
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 May 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1309803
Introduction: Since the first report, primary mediastinal seminoma has a low incidence in the population, and it mainly affects young and middle-aged men, is clinically rare, and accounts for a very small proportion of mediastinal tumors. In this study, we describe the first case of primary mediastinal seminoma with azoospermia and hypothesize that the coexistence of the two disorders may not be a coincidence.
Case report: A 16-year-old man presented with chest tightness and chest pain, a mediastinal mass on chest CT, and abnormal 18F-fluoro-deoxyglucose uptake on a PET-CT scan. By biopsy of the mass, the pathological diagnosis was a primary mediastinal seminoma. Because chemotherapy is included in the treatment of the tumor, the patient underwent sperm freezing before treatment, considering that chemotherapy can affect fertility, but the patient was diagnosed with azoospermia. Finally, the patient underwent tumor resection and postoperative chemotherapy. No tumor recurrence was observed at the current follow-up.
Conclusion: Primary mediastinal seminoma is mainly confirmed by histopathological examination, and surgery and chemoradiotherapy are the current treatments. In patients with mediastinal seminoma or azoospermia, doctors should be aware that the two disorders may coexist, especially in men who have fertility requirements or long-term infertility, and that examination of the mediastinum and semen may lead to unexpected findings in the diagnosis and treatment. For mediastinal germ cell tumors, genetic testing is of great value in the treatment of tumors and the prediction of associated diseases. Future studies exploring the potential correlation between mediastinal seminoma and azoospermia will be prospective.
Primary mediastinal seminoma was first reported by Woolner et al. in 1955. The tumor is an extragonadal malignant germ cell tumor that usually occurs in men aged 15~40 years. The incidence is low, the disease is relatively rare, and primary mediastinal seminoma accounts for approximately 1% ~ 4% of mediastinal tumors (1–3). The pathogenesis of primary mediastinal seminoma is not fully understood, but it may be related to ectopic gonadal tissue during embryonic development, retention and displacement of some germ cells or pluripotent stem cells to the mediastinum, and induction by carcinogenic factors (4–8). At present, there are many reports of a single tumor, but reports of other diseases are still rare. Here, we describe the first case of primary mediastinal seminoma with azoospermia and hypothesize that the coexistence of the two disorders may not be a coincidence.
A 16-year-old man presented to the department of thoracic surgery of the hospital with a 2-day history of chest pain and chest tightness. He was previously healthy and did not take any medications. The man’s physical examination was normal; a huge mass in the left anterior mediastinum with a maximum measurement of approximately 58 mm x 46 mm was found on the chest CT examination; and he was hospitalized. In the laboratory analysis results after admission, in addition to uric acid (UA) 528 µmol/l (normal 208–428 µmol/l), urine glucose (UGLU) positive (3+), human chorionic gonadotropin-beta subunit (HCG-β) 313.08 mIU/mL (normal 0–5 mIU/mL), the remaining liver and kidney function, AFP, blood lipids, serum glucose, and systemic inflammatory markers (erythrocyte sedimentation rate and C-reactive protein) were normal. Due to the abnormal HCG-β value, the man underwent a color ultrasound and CT examination of the reproductive system to exclude other diseases, but the results showed that there was no abnormality. To clarify the nature of the mediastinal mass, a needle biopsy was performed on the mass, and the diagnosis was mediastinal seminoma based on the pathological examination combined with immunohistochemistry. Considering that seminoma is a malignant tumor, the possibility of distant metastasis could not be ruled out, so a whole-body PET-CT examination was completed. The results showed that the 18F-fluoro-deoxyglucose metabolism of the left anterior mediastinal mass was increased, and there were no abnormalities in the examination of other organs and tissues (Figures 1A-D). To determine the next treatment plan, the hospital organized multidisciplinary treatment (MDT) meetings with the departments of thoracic surgery, urology, oncology, imaging, radiotherapy, and pathology. There are two treatment options that were discussed in the MDT meetings: chemoradiotherapy before surgery or chemoradiotherapy after surgery. Because of the young age of the patient, considering that chemoradiotherapy can affect fertility, it was recommended that the man undergo sperm freezing before treatment. Surprisingly, after rigorous testing by the human sperm bank institution, the man was finally diagnosed with azoospermia. The patient said that he had never had this test before, and the man also told that he had ejaculated spontaneously in the past, but no sperm loss occurred. Because no reproductive disorders were detected on imaging, gonadotropins and lactate dehydrogenase (LDH) were examined. His estradiol (E2) 100.0 pg/ml (normal 11.3–43.2 pg/ml), prolactin (PRL) 15.30 pg/ml (normal 4.04–15.2 pg/ml), follicle stimulating hormone (FSH) < 0.30 mIU/mL (normal 1.5–12.4 mIU/mL), luteinizing hormone (LH) < 0.30 IU/mL (normal 1.7-8.6 mIU/mL), progesterone (PROG), testosterone (T), and LDH levels were normal. Based on the condition, the patient eventually requested a hospital transfer.
