- Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, China
Background: Oxidative stress is strongly associated with the development, recurrence metastasis, and treatment of gastric cancer. It is yet unknown, though, how systemic oxidative stress levels relate to the surgically treated gastric cancer patients’ clinical results. This research aims to investigate the prognostic effect of systemic oxidative stress score, also known as systematic oxidative stress score (SOS), on gastric cancer patients undergoing surgical treatment.
Methods: Development of the SOS Formula through Least Absolute Shrinkage and Selection Operator LASSO Cox Regression. By using optimal cut-off values, the 466 patients included in the study had been split into high SOS and low SOS groups. Utilizing Chi-square test and the Wilcoxon rank sum test, this research examined the relationship between SOS and clinical traits. With the aid of Kaplan-Meier and COX regression analysis, the prognosis of patients with gastric cancer was examined.
Results: SOS consisted of four oxidative stress-related laboratory indices. Univariate and multivariate COX regression analyses revealed that SOS, Age, CA724, Radical resection and TNM stage were crucial prognostic factors for OS, and the independent prognostic factors for PFS included Age, CA724, TNM stage and SOS. They could have their prognosis correctly predicted using a nomogram built around SOS and independent prognostic variables.
Conclusion: SOS is a practical and reasonably priced tool for determining a patient’s prognosis for gastric cancer. More notably, SOS is an accurate prognostic factor for patients with advanced gastric cancer who has undergone radical surgery.
Introduction
The highest incidence of gastric cancer in the world is still found in East Asia, despite the fact that the mortality rate has been decreasing due to the development of a variety of cancer treatment modalities and the growing popularity of screening for Helicobacter pylori, a major risk factor for gastric cancer (1–4). Although the survival rates for gastric cancer in China have significantly increased since, 2000, the disease still poses a serious threat to the country’s public health (5).
The clinical results of patients with late gastric cancer and distant metastases are still a matter for concern, despite the fact that the adoption of immunotherapy and targeted therapy has improved patients’ survival (6–8). Although stage and severity of patients’ disease do have an impact on survival time, and the prognosis of gastric cancer, nutrition and inflammatory status also have a substantial effect on patients’ prognosis and course of therapy (9, 10).
For instance, cancer patients frequently experience cachexia and weight loss. Cachexia is a condition that causes individuals to lose weight while also losing skeletal muscle and adipose tissue (11). It is caused by a combination of enhanced wasting capacity, abnormally high catabolism, and inflammation. The patient’s life treatment, follow-up care, and length of survival are impacted by this (12). Chronic inflammation is a significant contributor to tumor growth and has a beneficial effect on the tumor micro-environment by encouraging the formation of tumor blood vessels and lymphatic vessels as well as boosting metastasis and tumor dissemination (13). The chronic inflammation mentioned above and cachexia are both closely related to oxidative stress. Studies have shown that oxidative stress can encourage protein catabolism by activating the nuclear factor-kb (NFkB) molecular mechanism and initiating the ubiquitin-proteasome pathway in skeletal muscle, both of which reduce the amount of protein in the muscle (14, 15). Patients with cachexia cancer experience increased oxidative stress due to decreased intake of nutrients (including antioxidants), altered metabolism impairing the production of reducing compounds, and increased ROS brought on by chronic inflammation’s overproduction of pro-inflammatory cytokines (16). In conclusion, oxidative stress influences patient prognosis and is a factor in cachexia development. The transcription factors NF-B, AP-1, p53, HIF-1, PPAR-, -linked protein/Wnt, and Nrf2 are also activated by persistent oxidative stress, which is the root cause of chronic inflammation. The expression of several inflammatory genes, such as IL-1, IL-6, and IL-8, which are produced by pro-inflammatory cells, is therefore increased as a result of these transcription factors (17). Oxidative stress is a significant factor in cancer.
Greater emphasis should be paid to the oxidative stress status of gastric cancer patients. From the perspective of the epidemiology causing gastric cancer, diet, smoking, and H. pylori infection can all lead to an imbalance in oxidative stress status (18, 19). Also, in terms of gastric cancer treatment, increased levels of oxidative stress might hasten the development of drug resistance to particular treatments in patients with gastric cancer (20). One example of this is the part that oxidative stress plays in the mechanism of oxaliplatin resistance (21–23). Consequently, treatment with medicines like cisplatin might increase oxidative stress levels in patients (24). What’s more to that, surgery, the most common treatment option for individuals with gastric cancer, generates a substantial number of oxygen and nitrogen radicals. The preoperative SOS in surgically treated patients was the focus of this study because of its prognostic potential.
Methods
Research population
This study retrospectively included surgically treated 466 patients with gastric cancer, 413 of whom underwent radical gastrectomy for gastric cancer. They all underwent surgery in, 2016-2022 at the Cancer Hospital of Harbin Medical University. Their detailed clinical information can be accessed through an electronic case system which archive patients’ clinical characteristics, blood biochemical indicators, tumor markers, and pathological staging.
Patients eligible for inclusion in the study were those whose pathology supported a diagnosis of gastric cancer, those who underwent surgical treatment and those without severe cardiovascular or psychiatric disease. Patients without clinical data or unable to undergo surgical treatment were excluded. The standards of the Declaration of Helsinki and its subsequrevisions were adhered to in this study, which was reviewed by the Institutional Review Board (IRB) and approved by the Harbin Medical University Oncology Affiliation. (Ethics number: 2019-57-IIT).
Follow-up
PFS and OS of the study subjects were obtained by telephone follow-up after data collection. The follow-up included: laboratory tests (routine blood, liver and kidney function, tumor markers), imaging (computed tomography, supraclavicular ultrasound); follow-up period: stage I: every 12 months; stage II: 6 months; stage III: 3 months; stage IV, recurrence: anytime. The term “progression-free survival” (PFS) refers to the period of time between the date of surgery and the date of disease progression, metastasis, or death, or the date of the final follow-up. The time from the date of operation until decease or the final check-up was known as overall survival (OS).
Statistical methods
Receiver operating characteristic (ROC) curves were used for the optimal cut-off values of SOS and its related laboratory indicators. The Wilcoxon rank sum test was used to compare variances between two groups for continuous variables that had a positive-terrestrial distribution. Continuous variables that did not have this distribution were marked as median and interquartile spacing. The chi-square test was applied to compare two groups of qualitative data, which were expressed as the percentage of cases (%). The Kaplan-Meier method along with the Log-rank test were conducted to calculate the survival curves for OS and PFS. With the use of Cox regression analysis, both univariate and multivariate, independent prognostic variables of patients were found. In multivariate Cox regression analysis, univariate Cox regression analysis P<0.05 was taken into account, and relative risk was assessed using HR and 95% confidence intervals. SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and R 4.2.3 (Vienna, Austria) were applied to build all statistical analyses, with P<0.05 considered a meaningful variance between the two groups.
