
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 07 February 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1305720
This article is part of the Research TopicTreatment of Brain Metastases from Non-Small Cell Lung Cancer: Preclinical, Clinical, and Translational ResearchView all 16 articles
 Lauren Julia Brown1,2,3,4
Lauren Julia Brown1,2,3,4 Victor Khou4,5,6
Victor Khou4,5,6 Chris Brown7
Chris Brown7 Marliese Alexander8,9
Marliese Alexander8,9 Dasantha Jayamanne4,5,10
Dasantha Jayamanne4,5,10 Joe Wei11
Joe Wei11 Lauren Gray11
Lauren Gray11 Wei Yen Chan12,13
Wei Yen Chan12,13 Samuel Smith12
Samuel Smith12 Susan Harden8,14
Susan Harden8,14 Antony Mersiades7,15
Antony Mersiades7,15 Lydia Warburton16,17
Lydia Warburton16,17 Malinda Itchins4,10,11
Malinda Itchins4,10,11 Jenny H. Lee12,13
Jenny H. Lee12,13 Nick Pavlakis4,10,11
Nick Pavlakis4,10,11 Stephen J. Clarke4,10,11
Stephen J. Clarke4,10,11 Michael Boyer4,12
Michael Boyer4,12 Adnan Nagrial2,3,4
Adnan Nagrial2,3,4 Eric Hau1,2,3,4
Eric Hau1,2,3,4 Ines Pires da Silva3,4,18
Ines Pires da Silva3,4,18 Steven Kao4,12
Steven Kao4,12 Benjamin Y. Kong4,11,19,20*
Benjamin Y. Kong4,11,19,20*Introduction: Brain metastases commonly occur in patients with non-small cell lung cancer (NSCLC). Standard first-line treatment for NSCLC, without an EGFR, ALK or ROS1 mutation, is either chemoimmunotherapy or anti-PD-1 monotherapy. Traditionally, patients with symptomatic or untreated brain metastases were excluded from the pivotal clinical trials that established first-line treatment recommendations. The intracranial effectiveness of these treatment protocols has only recently been elucidated in small-scale prospective trials.
Methods: Patients with NSCLC and brain metastases, treated with first-line chemoimmunotherapy or anti-PD-1 monotherapy were selected from the Australian Registry and biObank of thoracic cancers (AURORA) clinical database covering seven institutions. The primary outcome was a composite time-to-event (TTE) outcome, including extracranial and intracranial progression, death, or need for local intracranial therapy, which served as a surrogate for disease progression. The secondary outcome included overall survival (OS), intracranial objective response rate (iORR) and objective response rate (ORR).
Results: 116 patients were included. 63% received combination chemoimmunotherapy and 37% received anti-PD-1 monotherapy. 69% of patients received upfront local therapy either with surgery, radiotherapy or both. The median TTE was 7.1 months (95% CI 5 - 9) with extracranial progression being the most common progression event. Neither type of systemic therapy or upfront local therapy were predictive of TTE in a multivariate analysis. The median OS was 17 months (95% CI 13-27). Treatment with chemoimmunotherapy was predictive of longer OS in multivariate analysis (HR 0.35; 95% CI 0.14 – 0.86; p=0.01). The iORR was 46.6%. The iORR was higher in patients treated with chemoimmunotherapy compared to immunotherapy (58% versus 31%, p=0.01). The use of chemoimmunotherapy being predictive of iORR in a multivariate analysis (OR 2.88; 95% CI 1.68 - 9.98; p=0.04).
Conclusion: The results of this study of real-world data demonstrate the promising intracranial efficacy of chemoimmunotherapy in the first-line setting, potentially surpassing that of immunotherapy alone. No demonstrable difference in survival or TTE was seen between receipt of upfront local therapy. Prospective studies are required to assist clinical decision making regarding optimal sequencing of local and systemic therapies.
Brain metastases are an important clinical problem in the treatment of non-small cell lung cancer (NSCLC), occurring in up to 30% of cases at the time of diagnosis (1). The impact of brain metastases on the prognosis of NSCLC is mixed, linked to a poor prognosis in some studies (1), while other reports suggest it can lead to improved outcomes (2). Active, symptomatic or untreated brain metastases are almost universal criteria for exclusion from lung cancer clinical trials, therefore overall survival (OS) estimates from clinical trials do not accurately reflect that of patients with brain metastases.
In patients with driver mutations such as epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements, the incidence of brain metastases is higher than those without mutations (3). The intracranial activity of later generation tyrosine kinase inhibitors is high with the intracranial objective response rate (iORR) of EGFR inhibitor, osimertinib reported as 64% (4) and ALK inhibitor, lorlatinib up to 82% (5). Prior studies of combination EGFR inhibitors plus chemotherapy have also shown higher iORR of up to 85% (6).