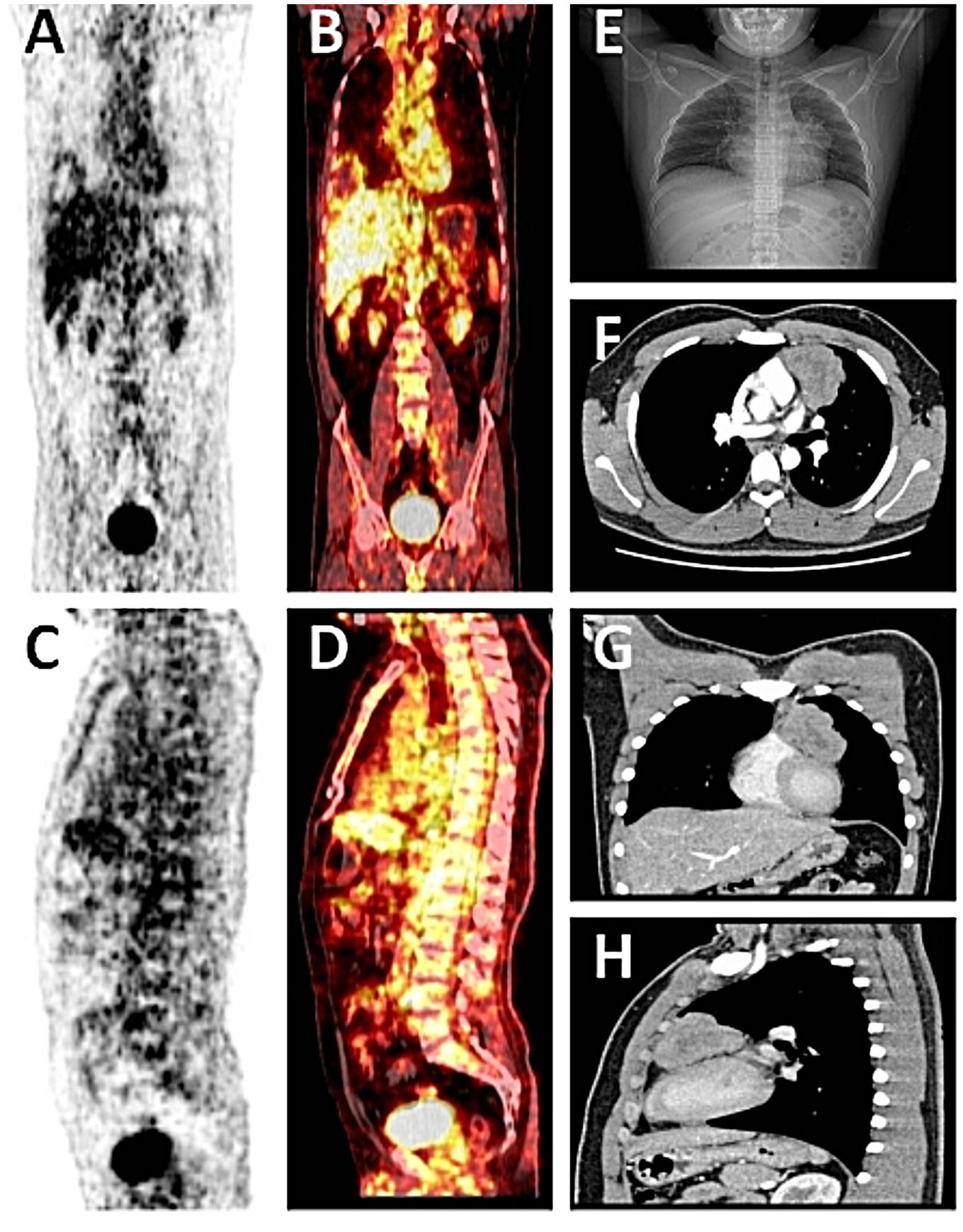
Figure 1 (A-D) Whole-body PET-CT showed a left anterior mediastinal soft tissue shadow, an uneven increase in radioactive uptake (SUVmax = 4.5), and no obvious abnormalities in other organ tissue examinations. (E) A chest X-ray showed a left anterior mediastinal tumor with illuminated edges. (F-H) Chest CT showed a large mass of soft tissue density in the left anterior upper mediastinum, with lobulated edges and uneven internal density, and mild to moderate enhancement of the solid part of the edge on enhanced scanning.