Result
Creation of SOS
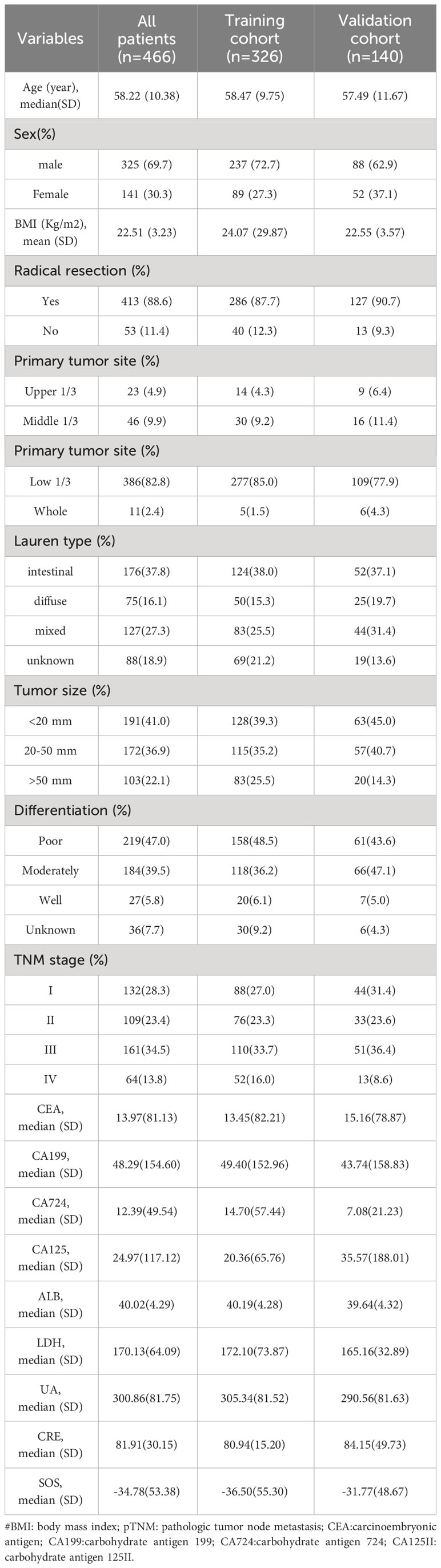
A total of 466 patients, with a mean age of 58.22 years, was enrolled in this investigation, of which 88.6% underwent radical surgery. The baseline demographic and clinical features of the patients, segregated into training (n = 326) and validation cohorts (n = 140), are detailed in Table 1. To assess the prognostic implications of systemic oxidative stress indices, these indices were dichotomized using critical values determined via ROC analysis. Kaplan-Meier survival analysis was conducted for oxidative stress-related biochemical indices, with those exhibiting P<0.05 then subjected to LASSO Cox regression analysis. Consequently, a Systemic Oxidative Stress (SOS) score was derived from variables with non-zero coefficients, where low albumin (ALB) and uric acid (UA) levels correlated with poorer OS and PFS, while high lactate dehydrogenase (LDH) and creatinine (CRE) levels were associated with adverse OS and PFS outcomes (all p<0.05) (Figures 1, 2). The SOS formula was established as SOS = 0.554 * LDH + 0.404 * CRE - 0.493 * ALB - 0.474 * UA, and its optimal cut-off value determined by ROC was -9.21 (Figure 3).
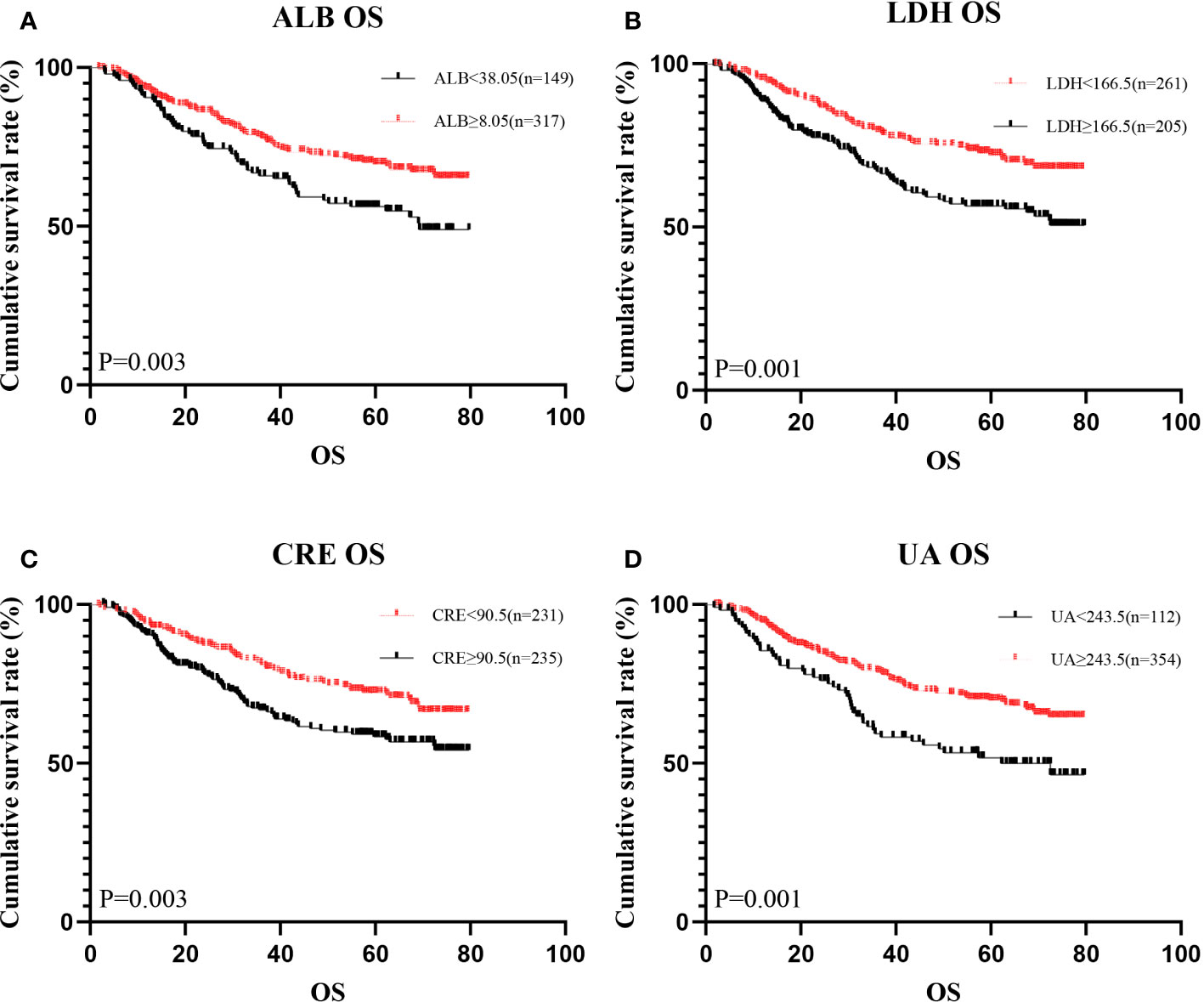
Figure 1 Kaplan-Meier analysis showing survival curve for OS of oxidative stress biomarker. #Kaplan–Meier curves for overall survival (OS), stratified by (A) ALB, (B) LDH, (C) CRE, (D) UA.