For patients who test negative for actionable biomarkers, multi-modal management with immune checkpoint inhibitors (ICI’s) with or without chemotherapy and local therapy is recommended by international guidelines (7). In contrast to the intracranial activity observed with targeted therapy, systemic treatment with chemotherapy alone is estimated to have a 28 – 42% iORR in asymptomatic brain metastases (8–10). However, first-line immunotherapy and chemoimmunotherapy regimens have rapidly become a standard of care for both adenocarcinoma and squamous cell carcinoma in patients with adequate performance status (11–16). A pooled analysis of the KEYNOTE-021, KEYNOTE-189 and KEYNOTE-407 trials, all of which enrolled patients with treated or stable brain metastases, revealed that the addition of immunotherapy to chemotherapy improved overall survival, progression-free survival (PFS) and iORR compared with chemotherapy alone (17). The recently published ATEZO-BRAIN (18) and CAP-BRAIN trials (19) have both demonstrated the intracranial activity of first-line anti-PD-(L)1 antibodies plus chemotherapy in select patients with non-squamous lung cancer with untreated brain metastases, without the need for upfront local therapy.
The intracranial activity of immunotherapy and chemoimmunotherapy regimens in non-squamous NSCLC has been recently established in small prospective studies. The ideal treatment sequencing between systemic (immunotherapy or chemoimmunotherapy) and local (radiotherapy and/or surgery) therapy remains unknown. In this study we have compared the clinical outcomes of the different treatment approaches for patients with both non-squamous and squamous NSCLC with brain metastases in a real-world setting. These approaches include chemoimmunotherapy versus immunotherapy, both with and without local therapy (comprising radiotherapy, surgery, or a combination of both).
Patients from seven institutions were retrospectively identified via the Australian Registry and biObank of thoracic cancers (AURORA). AURORA is approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee (HREC/17/PMCC/42). All patients had Stage IV NSCLC with brain metastases and received first-line systemic therapy with anti-PD-1 monotherapy or combination chemoimmunotherapy. All patients were negative for EGFR, ROS1, ALK oncogenes by institutional standard-of-care testing. Variables collected included baseline patient demographics, disease characteristics (including sites of extracranial disease, method of brain metastasis assessment, number and symptoms of brain metastases, size of brain metastases and presence or absence of symptoms) and treatment details (including systemic therapy dose, radiotherapy timing and dose and surgical details).
Intracranial disease progression may be manifested by asymptomatic radiological findings, symptomatic progression, or death. Thus, a composite time-to-event (TTE) primary endpoint was constructed to capture all progression events regardless of whether intracranial imaging was performed at the time of progression or death. This included investigator-assessed radiological intracranial or extracranial progressive disease (PD), need for local therapy (surgery or radiotherapy) or death due to any cause. Intracranial progression was defined as a ≥20% increase in the sum of the lesions in the brain or the presence of a new lesion as per RECIST 1.1 (20).
Secondary endpoints included OS, iORR and objective response rate (ORR). ORR and iORR were determined by best investigator-assessed extracranial and intracranial responses. ORR and iORR were defined as the proportion of patients who had a partial (PR) or complete response (CR) to treatment by best investigator-assessed response. This was defined as ≥30% reduction in the size of measured lesions or complete resolution of measured lesions, respectively as per RECIST 1.1 (20).
Descriptive statistics were used to summarize patients and disease characteristics, including medians and ranges for continuous variables and proportions for categorical variables. Chi-squared tests were used to examine associations between categorical variables. OS and TTE were calculated from the date of commencement of systemic treatment to the date of death and to the date of an event (either death, intracranial or extracranial PD or salvage radiotherapy). Patients without a clinical event were censored at the last known follow-up date.
Univariate and multivariate logistic and Cox-proportional hazards regression models were used to identify factors associated with the composite outcome and prespecified baseline and treatment characteristics. Factors included in the model included presence of symptoms, number of brain metastases, PD-L1 status, type of systemic therapy, receipt of local therapy, presence of extracranial disease and location of extracranial disease. All factors were considered in the multivariate analysis. A competing risk analysis was performed to assess the cumulative incidence of the composite TTE. All statistical analyses were performed using R (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria) and some figures were created using GraphPad Prism, version 10. A two-sided p-value of <0.05 was considered statistically significant. The reported analyses, including OS, were exploratory in nature. Final data analysis was performed on 23 March 2023.
One hundred and sixteen patients from seven Australian hospitals with NSCLC with brain metastases treated with first-line systemic therapy, diagnosed between December 2016 to January 2022, were included in this analysis (Table 1). The median age was 66 years (IQR 58-71). 65 (56%) were male and 101 (87%) had a European Cooperative Oncology Group Status (ECOG) of 0-1. The histology of the population included 103 (89%) with adenocarcinoma, 7 (6%) with squamous cell carcinoma and 6 (5%) with not-otherwise specified NSCLC. PD-L1 status was performed on 110 patients, 31(27%) had a PD-L1 status of <1%, 21 (18%) were PD-L1 1-49% and 61 (53%) were PD-L1 ≥50%. At the time of diagnosis, 64 patients (55%) were symptomatic of their brain metastases and 76 (66%) had multiple intracranial metastases. The sites and patterns of extra-thoracic metastatic disease were assessed at baseline, 54 (47%) had intracranial disease alone and 62 (53%) has ≥1 site of extracranial disease. Thirty-five (30%) had bone metastases, 23 (20%) had adrenal metastases, 18 (16%) had pleural metastases, 15 (13%) had liver metastases and 12 (10%) had other metastatic sites of disease (Supplementary Table 1).