A week later, the man, accompanied by his parents, came to our hospital for treatment. He told us about the previous diagnosis and treatment, and he decided to undergo surgery and then chemoradiotherapy. After admission, we evaluated and prepared the patient before surgery and found the following: HCG-β 403.47 IU/L, UA 586 µmol/l (normal 155-428 µmol/l), and UGLU positive (2+). A chest X-ray and a chest CT showed a large left anterior mediastinal mass; the maximum measurement was approximately 72 mm x 49 mm, and the remaining preoperative examination results were normal (Figures 1E-H). After all the preoperative preparations were complete, we opted for thoracoscopic mediastinal mass resection. During the operation, we observed that the mass was huge and closely adhered to the upper left lung lobe, which did not rule out the possibility that the tumor had invaded the lung lobe (Figure 2A). Finally, under the premise of resecting part of the upper left lung lobe, we successfully and completely removed this large tumor. The tumor was actually measured to be approximately 110 mm x 60 mm x 45 mm in size, resembling a kidney, dark red and grayish yellow, hard in texture, and with solid gray-white tissue inside (Figures 2B-D). The man remained stable after surgery, and we also compared changes in the laboratory analyses before and after surgery. We found that HCG-β decreased significantly to 111.11 mIU/mL on postoperative day 1 and to 11.2 mIU/mL on postoperative day 6 (Figure 3). In addition, LH 0.22 IU/mL (normal 1.24–8.62 mIU/mL), PROG 0.22 nmol/L (normal 0.30–2.60 nmol/L), T 1.75 nmol/L (normal 6.07–27.10 nmol/L), E2, FSH, PRL, and UGLU results are normal. The final pathological diagnosis was mediastinal seminoma involving the lungs (Figures 4A, B). Immunocytochemistry (IHC) showed the following: CD117 (+), LCA (-), CK-pan (-), CD99 (-), AFP (low+), PLAP (+), and ki-67 (80%+) (Figures 4C-I). A week after the operation, the man recovered well and was discharged from the hospital. During the 3rd week after surgery, the patient had a repeat semen analysis, and the result was still azoospermia. The fluorescence in situ hybridization (FISH) method showed t(12p12.1) (KRAS gene locus was abnormal, with KRAS (12p) gene polybody). At 4 weeks postoperatively, the patient started chemotherapy with the EP regimen, specifically etoposide 200 mg + cisplatin 40 mg (d1-d5, 21 days/cycle). At present, the patient has completed 4 cycles of chemotherapy, and no evidence of tumor recurrence has been found thus far.
