
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 29 February 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1308492
Immune checkpoint blockade (ICB) has indeed transformed the outlook for many advanced-stage solid tumors, yet its effectiveness in hematological malignancies has been particularly limited, with success predominantly demonstrated in classical Hodgkin lymphoma (cHL) and immune-privilege subtypes of non-Hodgkin lymphoma (NHL). In this report, we present an impactful case of a 71-year-old man grappling with refractory follicular lymphoma (rFL) that had progressed to a high-grade lymphoma, leaving no conventional treatment options on the table. Notably, the histological examination of the tumor tissue revealed a markedly elevated PD-L1 expression, illuminating the potential for immunotherapy to be effective. Additionally, comprehensive gene sequencing unveiled a moderate tumor mutational burden (TMB), deepening our understanding of the tumor’s molecular intricacies. As his health declined with no access to cell therapies or clinical trials at that time, a combination treatment of PD-1 ICB and an anti-CD20 drug surprisingly led to a significant improvement in his condition and long-term remission. While PD-1 ICB therapy has historically shown limited responses in non-Hodgkin lymphomas (NHLs), this case serves as a beacon of optimism, underscoring the promise of combining immunotherapy modalities and the potential of comprehensive molecular assessments in charting innovative treatments for extensively treated NHL patients. The quest for predictive biomarkers to gauge treatment response remains a formidable challenge. This report serves as a testament to the ever-evolving landscape of cancer treatment, where precision medicine and immunotherapy continue to unlock new possibilities for those confronting the most challenging malignancies.