Eighty (69%) patients received upfront local therapy followed by systemic therapy. Forty (34%) received CNS radiotherapy and 40 (34%) had intracranial surgery prior to the commencement of systemic therapy. Thirty-six (31%) patients received systemic therapy alone (Figure 1A). Of the patients that underwent CNS radiotherapy prior to systemic therapy, 25 (25 of 40, 63%) underwent stereotactic radiosurgery (SRS), 15 (15 of 40, 36%) underwent whole brain radiotherapy (WBRT). Of the patients who had surgery, 37 (37 of 40, 93%) also received radiotherapy and 3 (3 of 40, 7%) had surgery alone. Post-operative radiotherapy (PORT) was administered to 36 patients, with 22 (22 of 36, 61%) receiving cavity SRS, 9 (9 of 36, 25%) received post-operative hypofractionated radiotherapy and 5 (5 of 36, 14%) receiving WBRT. One patient received pre-operative SRS (Figure 1B). The median time between diagnosis of brain metastases and commencement of systemic therapy, was 36.5 days, allowing for local therapies.
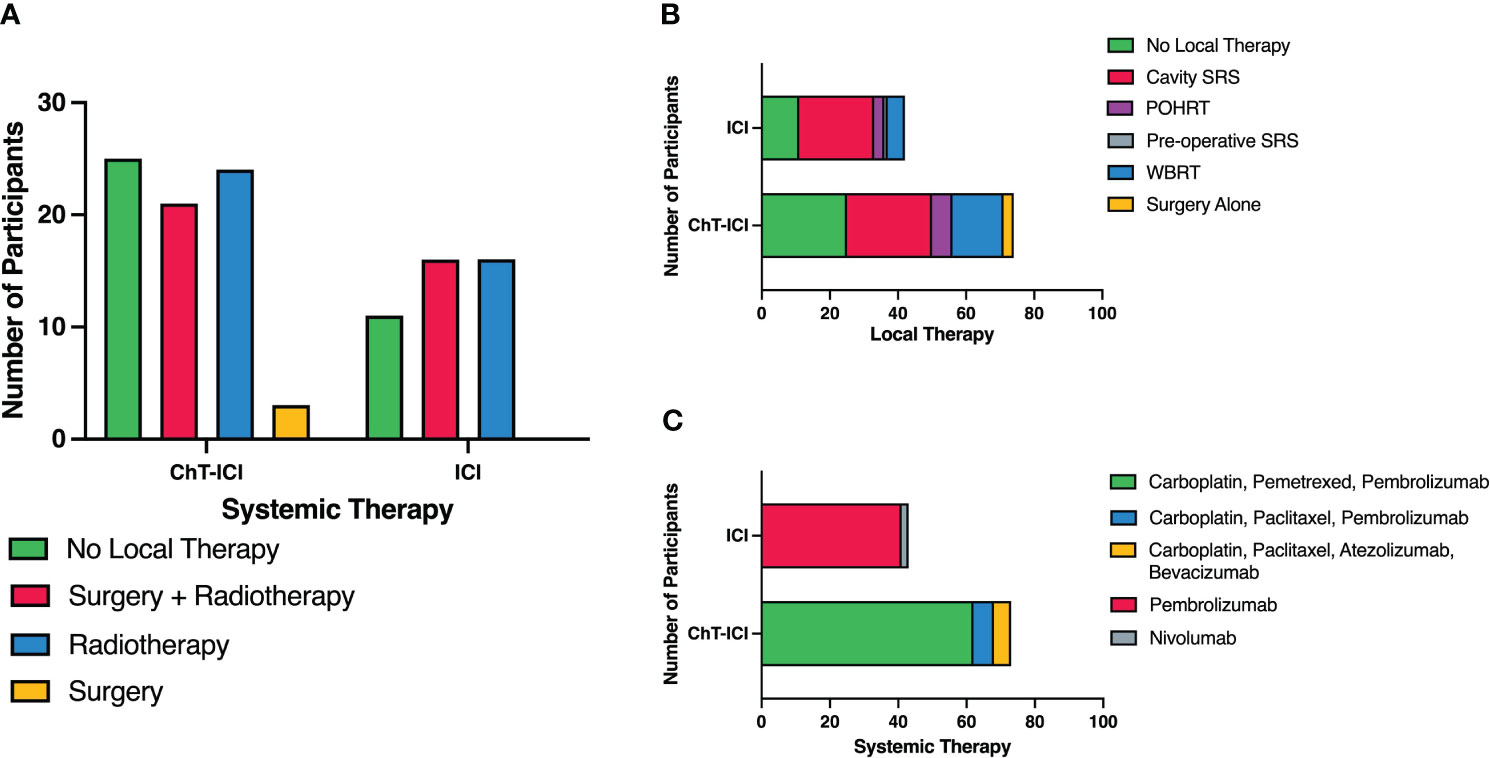
Figure 1 Summary of upfront systemic and local therapies. (A) Upfront Local Therapies by Systemic Therapy. (B) Upfront Local Therapies with Detailed Radiotherapy Modalities. (C) Types of Systemic Therapy. ChT, chemotherapy; ICI, Immune Checkpoint Inhibitor; SRS, Stereotactic radiosurgery; WBRT, whole brain radiotherapy; POHRT, Post-operative hypofractionated radiotherapy.