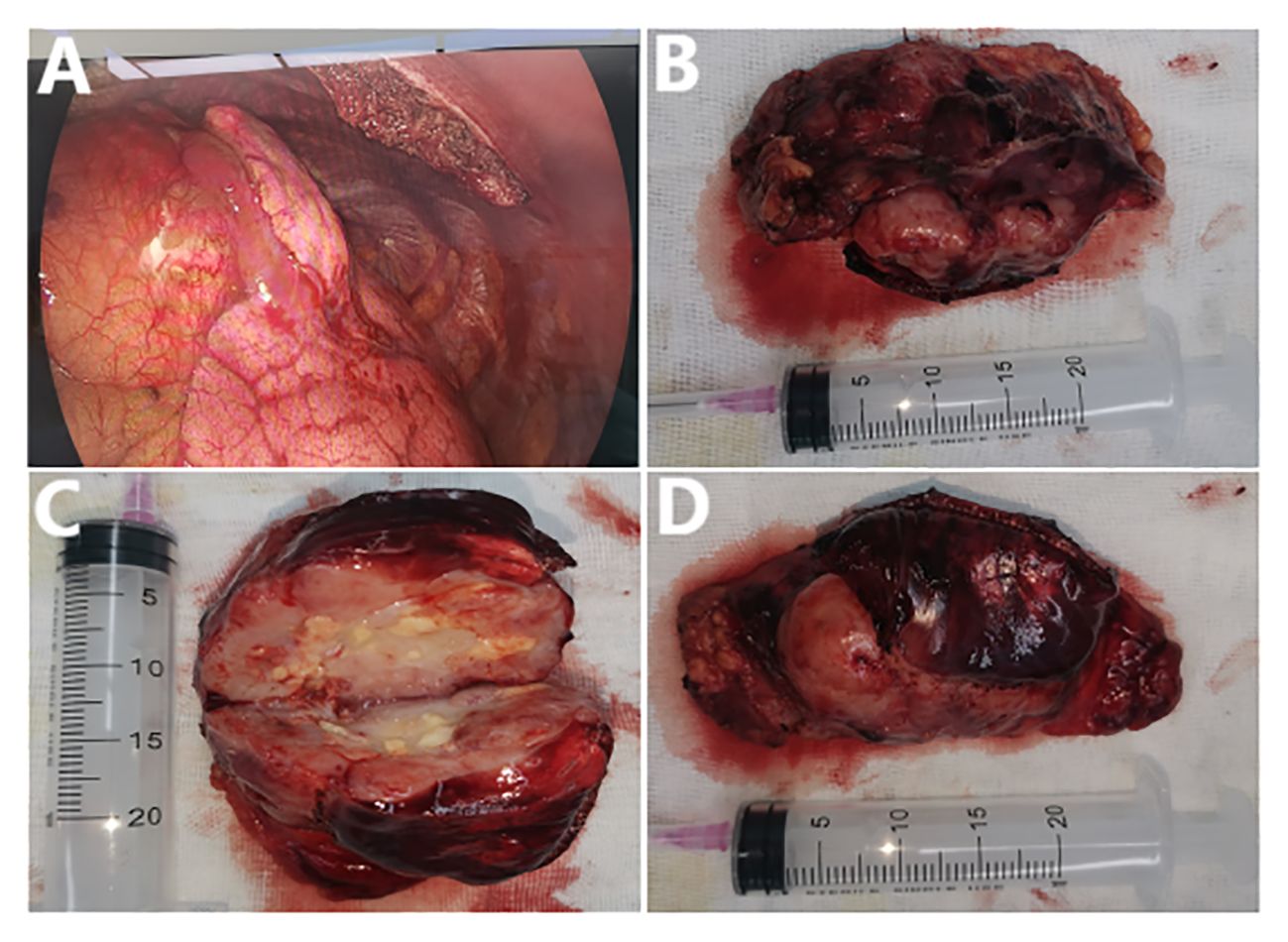
Figure 2 (A) The tumor seen during surgery. (B-D) The completely removed mediastinal tumor after surgery and the internal conditions after tumor incision.