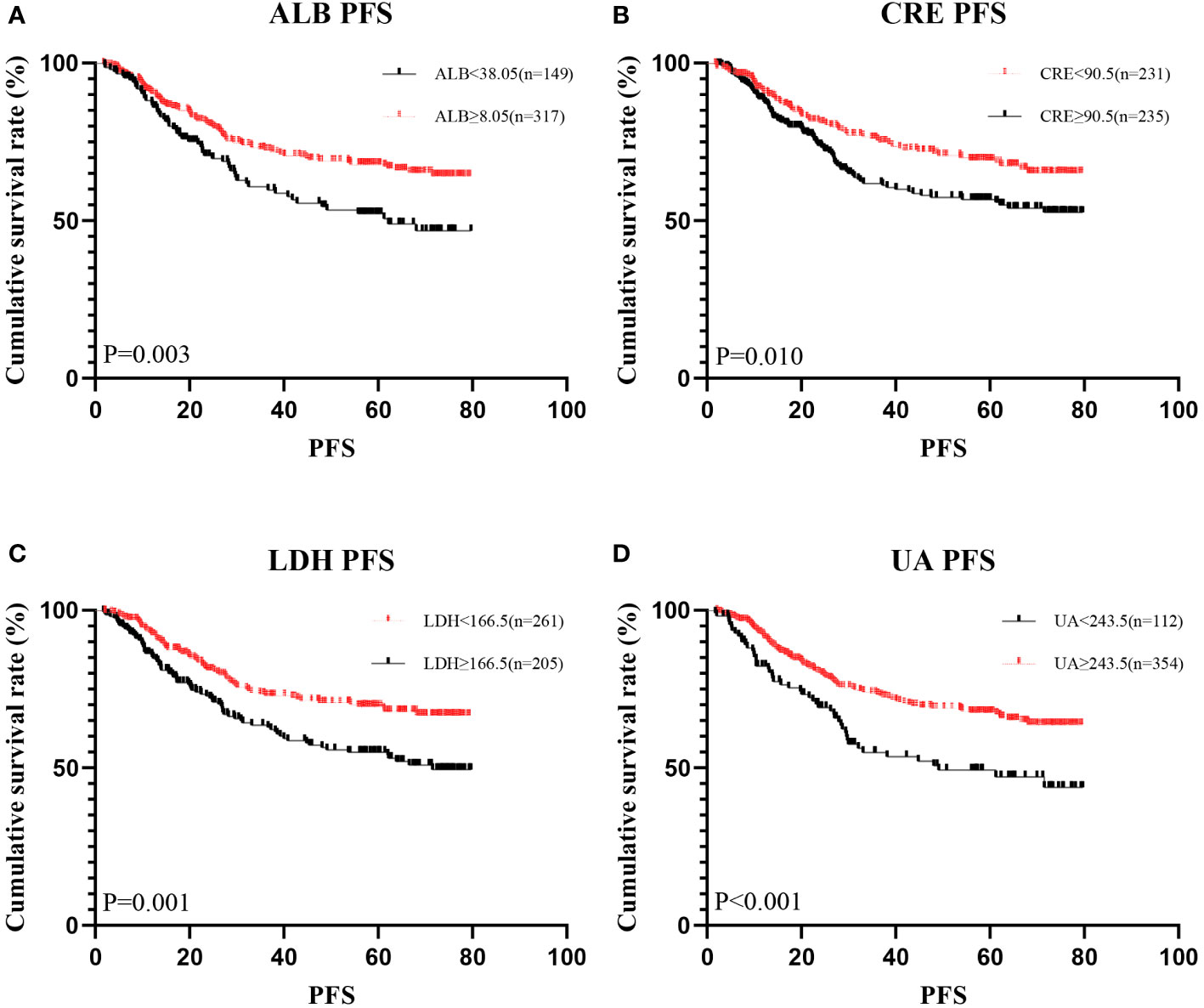
Figure 2 Kaplan-Meier analysis showing survival curve for PFS of oxidative stress biomarker. # Kaplan–Meier curves for progression-free survival (PFS), stratified by (A) ALB, (B) LDH, (C) CRE, (D) UA.
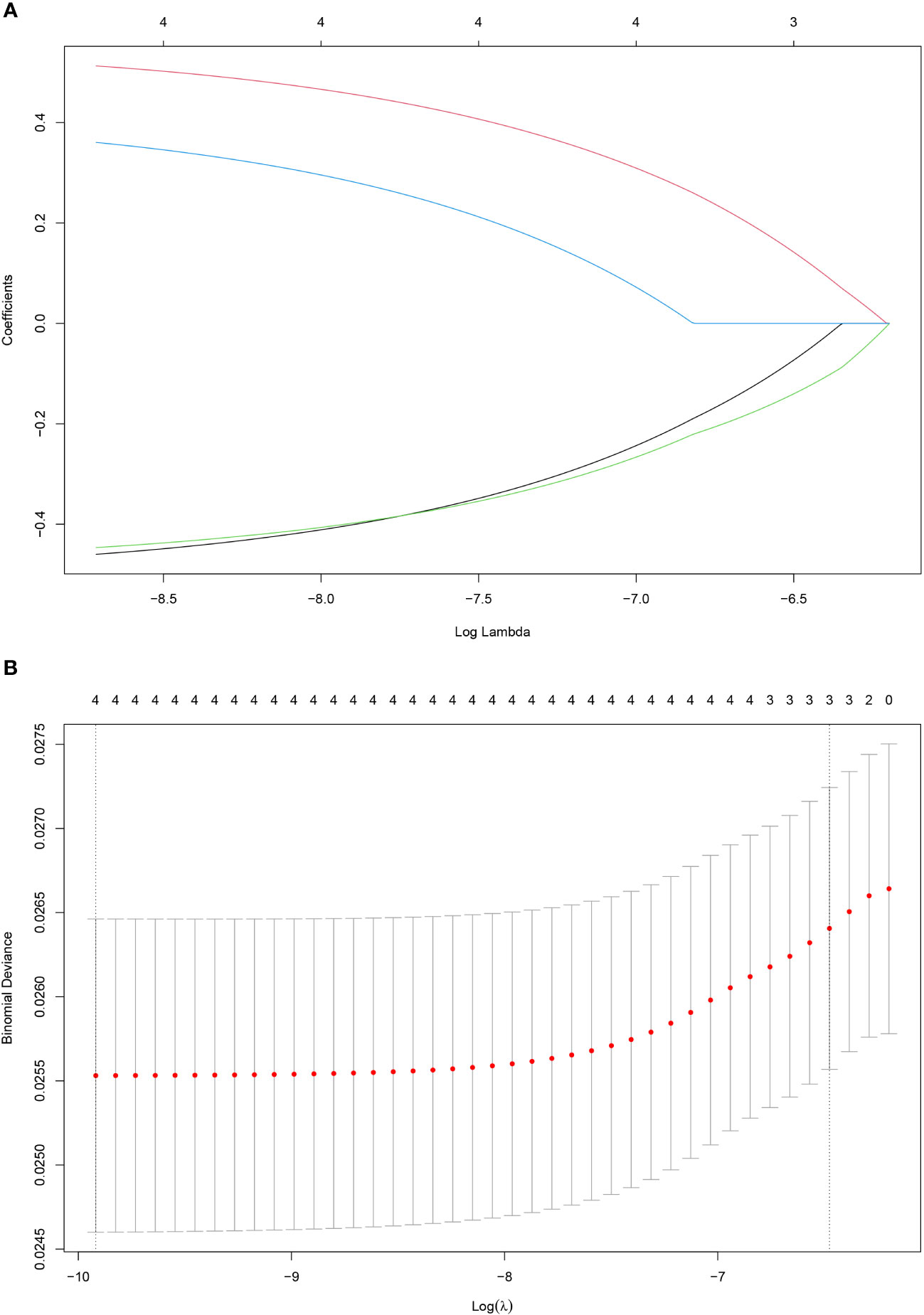
Figure 3 Construction of the SOS using the LASSO Cox regression model. Bias difference of LASSO coefficient profiles (A). Least Absolute Shrinkage and Selection Operator (LASSO) coefficient profiles (B) for 4 oxidative stress-related biomarkers.