Seventy-three patients received combination chemoimmunotherapy (63%) as their first-line systemic therapy. Sixty-two patients (53%) received carboplatin, pemetrexed and pembrolizumab, 6 (5%) received carboplatin, paclitaxel and pembrolizumab and 5 (4%) received carboplatin, paclitaxel, bevacizumab and atezolizumab. The remaining 43 (37%) patients were treated with anti-PD-1 monotherapy, 41 (34%) with pembrolizumab and 2 (1.7%) with nivolumab (Figure 1C). Two patients received concurrent chemoradiotherapy for stage III disease then progressed intracranially within 3-6 months of platinum-doublet chemotherapy, thus at clinician discretion were treated with anti-PD-1 monotherapy, despite one having an unknown PD-L1 status and one with PD-L1 with expression of 1-49%.
Thirty-two (28%) patients required salvage radiotherapy during or after progression on systemic therapy; 21 (21 of 32, 66%) of these were with treated with salvage SRS and 11 (11 of 32, 34%) with salvage WBRT (Supplementary Figure 1). Of these patients, 24 (24 of 32, 75%) had received upfront local therapy and 8 (8 of 32, 25%) did not receive upfront local therapy.
Whilst all patients were negative for EGFR, ALK and ROS1, 72 (62%) patients were also tested for KRAS and BRAF mutations with 25 (22%) patients undergoing further next generation sequencing for other mutations (Supplementary Table 2). Thirty-three patients (28%) had a KRAS mutation, of these 13 (13 of 33, 11%) were KRAS G12C. There were four patients had an ERBB2 amplification or insertion, three had a MET amplification/mutation, three with a PIK3CA mutation, two with a BRAF mutation, two with an NRASQ21R mutation.
The median follow-up for the study cohort was 28.6 months (IQR 20-37 months), the median TTE was 7.1 months (95% CI 5–9; Figure 2A). An event occurred in 68% of patients by 12 months (Supplementary Table 3). Extracranial progression of disease was the most common event to occur, followed by intracranial progression, need for stereotactic radiosurgery (SRS), death and need for whole brain radiotherapy (Figure 3).
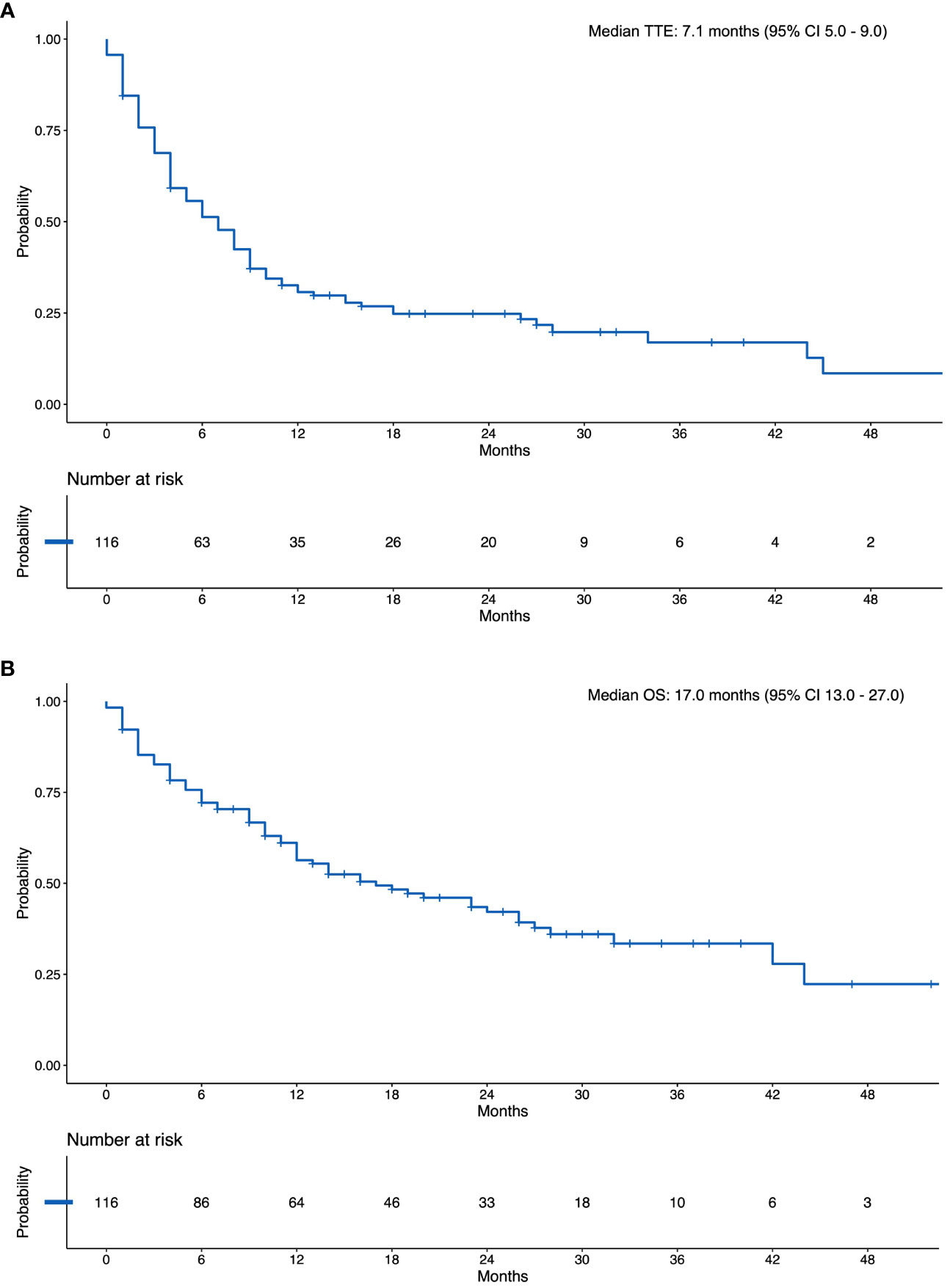
Figure 2 (A) Kaplan Meier Curve of the Composite Time-to-event Outcome. (B) Kaplan Meier Curve of Overall Survival. N, Number at Risk.