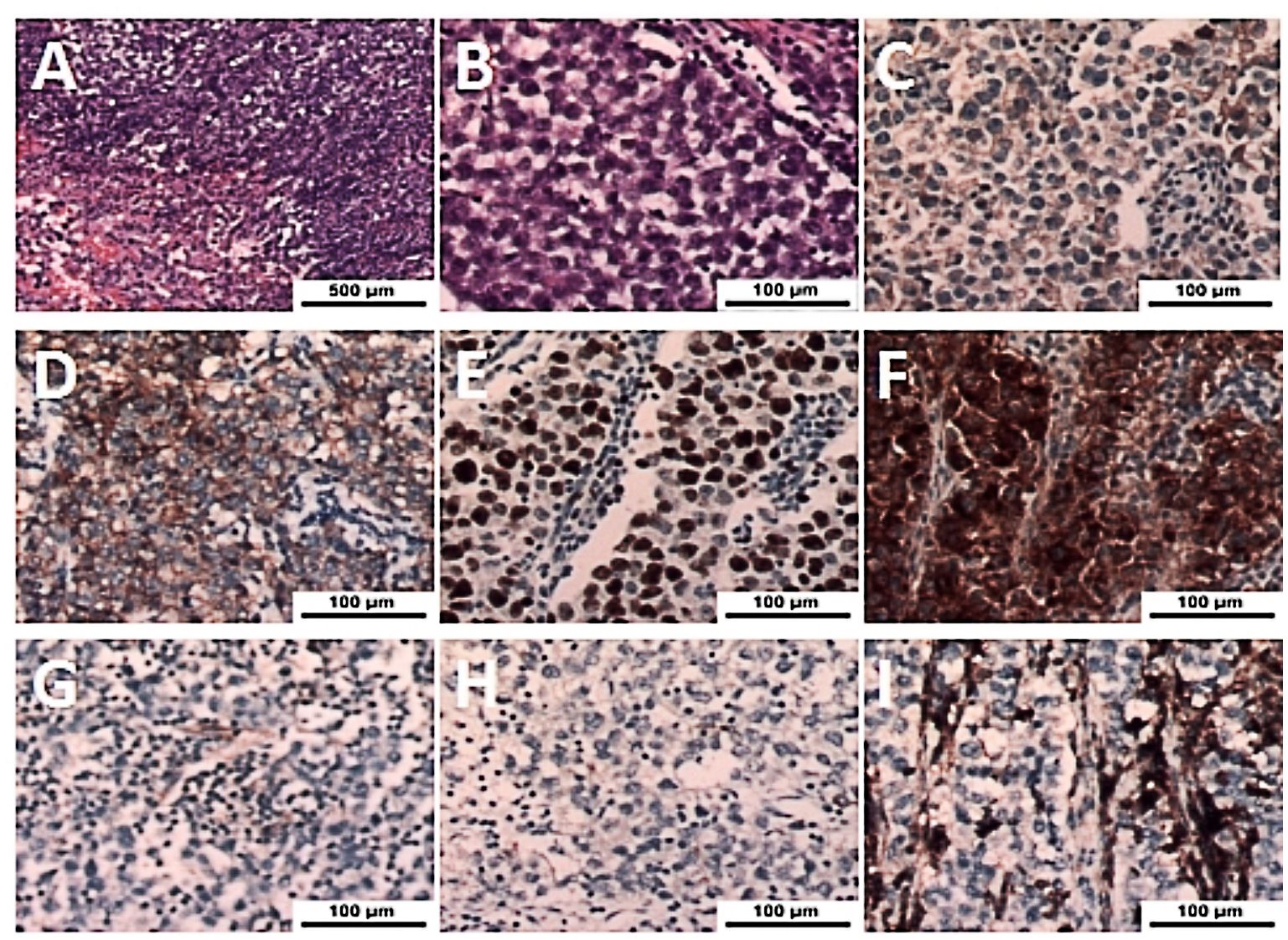
Figure 4 (A, B) Histologic features of seminoma with large tumor cells, round, polygonal appearance, deep nuclear staining, round foam nuclei, pathological mitotic images, and lymphocyte infiltration of the interstitium were observed (H&E, magnification x50 and x400). (C-F) Immunohistochemistry of tumor cells showed strong expression of AFP (low+), CD117 (+), ki-67 (+), and PLAP (+) (magnification x400). (G-I) Immunohistochemistry of tumor cells showed negative results for CD99 (-), CK-pan (-), and LCA (-) (magnification x400).
Seminoma is a malignant germ cell tumor that rarely occurs outside the gonads, and a small number of tumors can occur in extragonadal tissues or organs such as the retroperitoneum, mediastinum, and pineal gland (9, 10). The clinical manifestations of primary mediastinal seminoma are nonspecific, and common symptoms include chest pain, chest tightness, dyspnea, and facial swelling, usually related to tumor size and compression or invasion of adjacent tissues. In asymptomatic patients, smaller tumors are often found incidentally on imaging tests at physical examination (11–13).
