesthesia practice in Europe (NECTARINE). Br J Anaesth. (2021) 126:1157–72. doi: 10.1016/j.bja.2021.02.016
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Morray JP, Geiduschek JM, Caplan RA, Posner KL, Gild WM, Cheney FW. A comparison of pediatric and adult anesthesia closed malpractice claims. Anesthesiology. (1993) 78:461–7. doi: 10.1097/00000542-199303000-00009
16. Morray JP, Geiduschek JM, Ramamoorthy C, Haberkern CM, Hackel A, Caplan RA, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology. (2000) 93:6–14. doi: 10.1097/00000542-200007000-00007
17. Moons KG, Hooft L, Williams K, Hayden JA, Damen JA, Riley RD. Implementing systematic reviews of prognosis studies in Cochrane. Cochrane Database Syst Rev. (2018) 10:ED000129. doi: 10.1002/14651858
18. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
19. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
20. Beltman L, Roorda D, Backes M, Oosterlaan J, van Heurn LWE, Derikx JPM. Risk factors for short-term complications graded by Clavien-Dindo after transanal endorectal pull-through in patients with Hirschsprung disease. J Pediatr Surg. (2022) 57:1460–6. doi: 10.1016/j.jpedsurg.2021.07.024
21. Chou CM, Yeh CM, Huang SY, Chen HC. Perioperative parameter analysis of neonates and infants receiving laparoscopic surgery. J Chin Med Assoc. (2016) 79:559–64. doi: 10.1016/j.jcma.2016.05.005
22. Disma N, Engelhardt T, Hansen TG, de Graaff JC, Virag K, Habre W, et al. Neonates undergoing pyloric stenosis repair are at increased risk of difficult airway management: secondary analysis of the NEonate and Children audiT of Anaesthesia pRactice IN Europe. Br J Anaesth. (2022) 129:734–9. doi: 10.1016/j.bja.2022.07.041
23. Fraser JA, Briggs KB, Svetanoff WJ, Rentea RM, Aguayo P, Juang D, et al. Umbilical access in laparoscopic surgery in infants less than 3 months: A single institution retrospective review. J Pediatr Surg. (2022) 57:277–81. doi: 10.1016/j.jpedsurg.2021.11.010
24. Walsh CM, Ng J, Saxena AK. Comparative analysis of laparoscopic inguinal hernia repair in neonates and infants. Surg Laparoscopy Endoscopy Percutaneous Techniques. (2020) 30:459–63. doi: 10.1097/SLE.0000000000000815
25. Kalfa N, Allal H, Raux O, Lardy H, Varlet F, Reinberg O, et al. Multicentric assessment of the safety of neonatal videosurgery. Surg Endosc. (2007) 21:303–8. doi: 10.1007/s00464-006-0044-1
26. Landisch RM, Colwell RC, Densmore JC. Infant gastrostomy outcomes: The cost of complications. J Pediatr Surg. (2016) 51:1976–82. doi: 10.1016/j.jpedsurg.2016.09.025
27. Meng-Meng T, Xue-Jun X, Xiao-Hong B. Clinical effects of warmed humidified carbon dioxide insufflation in infants undergoing major laparoscopic surgery. Med (Baltimore). (2019) 98:e16151. doi: 10.1097/MD.0000000000016151
28. Onwubiko C, Weil BR, Bairdain S, Hall AM, Perkins JM, Thangarajah H, et al. Primary laparoscopic gastrojejunostomy tubes as a feeding modality in the pediatric population. J Pediatr Surg. (2017) 52:1421–5. doi: 10.1016/j.jpedsurg.2017.05.015
29. Ponsky TA, Rothenberg SS. Minimally invasive surgery in infants less than 5 kg: experience of 649 cases. Surg Endosc. (2008) 22:2214–9. doi: 10.1007/s00464-008-0025-7
30. MacLennan AI, Smith AF. An analysis of critical incidents relevant to pediatric anesthesia reported to the UK National Reporting and Learning System, 2006-2008. Paediatr Anaesth. (2011) 21:841–7. doi: 10.1111/j.1460-9592.2010.03421.x
31. de Graaff JC, Sarfo MC, van Wolfswinkel L, van der Werff DB, Schouten AN. Anesthesia-related critical incidents in the perioperative period in children; a proposal for an anesthesia-related reporting system for critical incidents in children. Paediatr Anaesth. (2015) 25:621–9. doi: 10.1111/pan.12623
32. Williams GD, Muffly MK, Mendoza JM, Wixson N, Leong K, Claure RE. Reporting of perioperative adverse events by pediatric anesthesiologists at a tertiary children's hospital: targeted interventions to increase the rate of reporting. Anesth Analg. (2017) 125:1515–23. doi: 10.1213/ANE.0000000000002208
33. Molenbuur B, Lemson J, Smeulers NJ, Vermeulen PM, Backus EMJM, Bruinenberg JFM, et al. (NVA) NVvA. Richtlijn Anesthesie bij kinderen. SKA (2017). Available at: https://www.anesthesiologie.nl/uploads/files/KD_Richtlijn_Anesthesie_bij_kinderen_defnw.pdf.