Figure 3 Cumulative risk of composite time-to-event outcome. PD, progressive disease; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
There were no significant differences in TTE on univariate and multivariate analysis when patients were stratified according to systemic therapy, presence of brain metastasis symptoms, number of brain metastases, PD-L1 score, whether they received upfront local therapy or presence and number of other sites of metastatic disease (Table 2 and Supplementary Table 4).
The median TTE was similar for patients who received upfront local therapy (7 months, 95% CI 5-10) versus no upfront local therapy (7 months, 95% CI 4-11; p=0.53) (Supplementary Figure 2). The TTE was also similar for patients who received chemoimmunotherapy (7 months, 95% CI 5-9) versus immunotherapy alone (6 months, 95% CI 3-18; p=0.82) (Supplementary Figure 3). The median TTE for the different subgroups based on patient characteristics and therapy type is included in Supplementary Table 5.
The median OS was 17 months (95% CI 13 -27; Figure 2B). The landmark 12-month survival was 61%.
In univariate analysis, age, ECOG, presence of brain metastases symptoms, number of brain metastases, PD-L1 score, type of systemic therapy, upfront local therapy, site and number of extracranial metastases did not influence OS (Supplementary Table 6). However, when all factors were assessed in a multivariate analysis, patients who received chemoimmunotherapy had a longer OS (HR 0.35; 95% CI 0.14–0.86, p=0.01) and patients with PD-L1 score of ≥50% also had longer OS (HR 0.25; 95% CI 0.10–0.65, p<0.01) (Table 2).
Of note, patients who received upfront local therapy followed by systemic therapy had a numerically longer median OS (23 months; 95% CI 12–NA) compared to those who had systemic therapy alone (16 months; 95% CI 10–NA; p=0.13) (Supplementary Figure 4). The median OS was similar for patients who received chemoimmunotherapy (16 months; 95% CI 12–28) versus immunotherapy (17 months; 95% CI 6-44; p=0.95) (Supplementary Figure 5).
Patients with PD-L1≥ 50% (26 months, 95% CI 14– NA) had a longer median OS than those who had PD-L1 1-49% (11 months, 95% CI 9-NA) or PD-L1 <1% (12 months, 95% CI 6–23; p=0.05) (Supplementary Figure 6). Longer median OS was also noted in patients who were PD-L1≥50% treated with chemoimmunotherapy (median OS not reached; 95% CI 26–NA) versus those treated with immunotherapy alone (17 months; 95% CI 7–NA; p = 0.03) (Supplementary Figure 7). The median OS data for the different subgroups based on patient characteristics and therapy type is included in Supplementary Table 5.
The iORR was 46.6%. The iORR was not evaluable in 28 patients (due to complete resection of intracranial disease or no available cranial imaging following commencement of systemic therapy). In the 88 patients with evaluable disease, 10 patients had CR (10 of 88, 11%), 31 had PR (31 of 88, 35%), 33 had stable disease (33 of 88, 38%) and 14 had PD (14 of 88, 16%). The median time to best response was 4.5 months (IQR 3-7 months). Of the 41 patients who had a response, 30 (30 of 41, 73%) had upfront local therapy (2 with surgery, 16 with radiotherapy and 12 with both upfront surgery and radiotherapy). The iORR was higher in patients who received chemoimmunotherapy (30 of 52, 58%) versus those who had immunotherapy alone (11 of 36, 31%, p=0.01). The iORR was similar in patients who received upfront local therapy (30 of 65, 46%) versus no upfront local therapy (11 of 23, 48% p=0.89).
Amongst the 36 patients who had systemic therapy only, there were 11 (11 of 36, 31%) patients with investigator-assessed iORR; 9 with PR (9 of 36, 25%) and 2 with CR (2 of 36, 8%) (Figure 4). Of the five patients who received bevacizumab in addition with chemoimmunotherapy, an iORR was noted in 3 patients, one of whom had upfront local therapy.