Follicular lymphoma (FL) constitutes a significant proportion of non-Hodgkin lymphoma (NHL) cases, primarily affecting older individuals. Despite the availability of diverse treatment modalities, FL is often considered incurable, with relapses posing a significant challenge, especially in frail patients. While immune checkpoint blockade (ICB) has revolutionized solid tumor treatment, its effectiveness in NHL, including FL, remains uncertain due to the lack of reliable predictive biomarkers.
A retired 71-year-old man, previously diagnosed with chronic kidney disease (CKD), received an incidental diagnosis of grade 1 stage IVA follicular lymphoma (FL) an astounding 16 years ago. At the time of this diagnosis, his FLIPI score was 3/5, meeting criteria based on age, stage, and involvement of more than four nodal areas (refer to Figures 1, 2 for the initial radiological and pathological assessments). Notably, the GELF criteria were not initially met (GELF score, 0/7), prompting a period of close observation without any treatment, which extended for nearly 3 years.
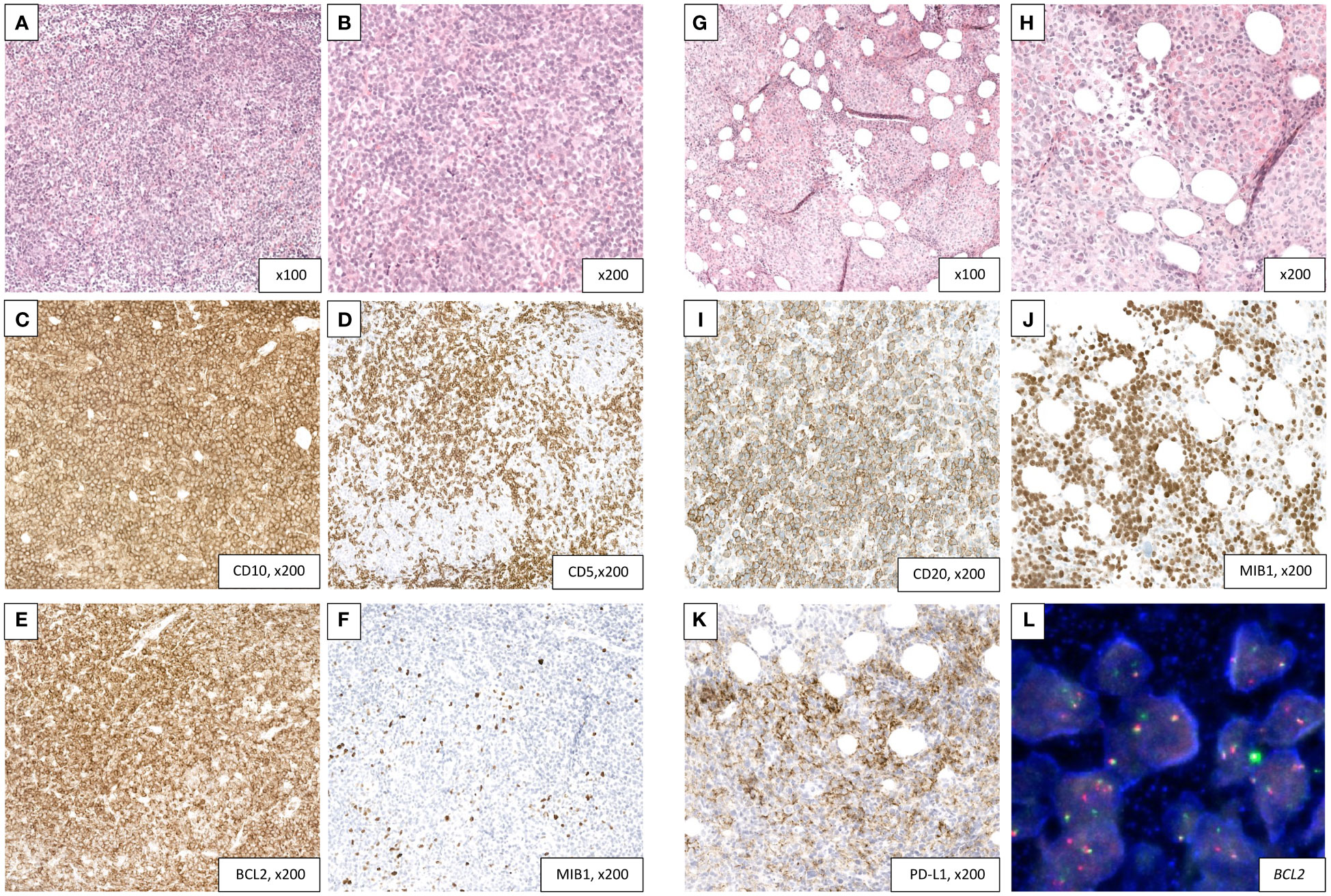
Figure 1 Transformation of a follicular lymphoma (A–F) into a DLBCL with high PD-L1 expression (G–L). (A–F) Follicular lymphoma diagnosed on a retroperitoneal lymph node biopsy. H&E sections (A, B) showing a vaguely nodular lymphoid proliferation composed mainly of centrocytes with rare centroblasts (grade I). The nodular architecture is better highlighted in immunohistochemistry with a T-cell marker (CD5, D). The tumor cells are diffusely reactive to CD10 (C) and Bcl2 (E) with a very low proliferation index (5%, F). (G–L) Transformation into a DLBCL with PD-L1 expression in a bone marrow core biopsy. H&E sections (G, H) showing a replacement of the hematopoietic lineages by a diffuse lymphoid proliferation consisting of large-size and atypical cells. The tumor cells retain a CD20 positivity (I) with half of them showing a PD-L1 expression (K). Proliferation index is high (70%, J). FISH analysis confirms a BCL2 rearrangement (L). BCL-2, B-cell lymphoma 2; DLBCL, diffuse large B-cell lymphoma; FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin; PD-L1, programmed death-ligand 1.