In the diagnosis and treatment of primary mediastinal seminoma, misdiagnosis and mistreatment are very likely to occur. Therefore, a comprehensive diagnosis is required based on clinical symptoms, imaging examinations, serum tumor markers, and pathological results. According to the clinical guidelines of the American Society of Clinical Oncology, serum AFP and β-hCG are not only considered important for the qualitative diagnosis and pathological classification, but are also closely related to the evaluation of the efficacy of primary mediastinal seminoma and monitoring of tumor recurrence and metastasis (14, 15). In this case report, our patient showed significant changes in β-hCG values before and after surgery. At present, new diagnostic methods on molecular, immunological, chromosomal, and other aspects are gradually being reported (16–18).
Treatment of primary mediastinal seminoma is mainly chemoradiotherapy combined with surgery to remove the tumor, but the timing of surgery is controversial. Chemoradiotherapy should be carried out first, followed by surgery to remove the residual tumor. It has also been argued that radical tumor resection should be performed before chemotherapy (19–21). In general, the timing of surgical treatment should be determined according to the actual situation of different patients. This tumor is mostly a low to moderately malignant tumor sensitive to chemoradiotherapy; it is also one of the tumors that can be cured by comprehensive treatment of chemoradiotherapy combined with surgery, and early correct diagnosis and standardized treatment are crucial to the prognosis of primary mediastinal seminoma (22, 23).
Azoospermia is the most refractory infertility and is clinically divided into nonobstructive and obstructive azoospermia. Its etiology and pathogenic mechanisms are complex and mostly related to the patient’s environment, chromosomes, sex hormones, and other factors (24–26). The treatment of azoospermia varies greatly due to different causes, and even with the rapid development of assisted reproductive technology, there is no standard treatment, but an individualized treatment plan based on the patient’s etiology can improve the treatment effect (27, 28).
In this paper, we report the first case of primary mediastinal seminoma with azoospermia, and no similar cases have been reported. Azoospermia has been associated with tumors and has a higher incidence of cancer in men with azoospermia (29–31). Although the specific cause of the association between mediastinal seminoma and azoospermia in male adolescents has not been clearly revealed, it may not be a coincidence that the two disorders coexist. In malignant germ cell tumors, mutations in chromosome 12p are most common (32–34). The mutant gene KRAS, which belongs to the Ras gene family and is also a key cause of cancer, is currently confirmed in studies of pancreatic cancer, colorectal cancer, and lung adenocarcinoma but is rarely reported in studies related to mediastinal seminoma and azoospermia. Notably, there is currently no relevant research to clearly explain whether the mutation of the KRAS gene can be used as direct evidence as the cause of these two diseases, but perhaps there will be more cases and studies to explore and confirm this in the future.
There are also limitations to the reporting of this study. First, we did not follow up for a long time, and the time from treatment to the recent follow-up of the cases reported this time was only 7 months. In this case, we have proposed the hypothesis of temporary azoospermia. Transient azoospermia occurs due to a disturbance of gonadal axis hormone levels due to mediastinal seminomas. When hormone levels in the body return to normal after the tumor is treated, azoospermia may resolve on its own. After a long period of follow-up, this hypothesis may be successfully validated. In addition, the patient did not undergo an extraction biopsy of testicular tissue. We were unable to find evidence at the cellular level that the patient’s sperm were hindered in the formation process. Therefore, it is difficult to further clarify the cause of the association between mediastinal seminoma and azoospermia, and it is not possible to provide more effective evidence that KRAS mutations directly affect spermatogenesis.