The association between SOS and clinical traits and laboratory data
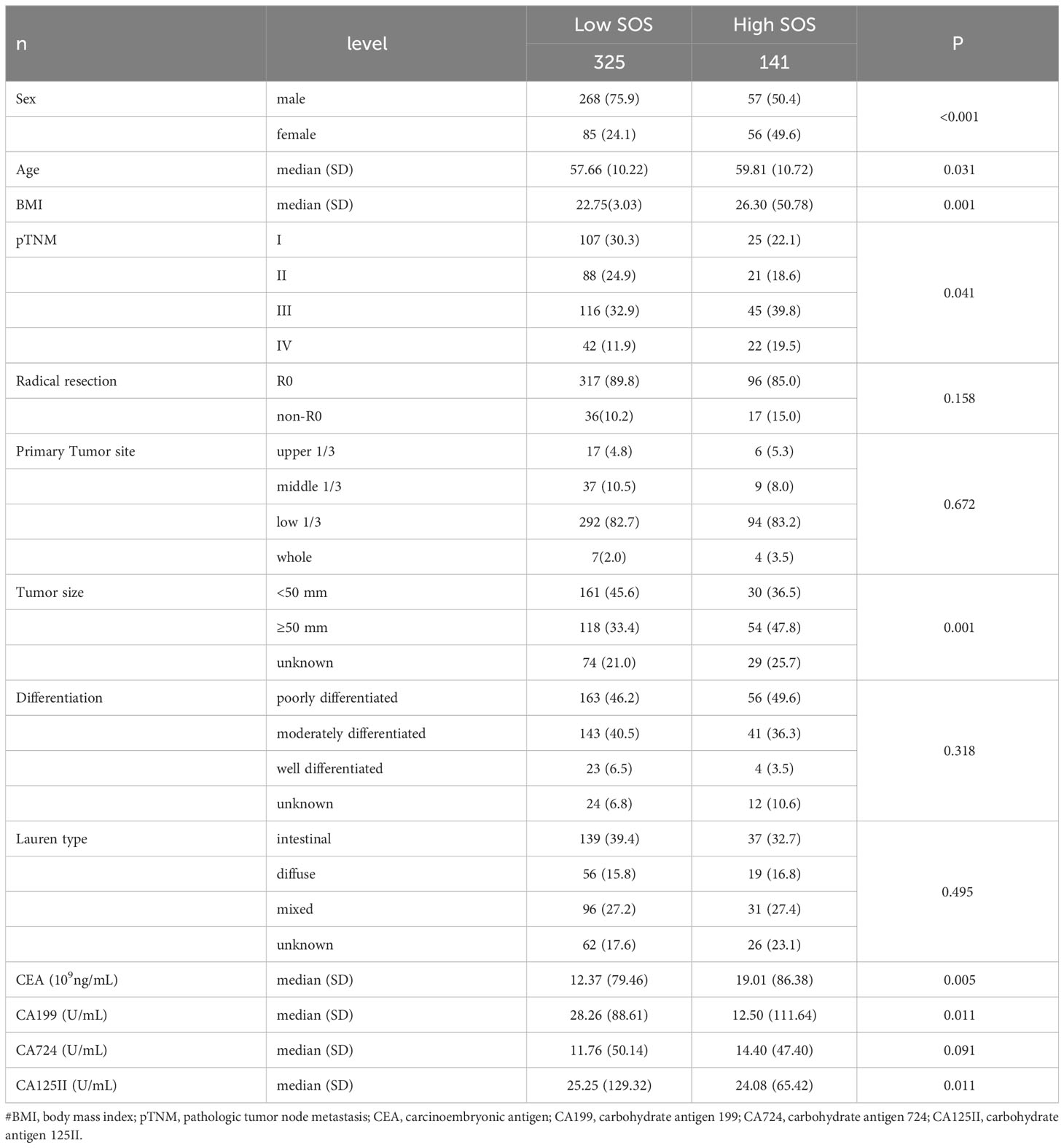
The cohort was stratified based on Systemic Oxidative Stress (SOS) cutoff values, resulting in 141 patients in the high SOS level group and 325 patients in the low SOS level group. The association between SOS levels and various clinical features and tumor markers is elucidated in Table 2. Noteworthy differences were observed in clinical characteristics, including Age, Sex, Body Mass Index (BMI), pathological TNM (pTNM) staging, and tumor size across distinct SOS groups. Regarding tumor markers, patients exhibiting high SOS levels displayed a tendency toward elevated Carcinoembryonic Antigen (CEA) levels, alongside reduced levels of Cancer Antigen 199 (CA199) and Cancer Antigen 125 (CA125II) (all p<0.05).