34. Murat I, Constant I, Maud'huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth. (2004) 14:158–66. doi: 10.1111/j.1460-9592.2004.01167.x
35. Tay CL, Tan GM, Ng SB. Critical incidents in paediatric anaesthesia: an audit of 10 000 anaesthetics in Singapore. Paediatr Anaesth. (2001) 11:711–8. doi: 10.1046/j.1460-9592.2001.00767.x
36. Ahmed A, Ali M, Khan M, Khan F. Perioperative cardiac arrests in children at a university teaching hospital of a developing country over 15 years. Paediatr Anaesth. (2009) 19:581–6. doi: 10.1111/j.1460-9592.2009.02992.x
37. Bharti N, Batra YK, Kaur H. Paediatric perioperative cardiac arrest and its mortality: database of a 60-month period from a tertiary care paediatric centre. Eur J Anaesthesiol. (2009) 26:490–5. doi: 10.1097/EJA.0b013e328323dac0
38. Gonzalez LP, Pignaton W, Kusano PS, Modolo NS, Braz JR, Braz LG. Anesthesia-related mortality in pediatric patients: a systematic review. Clinics (Sao Paulo). (2012) 67:381–7. doi: 10.6061/clinics/2012(04)12
39. Ramamoorthy C, Haberkern CM, Bhananker SM, Domino KB, Posner KL, Campos JS, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg. (2010) 110:1376–82. doi: 10.1213/ANE.0b013e3181c9f927
40. Mason DG WK, Gough MJ, Gough MJ, Lucas SB, Freeth H, Shotton H, et al. Are we there yet? report by the National Confidential Enquiry into Patient Outcome and Death (NCEPOD). National Confidential Enquiry into Patient Outcome and Death (NCEPOD (2011).
41. van der Griend BF, Lister NA, McKenzie IM, Martin N, Ragg PG, Sheppard SJ, et al. Postoperative mortality in children after 101,885 anesthetics at a tertiary pediatric hospital. Anesth Analg. (2011) 112:1440–7. doi: 10.1213/ANE.0b013e318213be52
42. Bhananker SM, Ramamoorthy C, Geiduschek JM, Posner KL, Domino KB, Haberkern CM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. (2007) 105:344–50. doi: 10.1213/01.ane.0000268712.00756.dd
43. Flick RP, Sprung J, Harrison TE, Gleich SJ, Schroeder DR, Hanson AC, et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center: a study of 92,881 patients. Anesthesiology. (2007) 106:226–37. doi: 10.1097/00000542-200702000-00009
44. Catre D, Lopes MF, Viana JS, Cabrita AS. Perioperative morbidity and mortality in the first year of life: a systematic review (1997-2012). Braz J Anesthesiol. (2015) 65:384–94. doi: 10.1016/j.bjane.2013.03.025
45. Morita K, Kawashima Y, Irita K, Iwao Y, Seo N, Tsuzaki K. [Perioperative mortality and morbidity in the year 2000 in 520 certified training hospitals of Japanese Society of Anesthesiologists: with a special reference to age–report of Japanese Society of Anesthesiologists Committee on Operating Room Safety]. Masui. (2002) 51:1285–96.
46. Morita K, Kawashima Y, Irita K, Kobayayashi T, Goto Y, Iwao Y, et al. [Perioperative mortality and morbidity in 1999 with a special reference to age in 466 certified training hospitals of Japanese Society of Anesthesiologists–report of Committee on Operating Room Safety of Japanese Society of Anesthesiologists]. Masui. (2001) 50:909–21.
47. Chan RP, Auler Junior JO. [Retrospective study of anesthetic deaths in the first 24 hours: review of 82,641 anesthesias.]. Rev Bras Anestesiol. (2002) 52:719–27. doi: 10.1590/S0034-70942002000600009
48. Bunchungmongkol N, Somboonviboon W, Suraseranivongse S, Vasinanukorn M, Chau-in W, Hintong T. Pediatric anesthesia adverse events: the Thai Anesthesia Incidents Study (THAI Study) database of 25,098 cases. J Med Assoc Thai. (2007) 90:2072–9.