Primary mediastinal seminoma is diagnosed mainly based on histopathological examination, and surgery and adjuvant chemoradiotherapy are the current treatments. In patients with mediastinal seminoma or azoospermia, doctors should be aware that the two disorders may coexist, especially in men who have fertility requirements or long-term infertility, and that examination of the mediastinum and semen may lead to unexpected findings in diagnosis and treatment. For mediastinal germ cell tumors, genetic testing is of great value in the treatment of tumors and the prediction of associated diseases. Future studies exploring the potential correlation between mediastinal seminoma and azoospermia will be prospective.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Suzhou Kowloon Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
ZL: Writing – review & editing, Writing – original draft, Supervision, Investigation, Data curation, Conceptualization. QZ: Writing – original draft, Methodology. SN: Writing – original draft. KX: Writing – review & editing. ZX: Writing – original draft. PY: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank the patient for his consent to the publication of the data. Appropriate written informed consent was obtained for the publication of this case report and accompanying images.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1309803/full#supplementary-material
1. Woolner LB, Jamplis RW, Kirklin JW. Seminoma (germinoma) apparently primary in the anterior mediastinum. N Engl J Med. (1955) 252:653–7. doi: 10.1056/NEJM195504212521602
2. Napieralska A, Majewski W, Osewski W, Miszczyk L. Primary mediastinal seminoma. J Thorac Dis. (2018) 10:4335–41. doi: 10.21037/jtd.2018.06.120
3. Ozgun G, Nappi L. Primary mediastinal germ cell tumors: A thorough literature review. Biomedicines. (2023) 11:487. doi: 10.3390/biomedicines11020487
4. El-Zaatari ZM, Ro JY. Mediastinal germ cell tumors: A review and update on pathologic, clinical, and molecular features. Adv Anat Pathol. (2021) 28:335–50. doi: 10.1097/PAP.0000000000000304
5. Xiu W, Pang J, Hu Y, Shi H. Immune-related mechanisms and immunotherapy in extragonadal germ cell tumors. Front Immunol. (2023) 14:1145788. doi: 10.3389/fimmu.2023.1145788
6. Ronchi A, Cozzolino I, Montella M, Panarese I, Zito Marino F, Rossetti S, et al. Extragonadal germ cell tumors: Not just a matter of location. A review about clinical, molecular and pathological features. Cancer Med. (2019) 8:6832–40. doi: 10.1002/cam4.2195
7. De Felici M, Klinger FG, Campolo F, Balistreri CR, Barchi M, Dolci S. To be or not to be a germ cell: the extragonadal germ cell tumor paradigm. Int J Mol Sci. (2021) 22:5982. doi: 10.3390/ijms22115982
8. Guida E, Tassinari V, Colopi A, Todaro F, Cesarini V, Jannini B, et al. MAPK activation drives male and female mouse teratocarcinomas from late primordial germ cells. J Cell Sci. (2022) 135(8):jcs259375. doi: 10.1242/jcs.259375
9. Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol. (2002) 20(7):1864–73. doi: 10.1200/JCO.2002.07.062
10. Resti G, Secondino S, Necchi A, Fornarini G, Pedrazzoli P. Primary mediastinal germ cell tumors. Semin Oncol. (2019) 46:107–11. doi: 10.1053/j.seminoncol.2019.04.001
11. Wang L, Zhao J, An T, Wang Y, Zhuo M, Wu M, et al. Clinical characteristics and outcomes of patients with primary mediastinal germ cell tumors: A single-center experience. Front Oncol. (2020) 10:1137. doi: 10.3389/fonc.2020.01137
12. Nichols CR. Mediastinal germ cell tumors. Clinical features and biologic correlates. Chest. (1991) 99:472–9. doi: 10.1378/chest.99.2.472
13. Albany C, Einhorn LH. Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol. (2013) 25:261–5. doi: 10.1097/CCO.0b013e32835f085d
14. AACR Cancer Progress Report 2022 Steering Committee. Cancer in 2022. Cancer Discovery. (2022) 12:2733–8. doi: 10.1158/2159-8290.CD-22-1134
15. Gilligan TD, Seidenfeld J, Basch EM, Einhorn LH, Fancher T, Smith DC, et al. American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. (2010) 28(20):3388–404. doi: 10.1200/JCO.2009.26.4481
16. Fischer A, Richter A, Filmar S, Kircher S, Rosenwald A, Küffer S, et al. Primary mediastinal germ cell tumours: an immunohistochemical and molecular diagnostic approach. Histopathology. (2022) 80(2):381–96. doi: 10.1111/his.14560
