
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 07 February 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1307458
 Elisa De Carlo1*
Elisa De Carlo1* Elisa Bertoli1,2
Elisa Bertoli1,2 Monica Schiappacassi3
Monica Schiappacassi3 Brigida Stanzione1
Brigida Stanzione1 Alessandro Del Conte1
Alessandro Del Conte1 Roberto Doliana3
Roberto Doliana3 Michele Spina1
Michele Spina1 Alessandra Bearz1
Alessandra Bearz1Over the past decade, molecular characterization has led to change the management of advanced non-small cell lung cancer (NSCLC) harboring driver mutations. Rearranged during transfection (RET) gene fusions, occurring in 1% to 2% of NSCLC, have emerged as an oncogenic druggable target. Systemic targeted therapies with highly selective RET inhibitors (RETi), selpercatinib and pralsetinib, represent a recent clinical breakthrough. While the development of RETi has improved survival, with their increasing use, it is crucial to be aware of the risks of rare but serious adverse events (AEs). A particular challenge for clinicians in applying targeted therapies is not only diagnosing but also interpreting rare mutations. Herein, we report a case of a 43-year-old Caucasian advanced NSCLC patient diagnosed with a rare RET gene fusion, ANK3::RET, identified with Next Generation Sequencing (NGS). Selpercatinib has been initiated at the recommended initial dose after one incomplete chemotherapy cycle due to a severe infusion reaction, but it subsequently required a dose adjustment following grade 3 (G3) AEs. During treatment, we used a particular selpercatinib dosage (160 mg in the morning and 80 mg in the evening) with good tolerance and without compromising effectiveness. Our finding broadens the range of RET fusion types in not-Asian NSCLC. To the best of our knowledge, our case demonstrates, for the first time, a clinical and radiological response to frontline highly selective RETi selpercatinib, expanding the spectrum of potential oncogenic RET fusion partners in newly diagnosed NSCLC patients. Furthermore, to our knowledge, this is the first case describing a RET fusion-positive (RET+) NSCLC patient treated with a modified selpercatinib dosage outside the drug data sheet and demonstrating a safe and effective use.
We are recently faced with a progressive paradigm shift in the treatment of advanced NSCLC, matching a specific targeted therapy for oncogenic driver alterations subsets (1). Molecular profiling with next-generation sequencing (NGS), detecting actionable driver mutations, is recommended by International Guidelines in newly diagnosed advanced NSCLC and led to the development of personalized therapeutic decision-making, specifically in adenocarcinoma (2, 3). RET is a proto-oncogene, located in the pericentromeric region of chromosome 10q11.2 that encodes a tyrosine kinase receptor involved in the development of neural crest-derived cell lineages, kidney, and male germ cells (4, 5). The RET oncogene activation occurs mainly in two different ways: chromosomal rearrangement and somatic or germline mutations. The RET fusions usually involve a 3’ sequence of the RET gene, encoding the kinase domain, and a 5’ sequence of other chaperone genes. The properties of the newly formed fusion proteins depend on the specific partner gene and the site of the RET breakpoints. Genomic rearrangements of RET gene could result in either ligand-independent activation or aberrant RET expression (4, 5).
The RET oncogene aberrations have been identified in various tumors. RET mutations have been reported in medullary thyroid carcinoma, with an incidence between 43% to 71% of sporadic cases; while germline mutations are a pathognomonic hallmark of multiple endocrine neoplasia (MEN), correlated with a high risk of developing a medullary thyroid carcinoma (6, 7). On the other hand, RET rearrangements are most common among papillary thyroid carcinoma in around 20-40% of patients (8, 9). Genomic rearrangements of RET gene occur in approximately 1-2% of NSCLC, mainly in young, non-smokers or light-smokers, adenocarcinoma patients (10–15). In NSCLC, the most frequently observed partners are kinesin family member 5B gene (KIF5B)::RET (7 variants) and coiled-coil domain containing 6 (CCDC6)::RET (16, 17). Other rearrangements are with Nuclear receptor coactivator 4 (NCOA4), Ephrin type-A receptor 5 (EPHA5), Unconventional myosin-Va (MYO5C), tripartite motif containing 33 (TRIM33), CAP-GLY domain containing linker protein 1 (CLIP), ELKS/RAB6-interacting/CAST family member 1 (ERC1), Phosphatidylinositol binding clathrin-assembly protein (PICALM), FERM Domain Containing 4A (FRMD4A), and RUN and FYVE domain containing 2 (RUFY2) (18, 19).