Univariate and multivariate Cox regression analyses
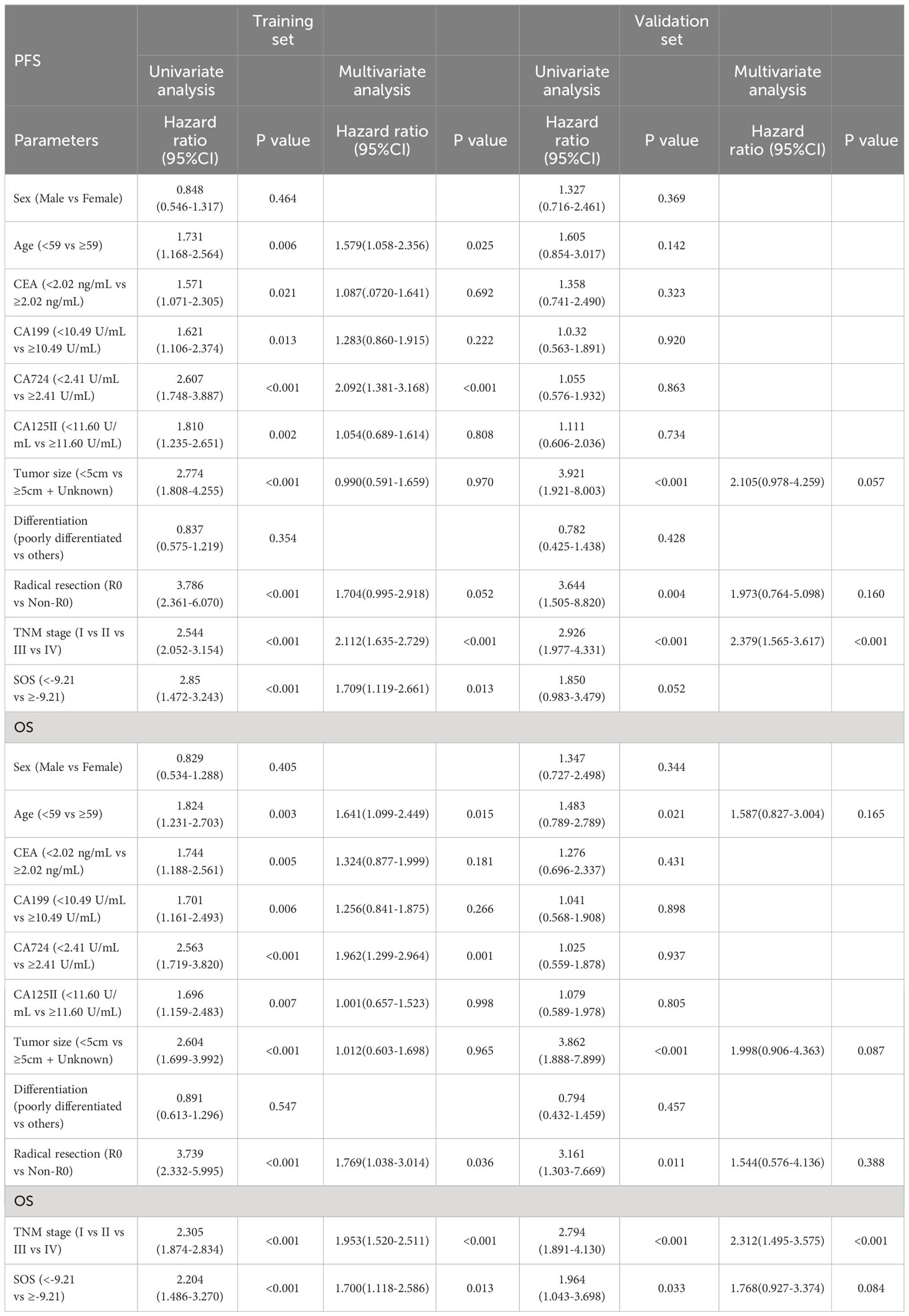
Univariate and multivariate Cox regression models were utilized to analyze the establishment of independent prognostic factors in these patients. The findings demonstrated that SOS was an independent prognostic factor for these patients in training cohorts. Among them, Age (HR = 1.641, P = 0.015), CA724 (HR = 1.962, P = 0.001), Radical resection (HR = 1.769, P = 0.036) and TNM stage (HR = 1.953, P < 0.001) were also independent prognostic factors for OS, and the independent prognostic factors for PFS included Age (HR = 1.578, P = 0.025), CA724 (HR = 2.092, P < 0.001) and TNM stage (HR = 2.112, P < 0.001) as well in Table 3.
Survival analysis
The prognostic significance of SOS was systematically investigated in gastric cancer patients undergoing surgical treatment, with distinct analyses conducted for both the training and validation cohorts. Within the training cohorts, lower SOS levels were notably associated with enhanced OS (median, not reached vs 43.40 months, P < 0.001) and PFS (median, not reached vs 32.07 months, P < 0.001) than high SOS levels (Figures 4A, B). Similarly, in the validation cohorts, lower SOS levels were linked to improved OS (median, not reached vs 62.70 months, P = 0.033) than high SOS levels, albeit without statistically significant predictive value for PFS (median, not reached vs 61.23 months, P = 0.052); nevertheless, the median survival in the low SOS group surpassed that in the high SOS group (Figures 4C, D).

Figure 4 Kaplan-Meier analysis for the PFS and OS of SOS. #Kaplan–Meier curves of OS (A) and PFS (B) for patients in the low and high groups according to the SOS in the training cohorts. Kaplan–Meier curves of OS (C) and PFS (D) for patients in the low and high groups according to the SOS in the validation cohorts. Kaplan-Meier analysis showing survival curve for OS (E) and PFS (F) of patients in R0 subgroup. Kaplan-Meier analysis showing survival curve for OS (G) and PFS (H) of patients in Non-R0 subgroup.
In-depth subgroup analyses were performed to discern the impact of SOS within the context of surgical resection outcomes, distinguishing between R0 (complete resection) and Non-R0 (incomplete resection) subgroups. Intriguingly, within the R0 subgroup, SOS exhibited a robust predictive effect (Figures 4E, F). However, in the Non-R0 subgroup, SOS failed to attain statistical significance in predicting clinical outcomes (Figures 4G, H).
Nomogram
Following the implementation of multivariate COX analysis to construct prognostic nomograms for both PFS and OS (Figure 5A and Figure 6A), the derived models exhibited robust predictive performance, as indicated by C-indexes of 0.780 and 0.799 for PFS and OS, respectively. To further assess the accuracy of these models in prognostication, additional analyses were conducted.