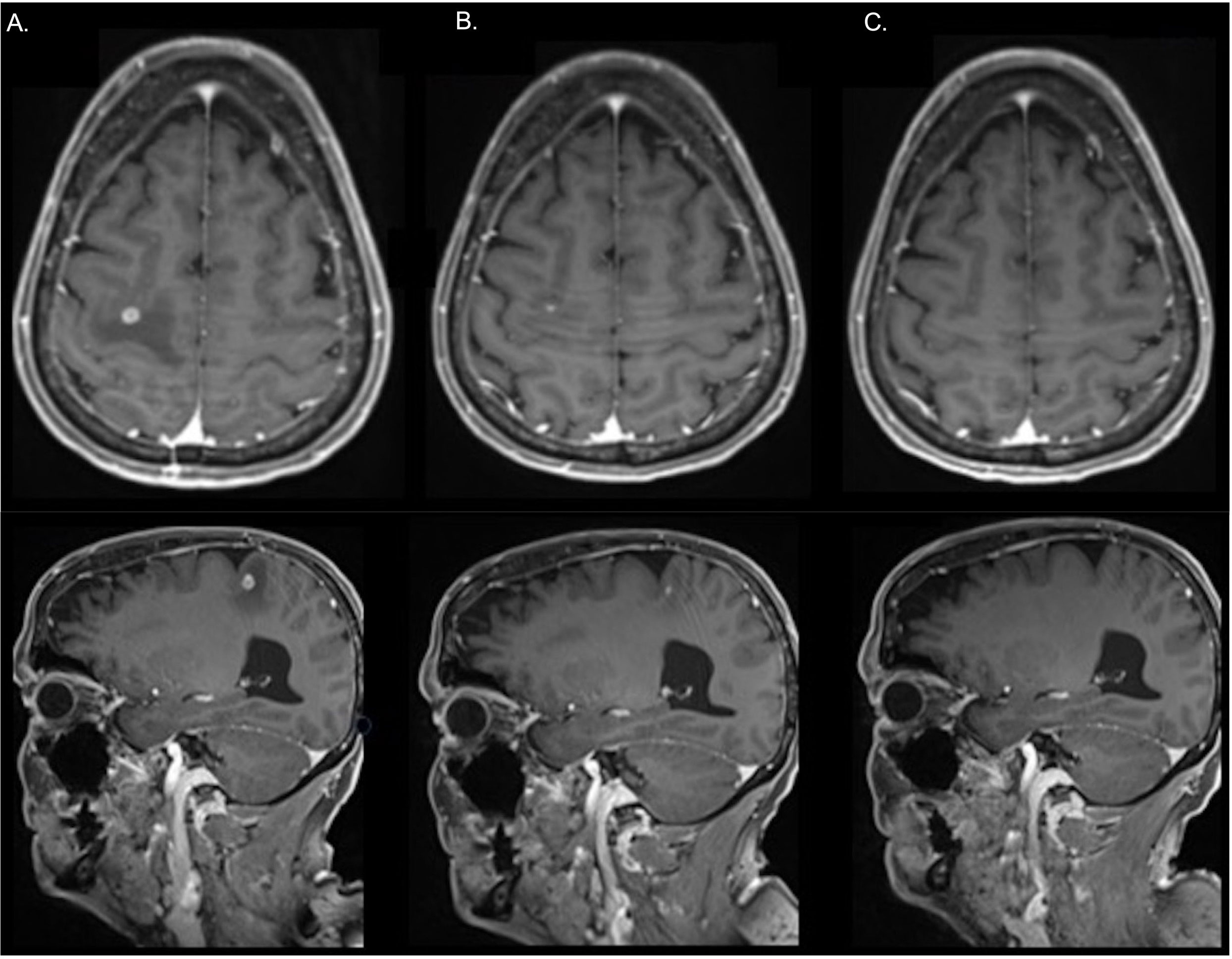
Figure 4 In a representative patient treated with carboplatin, pemetrexed and pembrolizumab without prior local therapy, a magnetic resonance imaging scan shows a solitary brain metastasis in the right parietal lobe at: (A) Pre-treatment (B) After 1 month of treatment (C) In complete response at 3 months following the commencement of systemic therapy.
On univariate analysis, patients who received chemoimmunotherapy had a higher iORR versus immunotherapy alone (OR 3.10, 95% CI 1.29 – 7.82; p=0.01). Other factors including upfront local therapy, PD-L1 status, number of intracranial metastases and symptoms of disease were not associated with higher iORR (Supplementary Table 7). Multivariate analysis confirmed chemoimmunotherapy was associated with improved iORR (HR 2.88, 95% CI 1.68 – 9.98, p=0.04) (Table 2).
The ORR was 50%. In 13 patients, the extracranial response data was not evaluable. Five patients had CR (5 of 103, 5%) and 53 had PR (53 of 103, 51%). Concordance between extracranial and intracranial response was evaluated in 84 patients (Supplementary Table 8). A concordant extracranial and intracranial response was recorded in 70 (70 of 84, 83%) patients. In 14 (14 of 84, 17%) patients, a discordant response was recorded.