As an inciting oncogenic event, RET fusions rarely co-occur with other activating mutations, although they might appear as a resistance mechanism during different tyrosine kinase inhibitors treatments for druggable alterations (20, 21).
Retrospectively, Pemetrexed-based chemotherapy demonstrated optimal responses in RET+ NSCLC (22). Furthermore, RET-rearranged adenocarcinomas usually have low Programmed death-ligand 1 (PD-L1) expression, not by chance response to immunotherapy is poor (23). In the past decade, different multitarget agents, such as cabozantinib, vandetanib, sorafenib and lenvatinib, have shown ancillary RETi activity, reflecting the lack of selectivity of these drugs (14, 24). In recent years, selective oral RETi, selpercatinib (LOXO-292) and pralsetinib (BLU-667), have been developed (25, 26). Two global phase 1/2 clinical trials, LIBRETTO-001 and ARROW (27–30), demonstrated the improved activity of these RETi in both platinum-treated and naïve RET+ NSCLC patients, leading to approvals by US Food and Drug Administration (FDA) and European Medicines Agency (EMA). Herein, we report the case of a Caucasian young woman presented with stage IV lung adenocarcinoma, harboring a rare RET rearrangement, demonstrating an impressive clinical and radiologic response to selpercatinib.
A 43-year-old female patient was admitted to the Emergency Department with epigastric pain, nausea and fever, with a temperature up to 38°C, in November 2022. She had a history of light smoking for a few years twenty years before. Chest and abdomen computed tomography CT scan revealed left pleural effusion, peritoneal carcinomatosis, liver metastasis and enlarged celiac and inter-aorto-caval lymphadenopathies. Brain magnetic resonance imaging (MRI) was negative for brain lesions.
The patient underwent thoracentesis with 1200 ml of serum blood fluid drainage and talc pleurodesis. Cytological examination showed the presence of suspicious features for malignancy of epithelial cells. Subsequently, a diagnostic laparoscopy was performed. Histopathological examination revealed a pulmonary origin of peritoneal lesions. In particular, the results of immunohistochemistry (IHC) staining were positive for Cytokeratin7 (CK7), protein16 (p16), Anti-Thyroid Transcription Factor (TTF1), Napsin and negative for Paired box gene 8 (PAX8), Calretinin, GATA Binding Protein 3 (GATA3), Cytokeratin20 (CK20), Estrogen receptor (ER), Wilms’ tumor 1 (WT1), Caudal-type homeobox transcription factor 2 (CD-X2), protein53 (p53), allowing a definitive diagnosis of peritoneal localization of lung adenocarcinoma with acinar growth pattern; PD-L1 was <1%. Molecular profiling of the biopsy sample was carried on performing NGS. Gene fusion analysis on tumor Ribonucleic acid (RNA) revealed a rare Ankyrin3 (ANK3) (Exon 33)::RET (Exon 12) rearrangement (in 96% of the analyzed reads). The gene fusion detected generates a chimeric protein ANK3-RET composed of the amino-terminus of ANK3 and the carboxyl-terminus of RET, including its kinase domain as depicted in Figure 1. Molecular alterations in driver genes, such as Epidermal growth factor receptor (EGFR), Kirsten rat sarcoma virus (KRAS), Erythroblastic oncogene B-2 (ERBB2), Mesenchymal Epithelial Transition factor (MET) and V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) were not detected on tumor Deoxyribonucleic acid (DNA).