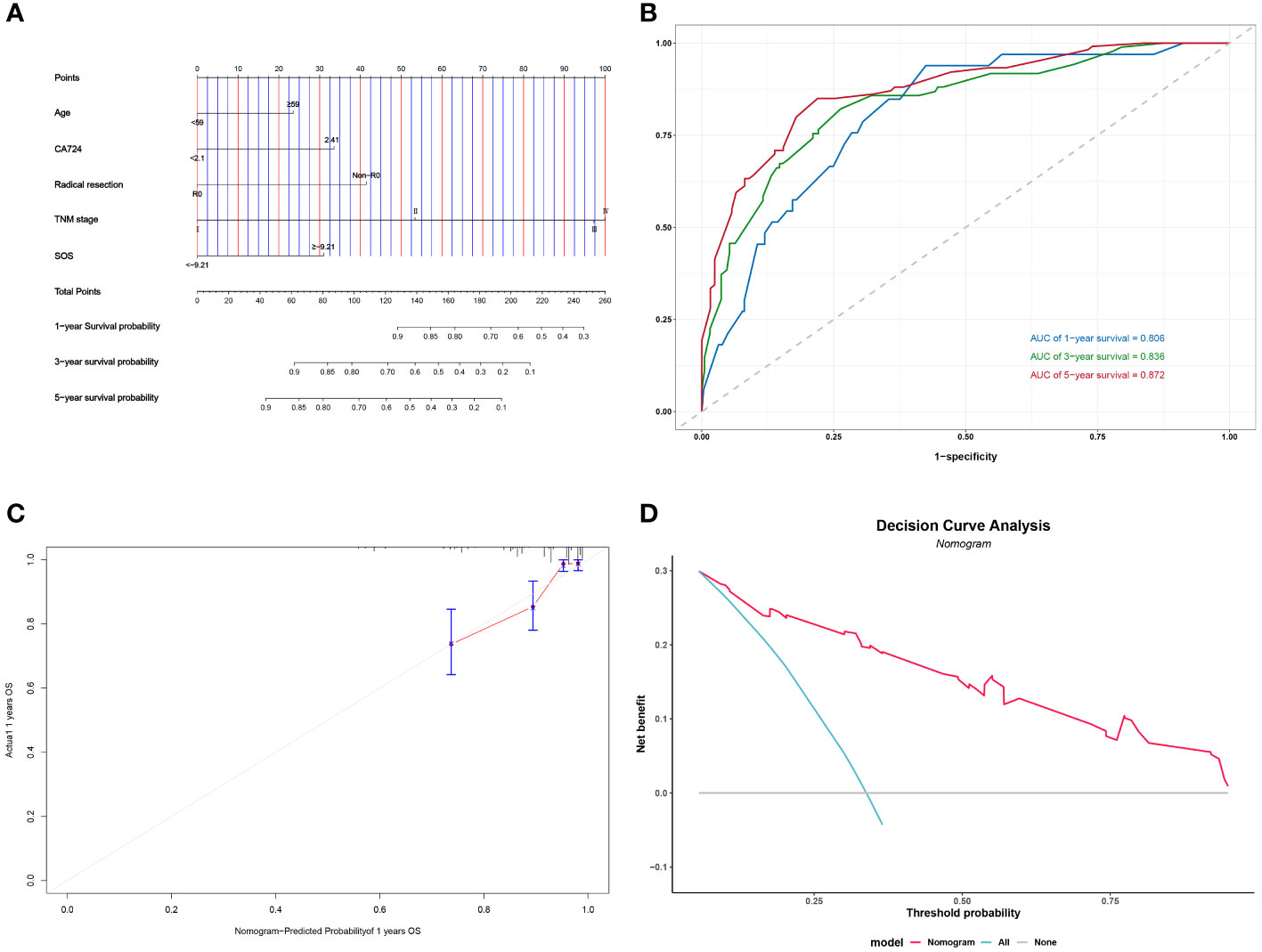
Figure 5 Construction and validation of the nomograms for OS. # Nomogram for OS (A); Time-dependent ROC for nomogram (B); 1-year calibration curve for nomogram (C); Decision Curve Analysis for nomogram (D).
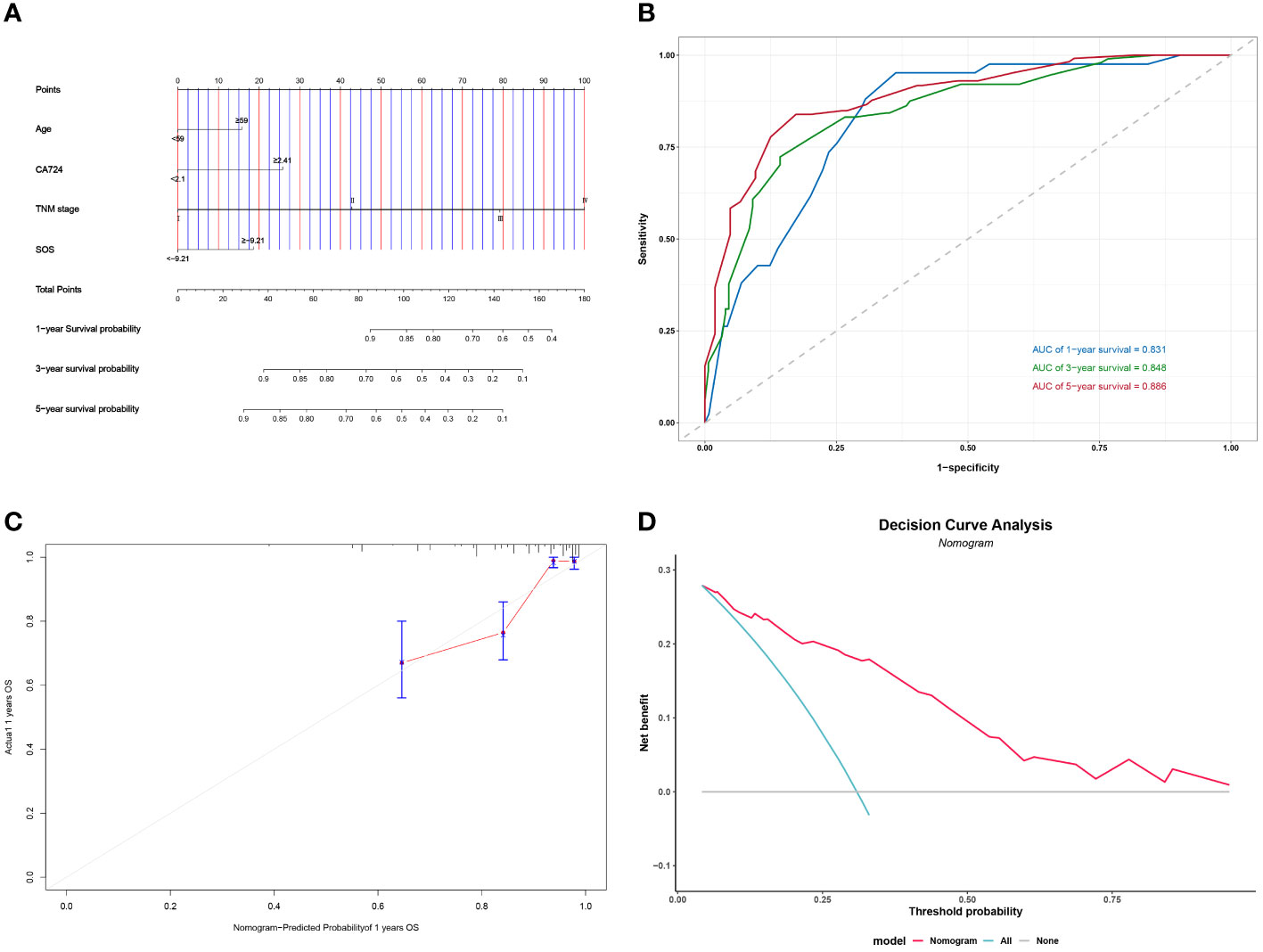
Figure 6 Construction and validation of the nomograms for PFS. # Nomogram for OS (A) Time-dependent ROC for nomogram (B) 1-year calibration curve for nomogram (C) Decision Curve Analysis for nomogram (D).