Treatment varied across institutions with some institutions favoring upfront local therapy followed by systemic therapy, regardless of the presence or absence of symptoms, and some favoring systemic therapy alone for patients who were asymptomatic (Supplementary Figure 8).
Patients who were symptomatic were more likely to receive upfront local therapy versus asymptomatic patients (74% versus 25%, p<0.001). Similarly, patients with multiple brain metastases were also more likely to receive upfront local therapy versus patients with a single brain metastasis (73% versus 25%, p=0.03). PD-L1 status, ECOG and age did not influence whether a patient received local therapy (Supplementary Table 9). Patients who were over the age of 65 years were also more likely to receive immunotherapy over chemoimmunotherapy (70% versus 49%, p=0.03). Number of metastases and presence of symptoms did not impact systemic treatment choice (Supplementary Table 10).
The data presented in this multi-institutional analysis provide valuable insights into the treatment patterns and outcomes of patients treated with first-line immunotherapy and chemoimmunotherapy for NSCLC and brain metastases. The median OS was 17 months whilst the median composite TTE was 7.1 months. In patients who received chemoimmunotherapy, an improved iORR was observed in the univariate and multivariate analysis (multivariate OR 2.88; p=0.04). Additionally, on multivariate analysis, receipt of chemoimmunotherapy (multivariate HR 0.35; p=0.01) and high PD-L1 (multivariate HR 0.25; p<0.01) were predictive of improved OS. This suggests that the combination of chemoimmunotherapy may be associated with enhanced intracranial activity and OS compared to immunotherapy alone. Despite differences in treatment sequencing across institutions, no significant difference in either outcome measure was observed based on receipt of upfront local therapy, symptoms at baseline, number of brain metastases.
The findings from our study support the results of recently prospective published studies assessing the activity of ICI’s with chemotherapy in NSCLC with brain metastases (18, 19, 21). The results of the Phase II ATEZO-Brain trial by Nadal et al. assessed the use of chemotherapy combined with atezolizumab for patients with non-squamous NSCLC and untreated brain metastases demonstrated a similar iORR of 42.7% (18). The Phase II CAP-Brain study by Hou et al. also assessed the use of chemotherapy combined with camrelizumab for patients with non-squamous NSCLC and untreated brain metastases (19). This study also allowed inclusion of patients who were symptomatic of their brain disease and/or on steroids or mannitol. The iORR was 52.5% and the median OS was 21.0 months (95% CI 15.9 – NR). In our cohort, there was a 31% iORR in patients who received systemic therapy alone without the need for upfront radiotherapy. The results of ATEZO-Brain, CAP-Brain (18, 19), and our study set a precedent for the use of chemoimmunotherapy prior to local therapy of radiotherapy or surgical management in select patient populations.
The median OS in our real-world population, although not directly comparable, was similar to trial populations of patients with pre-treated brain metastases treated with chemoimmunotherapy. In the CheckMate 9LA trial, patients treated with nivolumab plus ipilimumab and chemotherapy versus chemotherapy, the median OS (19.3 versus 6.8 months, HR 0.45, 95% CI 0.29–0.70), median intracranial PFS (13.5 versus 4.6 months, HR 0.36, 95% CI 0.22-0.60), iORR (39% versus 20%) and median time to development of new brain metastases (6.9 versus 5.3 months) were all improved (22). In pooled analyses of patients with brain metastases from the KEYNOTE -021, -189 and -407 trials of pembrolizumab plus chemotherapy also improved median OS versus chemotherapy (18.8 versus 7.6 months, HR 0.48, 95% CI 0.32-0.70) (17).
Immunotherapy alone has also been investigated as a potential approach for treating brain metastases in NSCLC. A trial of single-agent pembrolizumab in untreated brain metastases demonstrated an iORR of 29.7% in patients with PD-L1 ≥1% (23). In the CheckMate 227 study, nivolumab plus ipilimumab improved median OS (17.4 versus 13.7 months, HR 0.63, 95% CI 0.43 – 0.92) and decreased the rate of development of new brain metastases compared with chemotherapy (4% versus 20%) (24). This raises a hypothesis-generating question of whether anti-CTLA-4 has an intracranial priming effect. This hypothesis is best demonstrated in melanoma combination immunotherapy, which has a higher intracranial response rate compared with single-agent therapy (25). In contrast to studies containing nivolumab, the combination of pembrolizumab plus ipilimumab in the KEYNOTE-598 did not demonstrate any significant differences in survival between the two treatment groups in the subgroup of patients with brain metastases (26). However, this could represent differences in the baseline patient characteristics or the lower dose of ipilimumab (1mg/kg, 6 weekly, rather than 3mg/kg, 3 weekly, which was utilized in the melanoma study) and not lack of intracranial activity. In our study, although chemoimmunotherapy was not compared to chemotherapy alone, chemotherapy may fulfil the priming effect that has been achieved by anti-CTLA-4 in melanoma.