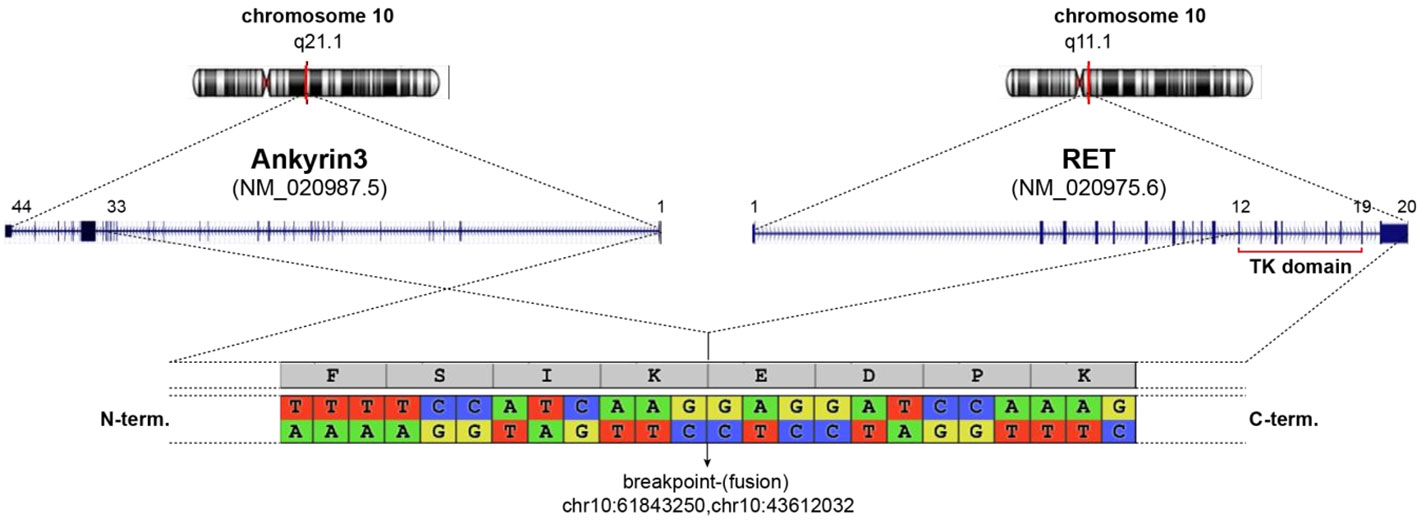
Figure 1 Schematic representation of ANK3-RET fusion detected. Both genes are located on chromosome 10 (upper graph). The detected fusion occurs as depicted in central graph linking ANK3 (exon 1-33) to RET (exon 12-20) given a chimeric protein (below). Breakpoint area is enlarge showing nucleotide sequence and coding aminoacids.
The patient was subsequently prescribed standard first-line treatment with carboplatin (AUC 5 i.v. every three weeks) and pemetrexed (500 mg/m² i.v. every three weeks). During first infusion of carboplatin, the patient reported a grade 3 infusion-related reaction after a few minutes; therefore, the chemotherapy infusion was interrupted (pemetrexed was completely administered while carboplatin only for few milligrams). Due to infusion reaction and to patient refusal, chemotherapy was definitively interrupted after the first cycle. In December 2022, because of worsening of dyspnoea needing oxygen therapy and uncontrolled abdominal pain, the patient started treatment with Selpercatinib at at the recommended initial dose of 160 mg twice a day, with immediate clinical benefit. On February 2023, a CT scan revealed a partial response (PR), with a reduction of pleural thickening, left pleural effusion and in the size of most omental nodules and liver lesions. The patient no longer required oxygen therapy.
In March 2023, the patient reported grade 3 (G3) fatigue, G2 chest, pelvic pain and dyspnoea and G3 hypertension. Electrocardiogram (ECG) revealed iatrogenic QT corrected (QTc) interval prolonged (516, G3); laboratory documented an Aspartate aminotransferase (AST), Alanine aminotransferase (ALT) and Gammaglutanyltransferase (GGT) G3 elevation. Other possible concomitant contributing factors for QTc prolongation were excluded: there were neither electrolyte disorders nor concomitant medications knowning to prolong QTc interval. The patient was hospitalized and Selpercatinib was temporarily discontinued. During hospitalization, a cardiological evaluation with ECG and echocardiography was performed, with evidence of non-specific inverted T waves, negative troponin and prescription of amlodipine 5 mg daily for hypertension. Moreover, abdominal MRI confirmed the stability of liver metastasis and did not show new pathological findings and other possible causes of hepatitis were excluded. After complete resolution of G3 hypertransaminasemia and QTc prolongation, returning to normal values, Selpercatinib was prescribed at the reduced dose of 80 mg twice a day. On April 2023, the CT scan displayed pleural effusion complete resolution with pleural thickening stability, liver lesions reduction mediastinal and abdominal lymphadenopathies disappearance and omental nodules stability.