Time-dependent ROC curves were plotted, demonstrating impressive predictive capabilities over 1-, 3-, and 5-year intervals for both PFS and OS, with corresponding AUC areas ranging from 0.806 to 0.886 (Figure 5B and Figure 6B). Calibration curves for the initial year affirmed the nomogram’s precise predictive properties (Figure 5C and Figure 6C).
Moreover, Decision Curve Analysis (DCA) revealed that the nomogram could provide tangible clinical benefit to patients undergoing gastric cancer surgery (Figure 5D and Figure 6D). These comprehensive validations underscore the reliability and utility of the prognostic models, highlighting their potential to guide clinical decision-making and improve patient outcomes.
Discussion
Gastric cancer is still a serious global public health risk (25). Despite the advent of new treatment modalities, recurrence and metastasis continue to be the cause of death in patients with gastric cancer (26). It’s learnt from a previous study that stress from perioperative events can lead to the spread and metastasis of gastric cancer even in patients undergoing radical gastric cancer surgery (27). In addition, oxidative stress contributes an equally crucial factor in the progression of gastric cancer (28, 29). In this study, the predictive value of SOS on the prognosis of patients undergoing gastric cancer surgery was investigated for the first time.
Relying solely on a singular oxidative stress-related biochemical index for prognostic prediction in gastric cancer patients undergoing surgical treatment presents inherent limitations. To overcome this, our study employed a multi-faceted approach. Utilizing Kaplan-Meier survival analysis, potential oxidative stress-related biochemical indices linked to prognosis were identified. Subsequently, employing the LASSO COX regression, we amalgamated these indices to formulate the SOS formula. The study robustly substantiates the efficacy of SOS in prognosticating outcomes in gastric cancer patients undergoing surgical treatment, a validation reinforced by its applicability in assessing OS within the validation cohorts. Significantly, subgroup analyses focused on the nature of surgical resection, distinguishing between R0 and Non-R0 subgroups. Within the R0 subgroup, patients with elevated SOS exhibited an inferior prognosis compared to their low SOS counterparts. However, in the Non-R0 subgroup, the predictive value of SOS for both PFS and OS did not achieve statistical significance. This observed trend may be attributed to the relatively diminutive sample size within the Non-R0 group and the heterogeneity introduced by the inclusion of palliative resections and gastrointestinal reconstructions. Furthermore, the impact of tumor load reduction on host oxidative stress levels within the Non-R0 subgroup is acknowledged as a potential confounding factor.
Studies addressing the prognostic impact of patients’ oxidative stress levels have been conducted in previous clinical studies. For example, Kaiming Zhang et al, used a prognostic model including SOS to predict the prognosis of breast cancer patients treated with surgery (30). Yinghao Cao et al. followed Kaiming Zhang et al’s study and constructed oxidative stress-related indicators for colorectal cancer in the same way, and used the oxidative stress indicator CIOSS to build a prognostic model to predict the prognosis of patients undergoing surgery for colorectal cancer (31).
ALB is also an important substance in the body, which not only responds to the inflammatory status of the organism, but also has its own antioxidant effect, and because of the free thiol group of albumin Cys34, it is able to engage reactive oxygen species in redox reactions (32, 33). The isoenzyme in lactate dehydrogenase: lactate dehydrogenase A. LDHA exist more abundantly in tumor tissues than in normal tissues. LDHA acts as a glycolytic gene prompting the conversion of pyruvate to lactate (34). Studies have shown that the production of LHDA can be reduced by short interfering RNA (siLDHA), and LDHA reduction alleviates oxidative stress in cells due to the conversion of pyruvate to lactate (35, 36). CRE is produced by muscle metabolism and eliminated via the kidneys, and elevated levels of oxidative stress lead to impaired CRE elimination (37). Cisplatin is a more than commonly used anti-cancer drug in the treatment of gastric cancer, but its side effect renal damage also limits clinical work. It was found that hesperidin attenuates oxidative stress induced by cisplatin by activating the NF2 signaling pathway (38). Therefore CRE can be used as a laboratory indicator to respond to the level of oxidative stress in the body. Uric acid is the product of purines catalyzed twice by xanthine dehydrogenase and xanthine oxidase, the latter using molecular oxygen as an electron acceptor to generate reactive oxygen products such as superoxide anion (39, 40). Uric acid has been proven to be an antioxidant substance. It can be excreted not only through the kidneys but also through the intestine (41, 42). Studies have demonstrated that UA and its oxidation product allantoin are presented as gastrointestinal contents, with the highest content of UA and allantoin in the duodenum and jejunum, and that activation of NF2 promotes the synthesis and secretion of UA, which can play an antioxidant role in the gastrointestinal tract (43).
Yet, there are still unavoidable limitations of this study for the following reasons. First, the relationship between the laboratory markers that make up SOS and oxidative stress and the mechanisms are unclear. Second, selection bias cannot be ruled out. Third, SOS is not a tumor-specific marker, which may also have significance for the diagnosis or prognosis of other diseases or cancers. Fourth, this study was conducted in a single-center retrospective method, and large-scale multicenter prospective studies are still required.
Conclusion
SOS is thought to be a significant predictor of survival and prognosis for individuals with gastric cancer undergoing surgical treatment. High SOS levels in patients typically result in less favorable clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the ethics committee of Harbin Medical University Cancer Hospital. All patients provided written informed consent before the study. (Ethics number: 2019-57-IIT).