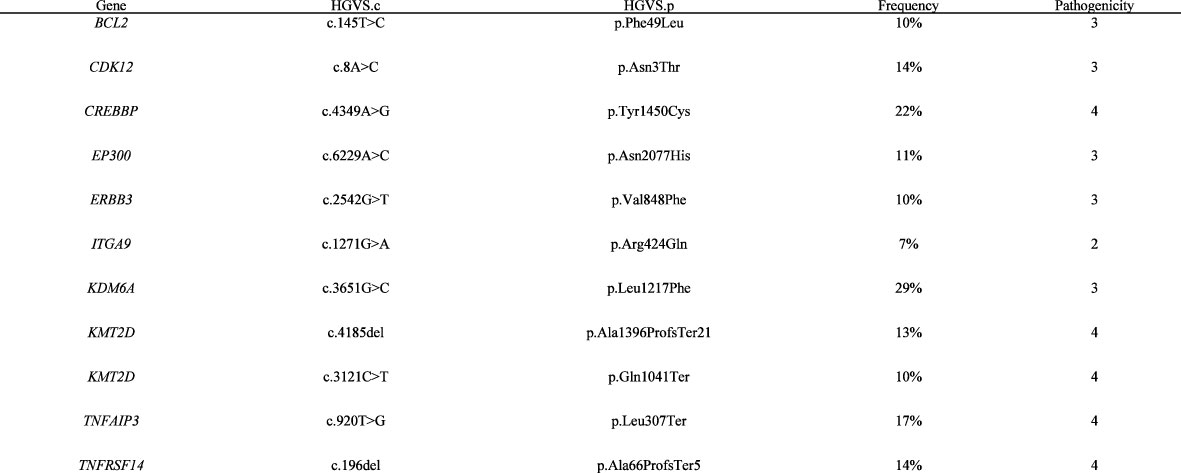
Figure 2 List of somatic mutations retained with a 400-gene NGS panel in the bone marrow sample. Bone marrow sample of patient was sequenced in 2020. The panel used for the sequencing was the NGS400v2, the custom capture panel based on Agilent SureSelect XT HS technology and covering all exons of 420 genes and the TERT promoter region. Sequencing was done on an Illumina NextSeq 500 System. The data were analyzed using HUG internal software (Soap 2.1.1)—Oncobench v4. with hg19 as a reference. The variant calling software detected 26 somatic mutations. The germline variants were detected by sequencing of blood or non-tumor tissue samples. Of the 26 somatic variants detected, only 16 had a potential impact on the protein, and of these 16 variants, two had an allelic frequency below 5% and were filtered out. Pathogenicity was assessed for the remaining 14 variants presented. The tumor mutation burden was 6.75 mut/Mb. Reference—ENSEMBL transcript ID, pathogenicity is classified as follows: 1, benign/2, probably benign/3, uncertain/4, probably pathogenic/5, pathogenic. HGVS, Human Genome Variation Society; HGVS.c, DNA variant, HGVS.p, protein variant; HUG, Hôpitaux Universitaires de Genève; VAF, variant allele frequency.
Subsequently, his medical journey took a more intense trajectory as he underwent a series of treatments in response to the disease’s progression. These treatments included R-CHOP (rituximab, cyclophosphamide, hydroxyadriamycin, vincristine, and prednisone) with rituximab maintenance, R-ICE (rituximab, ifosfamide, carboplatin, and etoposide), rituximab-bendamustine, pelvic VMAT radiotherapy, and idelalisib. Over this extended period, the patient encountered multiple infectious complications and ultimately reached end-stage CKD, necessitating hemodialysis.
While on idelalisib, the patient’s health took a dramatic downturn, resulting in severe illness characterized by febrile grade IV pancytopenia and significant dyspnea. A PET-CT scan revealed intensely diffuse osteo-medullary hypermetabolic activity, with a mixed response in previously identified areas of lymphadenopathy. A subsequent bone marrow biopsy confirmed the diagnosis of high-grade transformation from the prior FL to diffuse large B-cell lymphoma. This transformation was characterized by a diffuse proliferation of large-sized lymphoid cells, with CD20 positivity.