At the end of April 2023 abdominal pain, present at the time of diagnosis, recurred intensively, without other clinical symptoms or blood tests alterations. Considering that symptoms occurred two weeks after the execution of the CT showing the maintenance of a partial overall disease response, given the difficulty of radiologically evaluating carcinosis and to avoid the exposition of the patient to a second extremely close contrast agent and ionizing radiation potentially needlessly, it was not considered clinically appropriate to repeat a CT.On May 2023, on the suspicion that the reduced dose drug did not effectively control the disease, Selpercatinib was prescribed at a dose of 160 mg in the morning and 80 mg in the evening. The reason for the choice of this particular dosage is related to the need to reach symptom control without inducing severe adverse events, particularly cardiological toxicity. This Selpercatinib dosage was moderately tolerated; patient reported diarrhoea G1, without cardiological adverse events, hypertension and without hypertransaminasemia; QTC intervals were normal at subsequent ECG controls. Abdominal pain was slightly reduced. On August 2023, CT scan revealed further partial response of pleural thickenings and liver metastases with unvaried peritoneal carcinosis; an encephalic MRI showed no evidence of brain disease. The patient is still under Selpercatinib treatment at 160mg plus 80mg daily dosage. Figure 2 represents the patient’s clinical course.
Nucleic acids from the Formalin-Fixed Paraffin-Embedded (FFPE) biopsy tissue were extracted using QIAmpDNA FFPE extraction kit (Qiagen) and Maxwell 16 LEV RNA FFPE kit (Promega) according to the manufacturer’s protocols.
Molecular profiling was performed on extracted DNA using an “in-house” developed NGS panel covering multiple hot spots on EGFR, ERBB2, MET, KRAS and BRAF genes. Gene fusion analysis was performed on extracted RNA using Archer Fusion Plex Lung Cancer Kit which allows the detection of novel fusion partners. The library samples were run on an Illumina Miseq. Raw sequence data was analyzed using a pipeline based on Illumina Local Run manager and variant caller, Integrated Genome Viewer (IGV) and Varsome. For gene fusion analysis, fastq files were uploaded and analyzed on Archer Analysis 6.2 platform. Ref Sequences: ANK3: NM_020987.5, RET NM_020975.6).
RET fusion represents a new NSCLC subgroup with a targetable oncogenic driver. Many efforts have been made to target RET therapeutically over time; treatment for cancers harboring a RET fusion has evolved from traditional chemotherapy and non-selective multiple kinase inhibitors to selective RETi.
The new specific RETi, selpercatinib and pralsetinib have been registered by the US FDA and EMA on the basis of two multicenter, open-label, multicohort clinical trials. The activity of selpercatinib was demonstrated in the phase 1/2 LIBRETTO-001 trial (NCT03157128) in patients whose tumors had RET alterations (27). In this pivotal phase 1/2 clinical trial selpercatinib treatment was associated with remarkable responses in NSCLC patients both previously treated with platinum-based chemotherapy and treatment-naïve patients (28). The objective response rate (ORR) within RET+ NSCLC was 64% in patients treated with platinum-based chemotherapy and 85% in treatment-naive patients (28). In the phase 1/2 ARROW trial (NCT03037385), pralsetinib has been reported to be an effective treatment for RET+ NSCLC, demonstrating activity both in prior platinum-treated and treatment-naïve patients, with an ORR respectively of 61% and 70% (29, 30). It has been reported intracranial activity and a manageable safety profile, with mainly low-grade toxic effects, for both RETi (27–30).
Therefore, a precision treatment of RET+ NSCLC has been tailored.