17. Liu A, Cheng L, Du J, Peng Y, Allan RW, Wei L, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol. (2010) 34:697–706. doi: 10.1097/PAS.0b013e3181db84aa
18. Weissferdt A, Rodriguez-Canales J, Liu H, Fujimoto J, Wistuba II, Moran CA. Primary mediastinal seminomas: a comprehensive immunohistochemical study with a focus on novel markers. Hum Pathol. (2015) 46:376–83. doi: 10.1016/j.humpath.2014.11.009
19. Zhai Y, Chen B, Feng X, Liu K, Wang S, Hui Z, et al. Chemoradiotherapy is an alternative choice for patients with primary mediastinal seminoma. Radiat Oncol. (2022) 17(1):58. doi: 10.1186/s13014-022-02013-6
20. Childs WJ, Goldstraw P, Nicholls JE, Dearnaley DP, Horwich A. Primary Malignant mediastinal germ cell tumours: improved prognosis with platinum-based chemotherapy and surgery. Br J Cancer. (1993) 67:1098–101. doi: 10.1038/bjc.1993.201
21. Koizumi T, Kanda S, Nihonmatu R, Gomi D, Sekiguchi N, Noguchi T, et al. Primary mediastinal germ cell tumors - A retrospective analysis of >30 years of experience in a single institution. Thorac Cancer. (2021) 12:807–13. doi: 10.1111/1759-7714.13859
22. Bokemeyer C, Droz JP, Horwich A, Gerl A, Fossa SD, Beyer J, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer. (2001) 91(7):1394–401. doi: 10.1002/(ISSN)1097-0142
23. Sigg S, Heidenreich A, Papachristofilou A, Fankhauser CD. How much chemotherapy is required to optimise long-term outcomes in clinical stage 2 seminoma? Eur Urol. (2023) 84(1):32–5. doi: 10.1016/j.eururo.2022.12.010
24. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU working group on male sexual and reproductive health. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. (2021) 80(5):603–20. doi: 10.1016/j.eururo.2021.08.014
25. Cioppi F, Rosta V, Krausz C. Genetics of Azoospermia. Int J Mol Sci. (2021) 22:3264. doi: 10.3390/ijms22063264
26. Jiang H, Zhang Y, Ma H, Fan S, Zhang H, Shi Q. Identification of pathogenic mutations from nonobstructive azoospermia patients. Biol Reprod. (2022) 107:85–94. doi: 10.1093/biolre/ioac089
27. Mazelli R, Rucci C, Vaiarelli A, Cimadomo D, Ubaldi FM, Foresta C, et al. Male factor infertility and assisted reproductive technologies: indications, minimum access criteria and outcomes. J Endocrinol Invest. (2023) 46(6):1079–85. doi: 10.1007/s40618-022-02000-4
28. Esteves SC, Achermann APP, Simoni M, Santi D, Casarini L. Male infertility and gonadotropin treatment: What can we learn from real-world data? Best Pract Res Clin Obstet Gynaecol. (2023) 86:102310. doi: 10.1016/j.bpobgyn.2022.102310
29. Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. (2013) 100:681–5. doi: 10.1016/j.fertnstert.2013.05.022
30. Glazer CH, Eisenberg ML, Tøttenborg SS, Giwercman A, Flachs EM, Bräuner EV, et al. Male factor infertility and risk of death: a nationwide record-linkage study. Hum Reprod. (2019) 34(11):2266–73. doi: 10.1093/humrep/dez189
31. Yumura Y, Takeshima T, Komeya M, Karibe J, Kuroda S, Saito T. Long-term fertility function sequelae in young male cancer survivors. World J Mens Health. (2023) 41(2):255–71. doi: 10.5534/wjmh.220102
32. Heidenreich A, Srivastava S, Moul JW, Hofmann R. Molecular genetic parameters in pathogenesis and prognosis of testicular germ cell tumors. Eur Urol. (2000) 37:121–35. doi: 10.1159/000020128
33. de Vries G, Rosas-Plaza X, van Vugt MATM, Gietema JA, de Jong S. Testicular cancer: Determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat Rev. (2020) 88:102054. doi: 10.1016/j.ctrv.2020.102054
Keywords: mediastinum, seminoma, azoospermia, germ cell tumor, mediastinal tumor
Citation: Li Z, Zhu Q, Niu S, Xiao K, Xiao Z and Yang P (2024) Primary mediastinal seminoma with azoospermia: case report and review of the literature. Front. Oncol. 14:1309803. doi: 10.3389/fonc.2024.1309803
Received: 08 October 2023; Accepted: 03 May 2024;
Published: 17 May 2024.
Edited by:
Anand Sharma, Mount Vernon Cancer Centre, United KingdomReviewed by:
Susanna Dolci, University of Rome Tor Vergata, ItalyCopyright © 2024 Li, Zhu, Niu, Xiao, Xiao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pang Yang, cHlhbmczOTJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.