49. Braz LG, Braz DG, Cruz DS, Fernandes LA, Modolo NS, Braz JR. Mortality in anesthesia: a systematic review. Clinics (Sao Paulo). (2009) 64:999–1006. doi: 10.1590/S1807-59322009001000011
50. Piening N, Osei H, Munoz Abraham AS, Piening A, Greenspon J, Villalona GA. Open gastrostomy tube placement is associated with higher complications in infants: A national surgical quality improvement program database analysis. J Surg Res. (2021) 260:345–9. doi: 10.1016/j.jss.2020.10.033
51. Al-Qahtani AR, Almaramhi H. Minimal access surgery in neonates and infants. J Pediatr Surg. (2006) 41:910–3. doi: 10.1016/j.jpedsurg.2006.01.009
52. Shariff F, Kiely E, Curry J, Drake D, Pierro A, McHoney M. Outcome after laparoscopic fundoplication in children under 1 year. J Laparoendosc Adv Surg Tech A. (2010) 20:661–4. doi: 10.1089/lap.2010.0213
53. Slater B, Rangel S, Ramamoorthy C, Abrajano C, Albanese CT. Outcomes after laparoscopic surgery in neonates with hypoplastic heart left heart syndrome. J Pediatr Surg. (2007) 42:1118–21. doi: 10.1016/j.jpedsurg.2007.01.049
54. Cho MJ, Kim DY, Kim SC, Namgoong JM. Transition from laparotomy to laparoscopic repair of congenital duodenal obstruction in neonates: our early experience. Front Pediatr. (2017) 5:203. doi: 10.3389/fped.2017.00203
55. Esposito C, Montupet P, Reinberg O. Laparoscopic surgery for gastroesophageal reflux disease during the first year of life. J Pediatr Surg. (2001) 36:715–7. doi: 10.1053/jpsu.2001.22943
56. Marret JB, Dupont-Lucas C, Petit T, Menahem B, Godet C, Ravasse P, et al. Safety of laparoscopic fundoplication in children under 5 kg: a comparative study. Surg Endosc. (2018) 32:4191–9. doi: 10.1007/s00464-018-6164-6
57. Wall JK, Sinclair TJ, Kethman W, Williams C, Albanese C, Sylvester KG, et al. Advanced minimal access surgery in infants weighing less than 3kg: A single center experience. J Pediatr Surg. (2018) 53:503–7. doi: 10.1016/j.jpedsurg.2017.05.006
58. Sato T, Nishiwaki K. Retrospective investigation about anesthetic management of biliary atresia in children: laparoscopic versus conventional Kasai portoenterostomy. Ja Clin Rep. (2019) 5:7. doi: 10.1186/s40981-019-0228-z
59. Li Y, Xiang B, Wu Y, Wang C, Wang Q, Zhao Y, et al. Medium-term outcome of laparoscopic kasai portoenterostomy for biliary atresia with 49 cases. J Pediatr Gastroenterol Nutr. (2018) 66:857–60. doi: 10.1097/MPG.0000000000001934
60. Stey AM, Kenney BD, Moss RL, Hall BL, Berman L, Cohen ME, et al. A risk calculator predicting postoperative adverse events in neonates undergoing major abdominal or thoracic surgery. J Pediatr Surg. (2015) 50:987–91. doi: 10.1016/j.jpedsurg.2015.03.023
61. Valero J, Buitrago G, Eslava-Schmalbach J, Rincon CJ. Prognostic factors associated with clinical and economic outcomes of appendectomies in children: A multilevel analysis in a national retrospective cohort study. World J Surg. (2020) 44:303–12. doi: 10.1007/s00268-019-05182-w
62. Kauffman JD, Danielson PD, Chandler NM. Risk Factors for Adverse Outcomes after Ostomy Reversal in Infants Less than Six Months Old. Am Surgeon. (2019) 85:1253–61. doi: 10.1177/000313481908501132
63. Chu DI, Tan JM, Mattei P, Costarino AT, Rossano JW, Tasian GE. Mortality and Morbidity after Laparoscopic Surgery in Children with and without Congenital Heart Disease. J Pediatr. (2017) 185:88–93 e3. doi: 10.1016/j.jpeds.2017.02.011
64. Chu DI, Tan JM, Mattei P, Simpao AF, Costarino AT, Shukla AR, et al. Outcomes of laparoscopic and open surgery in children with and without congenital heart disease. J Pediatr Surg. (2018) 53:1980–8. doi: 10.1016/j.jpedsurg.2017.10.052
65. Short HL, Travers C, McCracken C, Wulkan ML, Clifton MS, Raval MV. Increased morbidity and mortality in cardiac patients undergoing fundoplication. Pediatr Surg Int. (2017) 33:559–67. doi: 10.1007/s00383-016-4033-8
66. Miller R, Tumin D, Tobias JD, McKee C. Estimating surgical risk in younger and older children with congenital heart disease. J Surg Res. (2018) 232:298–307. doi: 10.1016/j.jss.2018.06.050
67. Braz LG, Modolo NS, do Nascimento P Jr., Bruschi BA, Castiglia YM, Ganem EM, et al. Perioperative cardiac arrest: a study of 53,718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth. (2006) 96:569–75. doi: 10.1093/bja/ael065
68. Edomwonyi NP, Ekwere IT, Egbekun R, Eluwa B. Anesthesia-related complications in children. Middle East J Anaesthesiol. (2006) 18:915–27.