Despite a poor performance status (ECOG 4) and significant comorbidities (Cumulative Illness Rating Scale (CIRS) score of 14), the patient exhibited strong determination to explore a new palliative therapeutic option. Remarkably, the histological examination of his transformed FL revealed a pronounced and widespread expression of PD-L1 by the tumor cells (TPS > 50) (PD-L1 (E1L3N) XP Rabbit mAb, Cell Signaling) (Figure 1). Complementary molecular analysis using a 400-gene next-generation sequencing (NGS) panel (Supplementary Figure S1) unveiled classical mutations commonly associated with transformed FL, including CREBBP, KMT2D, BCL2, EP300, and TNFSRF14 genes; however, these mutations were not actionable. Notably, large deletions or insertions, particularly those described in genes such as KIT, CTNNB1, TP53, CDKN2A, PTEN, and FLT3, were not detected. Furthermore, the analysis revealed a moderate tumor mutational burden (TMB) of 6.75 mutations per megabase (mut/Mb) (Figure 2). Furthermore, we conducted Oncoscan Affymetrix analysis, which unveiled gains in 17q, 18q, and 18p, along with loss of heterozygosity (LOH) in Xq and Xp. Interestingly, none of these identified anomalies exhibited a direct correlation with the expression of PD1/PD-L1.
Based on these findings and informed by published results from a small phase 2 study in Richter syndrome (1), the patient received one cycle of rituximab (375 mg/m2) and cyclophosphamide (1,000 mg/m2), followed by pembrolizumab 200 mg and rituximab, administered every 3 weeks. Astonishingly, the patient’s overall condition improved dramatically, culminating in a complete metabolic response at the 3-month mark (Figure 3).
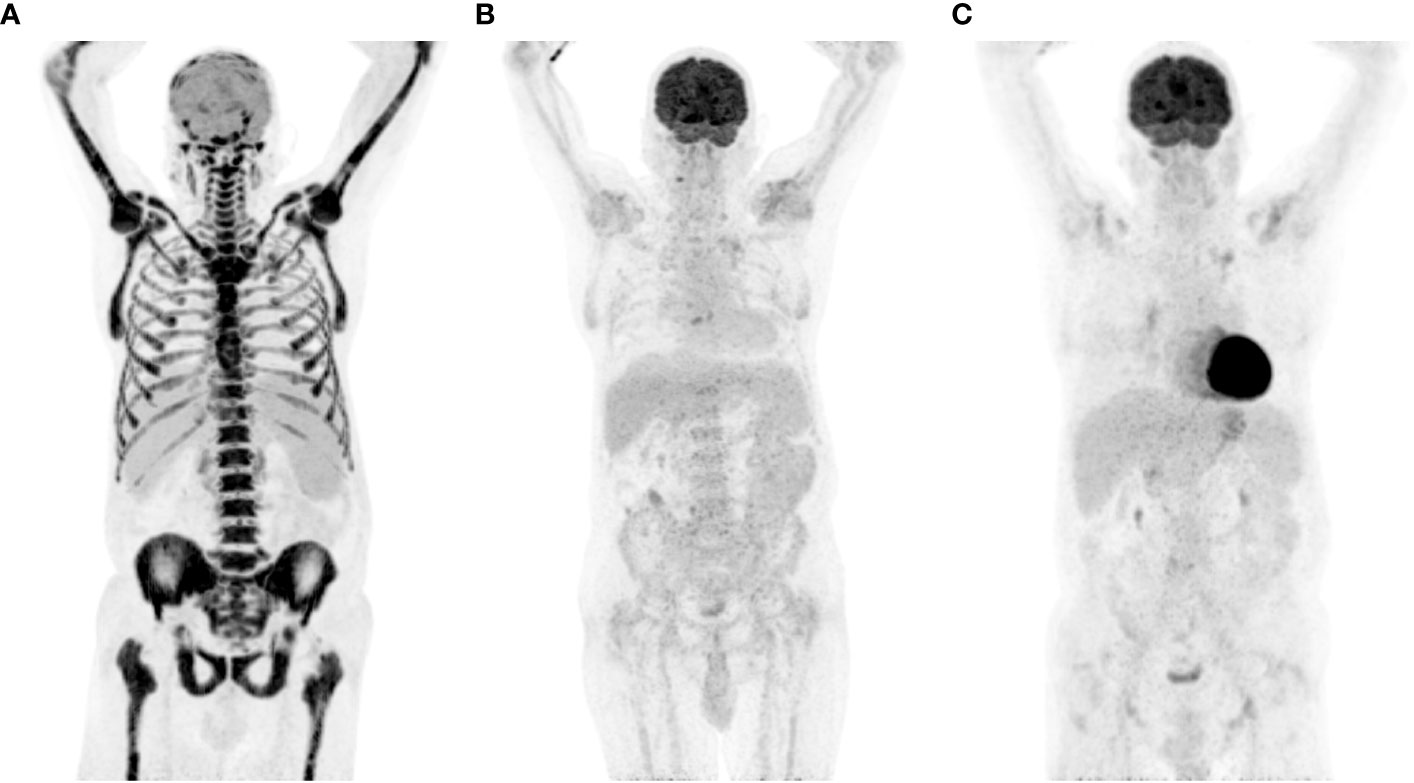
Figure 3 PET-CT response assessment of transformed FL on PD-1 ICB. (A) Intense bone marrow diffuse metabolism and mild lymph nodes, at diagnosis of transformation; (B) 3-month response assessment; (C) 9-month response assessment. FL, follicular lymphoma; ICB, immune checkpoint blockade; PD-1, programmed death-1; PET-CT, positron emission tomography and computed tomography; SUV max, maximum standardized uptake value.