In our case report, NGS was used to perform panel sequencing allowing the detection of a rare RET rearrangement, the ANK3(33)::RET(12) fusion. This rare rearrangement has been found only in 2 patients, with different fusions, in a large retrospective study of 1162 Chinese NSCLC patients (31). A case report has described ANK3::RET fusion as a druggable mechanism of osimertinib resistance; pralsetinib has demonstrated potent clinical activity when combined with osimertinib in treating an EGFR-mutated NSCLC Caucasian patient with RET-mediated mutation resistance (32). Another case report presents a case of RET dual fusion, ANK3::RET and CCDC6::RET, in an advanced Chinese NSCLC patient treated with pralsetinib after the failure of other treatments, including platinum-based chemotherapy (33).
Currently there is a limited understanding in the genomic landscape of NSCLC and other solid tumors harboring RET fusions. A retrospective multi-center study has recently investigated clinic-biological features and treatment outcomes of patients with any-stage RET+ NSCLC from 31 centers, 30 European and one from Argentina. The most frequent fusion partner was KIF5B followed by CCDC6; no cases were detected with ANK3::RET fusion (34). RET translocations/fusion genes are predominantly reported in papillary thyroid carcinoma and NSCLC, less in other solid tumors, such as colorectal carcinoma, salivary gland carcinoma, serous ovarian carcinoma, pancreatic and breast carcinoma, in global multicenter networks and reviews; ANK3::RET fusion was seen only in a papillary thyroid cancer (12, 17, 34, 35). The study of Parimi et al, reported the clinicopathologic and genomic landscape of a large cohort of RET fusion positive solid tumors (523 patients with NSCLC and 368 with other solid tumors), including the discovery of 61 novel fusions, detected by a DNA tissue-based NGS assay; no case of ANK3::RET rearrangement was detected in all African, Central and South American, East Asian, and South Asian patients (36).
The sensibility to highly selective RETi, selpercatinib and pralsetinib, was not demonstrated in naïve treatment lung adenocarcinoma with ANK3::RET rearrangement as unique oncogenic druggable target. In the Chinese study, the two ANK3::RET+ NSCLC patients did not receive targeted therapies (31) and in the two case reports pralsetinib was not administered as frontline treatment (32, 33).
To the best of our knowledge, this was the first case reported a novel rare RET fusion, ANK(33)::RET(12), in a not-Asian newly diagnosed NSCLC patient; furthermore, because of in our case chemotherapy was incomplete administered for only one cycle due to severe infusion reaction, we could conclude that this is the first report describing clinical and radiological response to highly selective RETi in naïve treatment ANK3::RET positive NSCLC.
In the current era of precision medicine, this case exemplifies how comprehensive genomic profiling with NGS displays greater strengths in identifying fusion variants and may provide important treatment options also for rare molecular alterations.
In addition, this case highlights the unique challenge of using a modified schedule of selpercatinib (160 mg in the morning and 80 mg in the evening) not included in the drug data sheet, reporting patient benefit with low-grade side effects.
Selpercatinib is a first-in-class, highly selective, and potent intracranial RET kinase inhibitor. In the phase I-II LIBRETTO-001 trial selpercatinib was orally administered in a continuous 28-day cycle. Patients enrolled in the phase 1 dose-escalation portion received between 20 mg once daily or 20–240 mg twice daily; the phase 2 recommended dose was 160 mg twice daily (27, 28, 37). Selpercatinib had a manageable tolerability profile and was associated with mainly low-grade toxic effects; this finding is consistent with its RET-selective profile and lack of substantial off-target activity. Adverse events could generally be managed with dose reductions and only a small proportion of patients discontinued selpercatinib because of drug-related adverse events. The most common treatment-related AEs that were grade 3-4 in severity were hypertension, elevated alanine aminotransferase and elevated aspartate aminotransferase. The majority of grade 3 AEs were reversible with dose modifications; this finding suggests that long-term treatment is feasible, particularly in light of the responses observed with selpercatinib at doses as low as 20 mg once daily (27, 28, 37). Based on this case of treating a rare RET-fusion NSCLC patient, we retain that this novel rare fusion might be a potential oncogenic driver, sensitive to frontline selective RETi as selpercatinib. Furthermore, the peculiar dosage of selpercatinib might be safe and efficacious, although a careful risk-benefit discussion with the patient is needed. Despite its limited generalizability, our case reported a novel molecular target in Caucasian RET fusion NSCLC patients and demonstrated the efficacy of a modified and tailored selpercatinib dosage.