69. Samake B, Keita M, Magalie IM, Diallo G, Diallo A. [Adverse events of anesthesia in pediatric surgery scheduled at Gabriel Toure hospital]. Mali Med. (2010) 25:1–4.
70. Lingappan K, Arnold JL, Fernandes CJ, Pammi M. Videolaryngoscopy versus direct laryngoscopy for tracheal intubation in neonates. Cochrane Database Syst Rev. (2018) 6:CD009975. doi: 10.1002/14651858.CD009975.pub3
71. Sun Y, Lu Y, Huang Y, Jiang H. Pediatric video laryngoscope versus direct laryngoscope: a meta-analysis of randomized controlled trials. Paediatr Anaesth. (2014) 24:1056–65. doi: 10.1111/pan.12458
72. Fiadjoe JE, Nishisaki A, Jagannathan N, Hunyady AI, Greenberg RS, Reynolds PI, et al. Airway management complications in children with difficult tracheal intubation from the Pediatric Difficult Intubation (PeDI) registry: a prospective cohort analysis. Lancet Respir Med. (2016) 4:37–48. doi: 10.1016/S2213-2600(15)00508-1
73. Warwicker SJ, Lobo CA, Dailami N, Young AE. The safety of general anaesthesia in paediatric patients undergoing the application of Biobrane(R) for small scalds. Burns. (2015) 41:1221–6. doi: 10.1016/j.burns.2015.02.007
74. Ozden ES, Meco BC, Alanoglu Z, Alkis N. Comparison of ProSeal laryngeal mask airway (PLMA) with cuffed and uncuffed endotracheal tubes in infants. Bosn J Basic Med Sci. (2016) 16:286–91. doi: 10.17305/bjbms.2016.1219
75. Drake-Brockman TF, Ramgolam A, Zhang G, Hall GL, von Ungern-Sternberg BS. The effect of endotracheal tubes versus laryngeal mask airways on perioperative respiratory adverse events in infants: a randomised controlled trial. Lancet. (2017) 389:701–8. doi: 10.1016/S0140-6736(16)31719-6
76. Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. (2010) 105 Suppl 1:i61–8. doi: 10.1093/bja/aeq302
77. Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth. (2011) 21:716–21. doi: 10.1111/j.1460-9592.2010.03506.x
78. Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. (2009) 110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d
79. DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. (2011) 113:1143–51. doi: 10.1213/ANE.0b013e3182147f42
80. Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. (2011) 128:e1053–61. doi: 10.1542/peds.2011-0351
81. Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. (2009) 12:246–53. doi: 10.1375/twin.12.3.246
82. McCann ME, Schouten AN, Dobija N, Munoz C, Stephenson L, Poussaint TY, et al. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. (2014) 133:e751–7. doi: 10.1542/peds.2012-0973
83. Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth. (2013) 110 Suppl1:i53–72. doi: 10.1093/bja/aet054
84. Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA. Anesthetic neurotoxicity–clinical implications of animal models. N Engl J Med. (2015) 372:796–7. doi: 10.1056/NEJMp1414786
85. SmartTots, Ramsay J RB, Brown E. CONSENSUS STATEMENT ON THE USE OF ANESTHETICS AND SEDATIVES IN CHILDREN (2012). Available online at: https://birthmark.org/wp-content/uploads/2018/06/Consensus_Statement_Dec_2012.pdf.
86. McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. (2019) 393:664–77. doi: 10.1016/S0140-6736(18)32485-1
87. Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. (2016) 315:2312–20. doi: 10.1001/jama.2016.6967
88. Biboulet P, Aubas P, Dubourdieu J, Rubenovitch J, Capdevila X, d'Athis F. Fatal and non fatal cardiac arrests related to anesthesia. Can J Anaesth. (2001) 48:326–32. doi: 10.1007/BF03014958
89. Lienhart A, Auroy Y, Pequignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. (2006) 105:1087–97. doi: 10.1097/00000542-200612000-00008
90. Newland MC, Ellis SJ, Lydiatt CA, Peters KR, Tinker JH, Romberger DJ, et al. Anesthetic-related cardiac arrest and its mortality: a report covering 72,959 anesthetics over 10 years from a US teaching hospital. Anesthesiology. (2002) 97:108–15. doi: 10.1097/00000542-200207000-00016
91. Olsson GL, Hallen B. Cardiac arrest during anaesthesia. A computer-aided study in 250,543 anaesthetics. Acta Anaesthesiol Scand. (1988) 32:653–64. doi: 10.1111/j.1399-6576.1988.tb02804.x
92. Tiret L, Desmonts JM, Hatton F, Vourc'h G. Complications associated with anaesthesia–a prospective survey in France. Can Anaesth Soc J. (1986) 33:336–44. doi: 10.1007/BF03010747
93. Tikkanen J, Hovi-Viander M. Death associated with anaesthesia and surgery in Finland in 1986 compared to 1975. Acta Anaesthesiol Scand. (1995) 39:262–7. doi: 10.1111/j.1399-6576.1995.tb04054.x
94. Mir Ghassemi A, Neira V, Ufholz LA, Barrowman N, Mulla J, Bradbury CL, et al. A systematic review and meta-analysis of acute severe complications of pediatric anesthesia. Paediatr Anaesth. (2015) 25:1093–102. doi: 10.1111/pan.12751
95. Corkum KS, Rhee DS, Wafford QE, Demeestere I, Dasgupta R, Baertschiger R, et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: A systematic review. J Pediatr Surg. (2019) 54:2200–9. doi: 10.1016/j.jpedsurg.2018.12.021
96. Harris CJ, Lautz TB, Rowell EE. Feasibility of laparoscopic ovarian tissue cryopreservation after open abdominopelvic tumor surgery. Am J Surg. (2020) 220:1249–52. doi: 10.1016/j.amjsurg.2020.06.040
97. Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. (2010) 16:617–30. doi: 10.1093/humupd/dmq010
98. Gueugniaud PY, Abisseror M, Moussa M, Godard J, Foussat C, Petit P, et al. The hemodynamic effects of pneumoperitoneum during laparoscopic surgery in healthy infants: assessment by continuous esophageal aortic blood flow echo-Doppler. Anesth Analg. (1998) 86:290–3. doi: 10.1213/00000539-199802000-00012
99. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. (2004) 364:1405–10. doi: 10.1016/S0140-6736(04)17222-X
100. Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. (2006) 21:1345–8. doi: 10.1093/humrep/del007
101. Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. (2004) 363:837–40. doi: 10.1016/S0140-6736(04)15728-0
102. Oktay K, Tilly J. Livebirth after cryopreserved ovarian tissue autotransplantation. Lancet. (2004) 364:2091–2. doi: 10.1016/S0140-6736(04)17541-7
103. Wallace WH, Pritchard J. Livebirth after cryopreserved ovarian tissue autotransplantation. Lancet. (2004) 364(9451):2093–4.
104. Matthews SJ, Picton H, Ernst E, Andersen CY. Successful pregnancy in a woman previously suffering from beta-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. (2018) 70:432–5. doi: 10.23736/S0026-4784.18.04240-5
105. Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril. (2021) 115:1102–15. doi: 10.1016/j.fertnstert.2021.03.008
Keywords: infants, pediatric oncology, ovarian tissue cryopreservation, fertility preservation, laparoscopy, perioperative complications
Citation: van der Perk MEM, van der Kooi ALLF, Broer SL, Mensink MO, Bos AME, van de Wetering MD, van der Steeg AFW and van den Heuvel-Eibrink MM (2024) A systematic review on safety and surgical and anesthetic risks of elective abdominal laparoscopic surgery in infants to guide laparoscopic ovarian tissue harvest for fertility preservation for infants facing gonadotoxic treatment. Front. Oncol. 14:1315747. doi: 10.3389/fonc.2024.1315747
Received: 10 October 2023; Accepted: 17 April 2024;
Published: 28 May 2024.
Edited by:
Lisa States, Children’s Hospital of Philadelphia, United StatesReviewed by:
Mindy S. Christianson, Johns Hopkins University, United StatesCopyright © 2024 van der Perk, van der Kooi, Broer, Mensink, Bos, van de Wetering, van der Steeg and van den Heuvel-Eibrink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. E. Madeleine van der Perk, bS5lLm0udmFuZGVycGVya0Bwcmluc2VzbWF4aW1hY2VudHJ1bS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.