In total, the patient received 24 months of pembrolizumab therapy and maintained a sustained clinical and metabolic remission. Regrettably, despite being in remission, the patient tragically succumbed to septic shock resulting from colitis with paralytic ileus, 3 years after the aggressive transformation from FL.
Despite promising responses to therapies like anti-CD20 immunotherapy and targeted agents, FL often relapses, especially when eligible cell therapies are unavailable (2). Additionally, FL can transform into a more aggressive lymphoma at an annual rate of 1.5%–2% (3). In our patient’s samples, we detected gene variants crucial for histone modification, including CREBBP, EP300, and KMT2D. These genes are commonly mutated in follicular lymphoma (FL) patients, with KMT2D being the most frequently altered (70%–80%), followed by CREBBP (70%) and EP300 (15%) (4, 5). CREBBP alterations reduce its acetyltransferase activity, affecting histone acetylation, including key proteins like TP53 and BCL6. EP300 encodes a histone acetyltransferase regulating gene expression (4). KMT2D mutations often lead to a loss of function and active transcription marks (5). Approximately 30% of FL patients exhibit genomic changes in genes related to the BCR/NF-κB signaling pathway, such as TNFAIP3 (4). TNFRSF14 gene disruptions are also common, modifying the microenvironment and promoting B-cell expansion in FL (6). Recently, Gao et al. notably reported an association between KMT2D, CREBBP, and TNFRSF14 variants and POD24 in follicular lymphoma (FL). However, to our knowledge, no correlation with transformation has been demonstrated (7).
PD-L1 expression serves as a potential prognostic indicator for immune checkpoint inhibitor (ICI) response (8). Tumor cells use PD-L1 to evade the immune system, influenced by interferon-γ from T cells and NK cells. This variable PD-L1 expression across tumors makes it valuable for assessing an inflamed microenvironment. Although PD-L1 is part of many clinical trials and associated with over 80% of FDA immunotherapy approvals, it predicted responses in only 28.9% of cases, with others lacking predictability or not conducting testing (8). Intriguingly, even in cases with absent PD-L1 expression, notable responses to ICI have occurred. PD-L1 expression, seen at varying levels and assessed using diverse techniques, remains essential in solid malignancies.