Author contributions
XW: Writing – original draft. LZ: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. (2020) 21:4012. doi: 10.3390/ijms21114012
2. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. (2020) 69:2113–21. doi: 10.1136/gutjnl-2020-320839
3. López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. (2023) 181:103841. doi: 10.1016/j.critrevonc.2022.103841
4. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
5. Li H, Zhang H, Zhang H, Wang Y, Wang X, Hou H, et al. Survival of gastric cancer in China from 2000 to 2022: A nationwide systematic review of hospital-based studies. J Glob Health. (2022) 12:11014. doi: 10.7189/jogh.12.11014
6. Jin X, Liu Z, Yang D, Yin K, Chang X. Recent progress and future perspectives of immunotherapy in advanced gastric cancer. Front Immunol. (2022) 13:948647. doi: 10.3389/fimmu.2022.948647
7. Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. (2022) 86:566–82. doi: 10.1016/j.semcancer.2021.12.004
8. Chang JS, Kim KH, Yoon HI, Hyung WJ, Rha SY, Kim HS, et al. Locoregional relapse after gastrectomy with D2 lymphadenectomy for gastric cancer. Br J Surg. (2017) 104:877–84. doi: 10.1002/bjs.10502
9. Wang HM, Wang TJ, Huang CS, Liang SY, Yu CH, Lin TR, et al. Nutritional status and related factors in patients with gastric cancer after gastrectomy: A cross-sectional study. Nutrients. (2022) 14:2634. doi: 10.3390/nu14132634
10. Qiu M, Zhou YX, Jin Y, Wang ZX, Wei XL, Han HY, et al. Nutrition support can bring survival benefit to high nutrition risk gastric cancer patients who received chemotherapy. Support Care Cancer. (2015) 23:1933–9. doi: 10.1007/s00520-014-2523-6
11. Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: Its mechanism and clinical significance. Int J Mol Sci. (2021) 22:8491. doi: 10.3390/ijms22168491
12. Santos JMO, Costa AC, Dias TR, Satari S, Costa E Silva MP, da Costa RMG, et al. Towards drug repurposing in cancer cachexia: Potential targets and candidates. Pharm (Basel). (2021) 14:1084. doi: 10.3390/ph14111084
13. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
14. Gomes-Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. (2002) 180:69–74. doi: 10.1016/S0304-3835(02)00006-X
15. Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M, et al. Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys. (2000) 380:360–6. doi: 10.1006/abbi.2000.1940
16. Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine activity in cancer-related anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate. Semin Oncol. (1998) 25:45–52.
17. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. (2010) 49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006
18. Kruk J, Aboul-Enein HY. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini Rev Med Chem. (2017) 17:904–19. doi: 10.2174/1389557517666170228115324
19. Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
20. Wang J, Sun Y, Zhang X, Cai H, Zhang C, Qu H, et al. Oxidative stress activates NORAD expression by H3K27ac and promotes oxaliplatin resistance in gastric cancer by enhancing autophagy flux via targeting the miR-433-3p. Cell Death Dis. (2021) 12:90. doi: 10.1038/s41419-020-03368-y
21. Quintanilha JCF, de Sousa VM, Visacri MB, Amaral LS, Santos RMM, Zambrano T, et al. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol. (2017) 80:223–33. doi: 10.1007/s00280-017-3358-x
22. Lu Y, Cederbaum AI. Cytochrome P450s and alcoholic liver disease. Curr Pharm Des. (2018) 24:1502–17. doi: 10.2174/1381612824666180410091511
23. Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. (2006) 89:515–23. doi: 10.1093/toxsci/kfj031
24. Senoner T, Schindler S, Stättner S, Öfner D, Troppmair J, Primavesi F. Associations of oxidative stress and postoperative outcome in liver surgery with an outlook to future potential therapeutic options. Oxid Med Cell Longev. (2019) 2019:3950818. doi: 10.1155/2019/3950818
25. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. (2020) 18:534–42. doi: 10.1016/j.cgh.2019.07.045
26. Gunderson LL. Gastric cancer–patterns of relapse after surgical resection. Semin Radiat Oncol. (2002) 12:150–61. doi: 10.1053/srao.2002.30817
27. Du XF, Zhang LL, Zhang DZ, Yang L, Fan YY, Dong SP. Clinical significance of serum total oxidant/antioxidant status in patients with operable and advanced gastric cancer. Onco Targets Ther. (2018) 11:6767–75. doi: 10.2147/OTT.S153946
28. Oliveira CP, Kassab P, Lopasso FP, Souza HP, Janiszewski M, Laurindo FR, et al. Protective effect of ascorbic acid in experimental gastric cancer: reduction of oxidative stress. World J Gastroenterol. (2003) 9:446–8. doi: 10.3748/wjg.v9.i3.446
29. Kim J, Lee J, Choi IJ, Kim YI, Kim J. Gastric cancer risk is reduced by a predominance of antioxidant factors in the oxidative balance: a hospital-based case-control study in Korea. Epidemiol Health. (2022) 44:e2022089. doi: 10.4178/epih.e2022089
30. Zhang K, Ping L, Du T, Wang Y, Sun Y, Liang G, et al. A novel systematic oxidative stress score predicts the prognosis of patients with operable breast cancer. Oxid Med Cell Longev. (2021) 2021:9441896. doi: 10.1155/2021/9441896
31. Cao Y, Deng S, Yan L, Gu J, Mao F, Xue Y, et al. An oxidative stress index-based score for prognostic prediction in colorectal cancer patients undergoing surgery. Oxid Med Cell Longev. (2021) 2021:6693707. doi: 10.1155/2021/6693707
32. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. (2012) 33:209–90. doi: 10.1016/j.mam.2011.12.002
33. Belinskaia DA, Voronina PA, Shmurak VI, Jenkins RO, Goncharov NV. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. (2021) 22:10318. doi: 10.3390/ijms221910318
34. Goldman RD, Kaplan NO, Hall TC. Lactic dehydrogenase in human neoplastic tissues. Cancer Res. (1964) 24:389–99.
35. Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. (1997) 11:388–95. doi: 10.1096/fasebj.11.5.9141507
36. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. (2010) 107:2037–42. doi: 10.1073/pnas.0914433107
37. Okamura DM, Pennathur S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol. (2015) 6:495–504. doi: 10.1016/j.redox.2015.09.039
38. Chen X, Wei W, Li Y, Huang J, Ci X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact. (2019) 308:269–78. doi: 10.1016/j.cbi.2019.05.040
39. Caliceti C, Calabria D, Roda A, Cicero AFG. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients. (2017) 9:395. doi: 10.3390/nu9040395
40. Kimura Y, Tsukui D, Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci. (2021) 22:12394. doi: 10.3390/ijms222212394
41. Su HY, Yang C, Liang D, Liu HF. Research advances in the mechanisms of hyperuricemia-induced renal injury. BioMed Res Int. (2020) 2020:5817348. doi: 10.1155/2020/5817348
42. Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. (2005) 11:4145–51. doi: 10.2174/138161205774913255
Keywords: oxidative stress, SOS, surgery, prognosis, gastric cancer
Citation: Wang X and Zhang L (2024) The systemic oxidative stress score has a prognostic value on gastric cancer patients undergoing surgery. Front. Oncol. 14:1307662. doi: 10.3389/fonc.2024.1307662
Received: 06 October 2023; Accepted: 26 February 2024;
Published: 08 March 2024.
Edited by:
Ji-Feng Feng, University of Chinese Academy of Sciences, ChinaReviewed by:
Bo Pang, China Academy of Chinese Medical Sciences, ChinaSylwia Dziegielewska-Gesiak, Medical University of Silesia, Poland
Copyright © 2024 Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Zhang, ODMxMjI2QGhyYm11LmVkdS5jbg==
†ORCID: Xinyu Wang, orcid.org/0000-0009-8284-1287
 Xinyu Wang
Xinyu Wang Limin Zhang*
Limin Zhang*