Our finding broadens the range of RET fusion types in not-Asian NSCLC population and provide the basis for the hypothesis that highly selective RETi, such as selpercatinib, represent the best treatment option in naïve treatment ANK3-RET positive lung adenocarcinoma. Selpercatinib dose adjustment, outside the drug data sheet, reduced the frequency and intensity of AEs without compromising effectiveness, highlighting the benefit of tailoring doses to optimize treatment outcomes and supporting clinical decision-making.
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ED: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Validation. EB: Data curation, Supervision, Validation, Writing – review & editing. MSc: Methodology, Supervision, Validation, Writing – review & editing. BS: Data curation, Validation, Writing – review & editing. AD: Validation, Visualization, Writing – review & editing. RD: Methodology, Validation, Writing – review & editing. MSe: Supervision, Validation, Writing – review & editing. AB: Supervision, Validation, Writing – review & editing.
This work was supported by the Italian Ministry of Health (Ricerca Corrente); no grant number was provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol (2022) 40(6):611–25. doi: 10.1200/JCO.21.01626
2. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34(4):358–76. doi: 10.1016/j.annonc.2022.12.013
3. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med (2018) 142(3):321–46. doi: 10.5858/arpa.2017-0388-CP
4. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev (2005) 16(4-5):441–67. doi: 10.1016/j.cytogfr.2005.05.010
5. Belli C, Anand S, Gainor JF, Penault-Llorca F, Subbiah V, Drilon A, et al. Progresses toward precision medicine in RET-altered solid tumors. Clin Cancer Res (2020) 26(23):6102–11. doi: 10.1158/1078-0432.CCR-20-1587
6. Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab (2008) 93(3):682–7. doi: 10.1210/jc.2007-1714
7. Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med (2011) 13(9):755–64. doi: 10.1097/GIM.0b013e318216cc6d
8. Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol (2006) 155(5):645–53. doi: 10.1530/eje.1.02289
9. Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology (2007) 148(3):936–41. doi: 10.1210/en.2006-0921
10. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol (2012) 0(35):4352–9. doi: 10.1200/JCO.2012.44.1477
11. Kazdal D, Hofman V, Christopoulos P, Ilié M, Stenzinger A, Hofman P. Fusion-positive non-small cell lung carcinoma: biological principles, clinical practice, and diagnostic implications. Genes Chromosomes Cancer (2022) 61(5):244–60. doi: 10.1002/gcc.23022
12. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(11):1209–21. doi: 10.1200/JCO.19.02551
13. Subbiah V, Cote GJ. Advances in targeting RET-dependent cancers. Cancer Discovery (2020) 10(4):498–505. doi: 10.1158/2159-8290.CD-19-1116
14. Belli C, Penault-Llorca F, Ladanyi M, Normanno N, Scoazec J-Y, Lacroix L, et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol (2021) 32(3):337–50. doi: 10.1016/j.annonc.2020.11.021
15. Chao BH, Briesewitz R, Villalona-Calero MA. RET fusion genes in non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30(35):4439–41. doi: 10.1200/JCO.2012.45.8240
16. Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discovery (2018) 12):1529–39. doi: 10.1158/2159-8290.CD-18-1022
17. Kim M, Na JM, Lee GW, Lee SJ, Kim JD, Yang JW. EGFR-mutated pulmonary adenocarcinoma with concurrent PIK3CA mutation, and with acquired RET fusion and EGFR T790M mutation after afatinib therapy. J Pathol Transl Med (2021) 55(1):79–82. doi: 10.4132/jptm.2020.11.02
18. Drilon A, Bergagnini I, Delasos L, Sabari J, Woo KM, Plodkowski A, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol (2016) 27(7):1286–91. doi: 10.1093/annonc/mdw163