PD-1/PD-L1 immune checkpoint blockade (ICB) has been scrutinized in the context of follicular lymphoma (FL), with a distinct study by Armand et al. reporting a notably lower overall response rate (ORR) of 4% (9, 10). In exploring combination approaches, the incorporation of PD-1/PD-L1 ICB with anti-CD20 monoclonal antibodies, whether administered as a single agent or in combination with other therapeutic agents, has demonstrated only modest response rates in relapsed/refractory FL (11–17). Overall, the efficacy and safety of PD-1/PD-L1 ICB in indolent lymphomas, particularly FL, exhibit varying outcomes, emphasizing the need for further research and identification of predictive biomarkers. Similarly, single-agent activity of PD-1/PD-L1 immune checkpoint blockade (ICB) has yielded only modest response rates in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) (18–20). Moreover, PD-1/PD-L1 ICB does not appear to confer substantial benefits when combined with frontline immunochemotherapy in newly diagnosed DLBCL (13, 14, 21) or grade 3b follicular lymphoma (FL) (22). For a comprehensive overview of the efficacy of PD-1/PD-L1 ICB in lymphomas, we recommend referring to our recent review in the field (10).
The correlation between tumor mutational burden (TMB) and immunotherapy response exists but lacks an ideal threshold (23). FDA approval requires a minimum of 10 mutations per megabase (mut/Mb) for pembrolizumab, while FoundationOne designates TMB over 20 mut/Mb as high (24). TMB links to neoantigen abundance recognized through MHC presentation by the immune system (24, 25). However, a TMB exceeding 20 mut/Mb results in only a 45% response rate (6). Factors include immunogenic neoantigen generation, immunoselection favoring antigenic mutant proteins, and the tumor microenvironment affecting T-cell infiltration and activation. TMB’s relation to PD-1/PD-L1 ICB response in hematological malignancies is evolving (10, 26). The median TMB in hematological malignancies is approximately 1.7 mut/Mb, with lymphoma TMB varying due to multiple factors (10, 26). In FL, Chalmers et al. reported a median TMB of 8.3 mut/Mb, with a maximum of 26.7. However, the confidence interval (CI) was notably wide (1.5–9.2), likely attributed to the relatively limited specimen size (n=107). Only 3% of these FL cases exhibited a TMB>20 (24). The same authors also noted a median TMB of 10 mut/Mb among 348 DLBCL samples (with a maximum of 251 mut/Mb), and 18% of cases had a TMB > 20 (24). The conventional TMB cutoffs have mainly been derived from comprehensive cohorts of solid tumors. However, we posit that these benchmarks may not precisely reflect the most appropriate thresholds for hematological diseases. We advocate for a customized, individualized approach for determining TMB thresholds tailored to each specific tumor type. This is particularly pertinent in our case, where we observe a moderate TMB of 6.75 mut/Mb.
Georgiou et al. emphasized that abnormalities within the PD-L1/PD-L2 locus, such as the fusion of PD-L1 with IGH, are detected in approximatively 20% of DLBCLs, with a notable prevalence in the non-germinal center B-cell subtype. They observed that specimens with cytogenetic modifications in the PD-L1/PD-L2 locus, especially those involving translocations or amplifications, exhibited a higher likelihood of manifesting overexpression of PD-L1 at both the mRNA and protein levels (27). However, in our case of DLBCL transformation from a preceding follicular lymphoma (FL), no copy number variations (CNVs) or translocations were detected, providing no apparent explanation for the remarkably positive response to PD-1 immunotherapy.
PD-1 serves as a surrogate marker for T-cell dysfunction, and the restoration of CD8+ T-cell function constitutes the antitumor mechanism of checkpoint blockers. In their study, Zhang et al. successfully elucidated additional mechanisms leading to PD-L1 upregulation in diffuse large B-cell lymphoma (DLBCL) (28). Their findings suggest that targeting the PD-1/PD-L1 immunosuppressive pathway in conjunction with CD73/A2aR inhibitors may offer added clinical benefits and partially overcome primary and secondary resistance to PD-1/PD-L1 blockade (28). In our specific case, the patient received cyclophosphamide shortly before pembrolizumab administration. It could be hypothesized that this distinctive infusion might have induced regulatory T-cell (Treg) depletion, potentially leading to a significant enhancement in antitumor immunity.
This case highlights the complex journey faced by individuals with transformed FL and the potential of immunotherapeutic approaches, even in challenging circumstances. It offers hope for the future of lymphoma treatment. The ongoing quest for reliable markers of response to PD1/PD-L1 immune checkpoint blockade remains a substantial challenge in hematological malignancy patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Cantonal Commission for Ethics in Research on Human Beings (CCER), Rue Adrien-Lachenal 8 1207 Genève Tél: +41 22 546 51 01 E-mail: Y2NlckBldGF0LmdlLmNo. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the patient was deceased, and previously signed the general institutional informed consent for reuse of his clinical data and material. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because the patient was deceased, and previously signed the general institutional informed consent for reuse of his clinical data and material.
NL: Supervision, Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. SP: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Open access funding by University of Geneva.
The authors thank Dr. Aimable Nahimana, Dr. Kathrin Vollmer, Dr. Ivana Racic, Prof. Thomas McKee, and Dr. Charles-Edouard Decorads.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1308492/full#supplementary-material
Supplementary Table 1 | PD-L1 scoring formulas. TPS tumor proportional score, IC tumor infiltrating immune cells, CPS combined positivity score.
Supplementary Figure 1 | Gene panel.
1. Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 129(26):3419–27. doi: 10.1182/blood-2017-02-765685
2. Brice P, Bastion Y, Lepage E, Brousse N, Haïoun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. JCO. 15(3):1110–7. doi: 10.1200/JCO.1997.15.3.1110
3. Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 32(3):298–308. doi: 10.1016/j.annonc.2020.11.008
4. Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 471(7337):189–95. doi: 10.1038/nature09730
5. Hübschmann D, Kleinheinz K, Wagener R, Bernhart SH, Lopez C, Toprak UM, et al. Mutational mechanisms shaping the coding and noncoding genome of germinal center derived B-cell lymphomas. Leukemia. 35(7):2002–16. doi: 10.1038/s41375-021-01251-z
6. Okosun J, Bödör C, Wang J, Araf S, Yang CY, Pan C, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 46(2):176–81. doi: 10.1038/ng.2856
7. Gao F, Liu H, Meng X, Liu J, Wang J, Yu J, et al. Integrative genomic and transcriptomic analysis reveals genetic alterations associated with the early progression of follicular lymphoma. Br J Haematol (202)(6), 1151–64. doi: 10.1111/bjh.18974
8. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J immunother Cancer. 7(1):278. doi: 10.1186/s40425-019-0768-9
9. Armand P, Janssens A, Gritti G, Radford J, Timmerman J, Pinto A, et al. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood. 137(5):637–45. doi: 10.1182/blood.2019004753
10. Perdikis-Prati S, Sheikh S, Bouroumeau A, Lang N. Efficacy of immune checkpoint blockade and biomarkers of response in lymphoma: A narrative review. Biomedicines. 11(6):1720. doi: 10.3390/biomedicines11061720
11. Barraclough A, Chong G, Gilbertson M, Grigg A, Churilov L, Fancourt T, et al. Immune priming with single-agent nivolumab followed by combined Nivolumab & Rituximab is safe and efficacious for first-line treatment of follicular lymphoma; interim analysis of the ‘1st FLOR’ Study. Blood. 134(Supplement_1):1523–3. doi: 10.1182/blood-2019-123908
12. Hawkes EA, Lee ST, Chong G, Gilbertson M, Grigg A, Churilov L, et al. Immune priming with nivolumab followed by nivolumab and rituximab in first-line treatment of follicular lymphoma: The phase 2 1st FLOR study. JCO. 39(15_suppl):7560–0. doi: 10.1200/JCO.2021.39.15_suppl.7560