19. Offin M, Guo R, Wu SL, Sabari J, Land JD, Ni A, et al. Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol (2019) 3:PO.18.00386. doi: 10.1200/PO.18.00386
20. Ferrara R, Auger N, Auclin E, Besse B. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol (2018) 13(1):27–45. doi: 10.1016/j.jtho.2017.10.021
21. Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J Hematol Oncol (2020) 13(1):143. doi: 10.1186/s13045-020-00977-0
22. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol (2018) 29(8):1869–76. doi: 10.1093/annonc/mdy137
23. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov (2018) 8(7):836–49. doi: 10.1158/2159-8290
24. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N Engl J Med (2020) 383(9):813–24. doi: 10.1056/NEJMoa2005653
25. Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol (2023) 41(2):385–94. doi: 10.1200/JCO.22.00393
26. Gainor GF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol (2021) 22(7):959–69. doi: 10.1016/S1470-2045(21)00247
27. Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol (2022) 33(11):1168–78. doi: 10.1016/j.annonc.2022.08.002
28. Binghao L, Qu H, Zhang J, Pan W, Liu M, Yan X, et al. Genomic characterization and outcome evaluation of kinome fusions in lung cancer revealed novel druggable fusions. NPJ Precis Oncol (2021) 5(1):81. doi: 10.1038/s41698-021-00221-z
29. Urbanska EM, Sørensen JB, Melchior LC, Costa JC, Santoni-Rugiu E. Durable response to combined osimertinib and pralsetinib treatment for osimertinib resistance due to novel intergenic ANK3-RET fusion in EGFR-mutated non–small-cell lung cancer. JCO Precis Oncol (2022) 6:e2200040. doi: 10.1200/PO.22.00040
30. Meng Y, Li L, Wang H, Chen X, Yue Y, Wang M, et al. Pralsetinib for the treatment of a RET-positive advanced non-small-cell lung cancer patient harboring both ANK-RET and CCDC6-RET fusions with coronary heart disease: a case report. Ann Transl Med (2022) 10(8):496. doi: 10.21037/atm-22-1237
31. Aldea M, Marinello A, Duruisseaux M, Zrafi W, Conci N, Massa G, et al. RET-MAP: an international multicenter study on clinicobiologic features and treatment response in patients with lung cancer harboring a RET fusion. J Thorac Oncol (2023) 18(5):576–86. doi: 10.1016/j.jtho.2022.12.0
32. Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol (2017) 35(13):1403–10. doi: 10.1200/JCO.2016.70.93
33. . Available at: https://atlasgeneticsoncology.org/gene/76/ret-(rearranged-during-transfection).
34. Parimi V, Tolba K, Danziger N, Kuang Z, Sun D, Lin DI, et al. Genomic landscape of 891 RET fusions detected across diverse solid tumor types. NPJ Precis Oncol (2023) 7(1):10. doi: 10.1038/s41698-023-00347-2
35. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol (2022) 23(10):1261–73. doi: 10.1016/S1470-2045(22)00541-1
36. Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol (2013) 31(8):1039–49. doi: 10.1200/JCO.2012.45.3753
Keywords: NSCLC, RET fusion, RET inhibitors, next-generation sequencing, selpercatinib
Citation: De Carlo E, Bertoli E, Schiappacassi M, Stanzione B, Del Conte A, Doliana R, Spina M and Bearz A (2024) Case report: First evidence of impressive efficacy of modulated dose selpercatinib in a young Caucasian with ANK3-RET fusion-positive NSCLC. Front. Oncol. 14:1307458. doi: 10.3389/fonc.2024.1307458
Received: 04 October 2023; Accepted: 18 January 2024;
Published: 07 February 2024.
Edited by:
Carminia Maria Della Corte, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Pasquale Pisapia, University of Naples Federico II, ItalyCopyright © 2024 De Carlo, Bertoli, Schiappacassi, Stanzione, Del Conte, Doliana, Spina and Bearz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa De Carlo, ZWxpc2EuZGVjYXJsb0Bjcm8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.