13. Younes A, Burke MJ, Diefenbach CS, Ferrari S, Kahn C, Sharman JP, et al. Safety and efficacy of atezolizumab in combination with obinutuzumab and bendamustine in patients with previously untreated follicular lymphoma: an interim analysis. Blood (2017) 130(Supplement 1):481. doi: 10.1182/blood.V130.Suppl_1.481.481
14. Younes A, Burke JM, Cheson B, Diefenbach S, Ferrari S, Hahn U, et al. Safety and efficacy of Atezolizumab in combination with rituximab plus CHOP in previously untreated patients with diffuse large B-cell lymphoma (DLBCL): primary analysis of a phase I/II study. Blood. 132(Supplement 1):2969–9. doi: 10.1182/blood-2018-99-116678
15. Jain N, Basu S, Thompson PA, Ohanian M, Ferrajoli A, Pemmaraju N, et al. Nivolumab combined with ibrutinib for CLL and richter transformation: A phase II trial. Blood. 128(22):59–9. doi: 10.1182/blood.V128.22.59.59
16. Jain N, Ferrajoli A, Basu S, Thompson PA, Burger JA, Kadia TM, et al. A phase II trial of nivolumab combined with ibrutinib for patients with richter transformation. Blood. 132(Supplement 1):296–6. doi: 10.1182/blood-2018-99-120355
17. Herrera AF, Goy A, Mehta A, Ramchandren R, Pagel JM, Svoboda J, et al. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large B-cell lymphoma. Am J Hematol. 95(1):18–27. doi: 10.1002/ajh.25659
18. Lesokhin AM, Ansell SM, Armand P, Scott EM, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic Malignancy: preliminary results of a phase Ib study. JCO. 34(23):2698–704. doi: 10.1200/JCO.2015.65.9789
19. Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. JCO. 37(6):481–9. doi: 10.1200/JCO.18.00766
20. Frigault MJ, Armand P, Redd RA, Jeter E, Merryman RW, Coleman KC, et al. PD-1 blockade for diffuse large B-cell lymphoma after autologous stem cell transplantation. Blood Adv. 4(1):122–6. doi: 10.1182/bloodadvances.2019000784
21. Nowakowski GS, Willenbacher W, Greil R, Larsen TS, Patel K, Jäger U, et al. Safety and efficacy of durvalumab with R-CHOP or R2-CHOP in untreated, high-risk DLBCL: a phase 2, open-label trial. Int J Hematol. 115(2):222–32. doi: 10.1007/s12185-021-03241-4
22. Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. 189(6):1119–26. doi: 10.1111/bjh.16494
23. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 16 11:2598–608. doi: 10.1158/1535-7163.MCT-17-0386
24. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9(1):34. doi: 10.1186/s13073-017-0424-2
25. Chabanon RM, Pedrero M, Lefebvre C, Marabelle A, Soria J-C, Postel-Vinay S. Mutational landscape and sensitivity to immune checkpoint blockers. Clin Cancer Res. 22(17):4309–21. doi: 10.1158/1078-0432.CCR-16-0903
26. Jeong A-R, Ball ED, Goodman AM. Predicting responses to checkpoint inhibitors in lymphoma: are we up to the standards of solid tumors? Clin Med Insights Oncol. 14:117955492097636. doi: 10.1177/1179554920976366
27. Georgiou K, Chen L, Berglund M, Ren W, Miranda NFCC, Lisboa S, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. 127(24):3026–34. doi: 10.1182/blood-2015-12-686550
Keywords: follicular lymphoma, aggressive transformation, NGS - next generation sequencing, PD-1 inhibitor, tumor mutational burden (TMB)
Citation: Bouroumeau A, Perdikis-Prati S and Lang N (2024) Case report: From sequence to solution: tailoring treatment for transformed follicular lymphoma (DLBCL) through next generation sequencing study. Front. Oncol. 14:1308492. doi: 10.3389/fonc.2024.1308492
Received: 06 October 2023; Accepted: 08 January 2024;
Published: 29 February 2024.
Edited by:
Christos Kosmas, Metaxa Hospital, GreeceReviewed by:
John Timmerman, University of California, Los Angeles, United StatesCopyright © 2024 Bouroumeau, Perdikis-Prati and Lang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noémie Lang, bm9lbWllLmxhbmdAaGN1Z2UuY2g=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.