In our cohort, chemoimmunotherapy was associated with improved iORR and OS on multivariate analysis over the use of immunotherapy alone. Interestingly, a difference was not observed in univariate analysis for OS. However, this phenomenon can be observed with multivariate modelling upon inclusion of other covariates to better delineate the true source of influence on median OS. Notably, there was no significant difference between systemic therapies on the composite TTE. The cumulative risk of extracranial progression was the most common progression event to occur and comprises a significant proportion of the events in TTE. These results suggest chemoimmunotherapy and immunotherapy have similar efficacy in the control of extracranial disease. However, the combination of chemoimmunotherapy improved intracranial response and potentially led to increased OS.
Whilst our study did not demonstrate any benefit for OS or TTE from the receipt of upfront local therapy, there were no quality-of-life data or assessment of symptomatic control in our study where additional benefits may have been noted. However, prior research has shown that radiotherapy can increase the efficacy of ICI’s through the release of tumor-associated antigens and stimulation of the antitumor response (27–30). The secondary analysis of NSCLC patients in KEYNOTE-001 who received pembrolizumab demonstrated longer PFS and OS in those who had prior radiotherapy (29). Chemotherapy in addition to immunotherapy is also thought to enhance T-cell activation and cancer cell infiltration, thereby increasing the efficacy of ICIs (31). With the further addition of radiotherapy to amplify immunotherapy responses it is logical that a combination strategy of chemoimmunotherapy following radiotherapy may lead to further improved responses.
The difference in sequencing of local therapy and management across institutions also highlights the complexity of decision making for this population. For a select group of patients, it may be reasonable to commence systemic therapy without local treatment. Several prospective clinical trials are underway to explore the impact of different chemotherapy, immunotherapy, radiotherapy and novel combinations on survival outcomes and iORR (Supplementary Table 11). Some of these trials allow for untreated and symptomatic brain metastases to be enrolled on trial, and may provide further insights into the optimal treatment and sequencing for this population.
There are several important differences between patients included in the described clinical trials compared with our real-world population. Firstly, symptomatic patients are generally excluded from clinical trials whilst in our cohort 55% of patients were symptomatic at baseline. The proportion of patients who received baseline CNS imaging with MRI was also high (87.1%). In contrast, whilst many clinical trial participants have MRI imaging at baseline, this is generally not mandated by protocol, thereby potentially underestimating the number of patients who have brain metastases at baseline albeit with no symptoms. Secondly, the number of patients who had local therapy with surgery or radiotherapy immediately before starting systemic therapy was likely to be higher in real-world patients since a washout period of 4 weeks is usually mandated in clinical trials. In real-world patients, this may have conferred some synergistic or antagonistic effects, due to the immunosuppressive effects of surgery or the synergy between radiotherapy and immunotherapy. Thirdly, we observed 53% of patients in our cohort had a PD-L1 ≥50%, which is generally higher than the reported rates of PD-L1 ≥50% which is closer to 30% (32). Lastly, a significant majority of the study population had adenocarcinoma (89%) and only a small proportion of patients had squamous cell carcinoma (6%). It is known that patients with non-squamous NSCLC are more likely to develop brain metastases (33, 34). This is consistent with combined data from the landmark immunotherapy trials in NSCLC (17, 24, 26, 35, 36), which also reveal a higher percentage of non-squamous patients with brain metastases, comprising 70%-85%, in contrast to those with squamous cell lung cancer, who represent 15%-30% of the brain metastasis cases. The retrospective nature of our study likely contributed to a lower proportion of cases with squamous cell carcinoma, and the applicability of this study should be interpreted with caution when considering this group.
This study provides a real-world insight into the outcomes for patients with NSCLC and brain metastases, however, there are some limitations to this methodology. The retrospective nature of this study is associated with unavoidable selection bias and although this was a multi-institutional study conducted at seven large cancer centers, the overall sample size is relatively small when compared to other real-world datasets. This limited sample size, along with the cohort’s heterogeneity, may have influenced the identification of predictive factors associated with the composite TTE.
It is also important to note that data regarding steroid use was not available and thus the influence of steroid use on outcomes was unable to be assessed in this study. Steroid use is often reflective of the symptom burden and is used to reduce peritumoral edema (37). Additionally, the use of steroids and the impact on the efficacy of immunotherapy is controversial with some studies suggesting steroid use can dampen the immune response (38–40). Multiple prospective studies are underway, allowing the use of steroids (Supplementary Table 11) which may help to guide clinical practice for the use of concurrent steroids and immunotherapy in patients with brain metastases.
There are also inherent challenges in the interpretation of imaging responses retrospectively in patients with brain metastases. This includes patients lost to follow-up, the difference in scan frequency and scan type between centers. Other potential confounders include the use of anti-angiogenic agents such as bevacizumab in chemotherapy regimens and its effect on reducing contrast enhancement that has been demonstrated in the setting of primary brain cancers (41, 42). MRI is also considered to have a higher sensitivity for the detection of intracranial metastatic disease, given a small proportion of our cohort had CT imaging, there may be a proportion of patients whose response was over- or underestimated. Furthermore, the timing of scans amongst our cohort differed. Notably, the RECIST and RANO-BM response assessment methods also differ in the timing of recommended scans. The most appropriate intracranial response criteria to use in clinical trials is the subject of ongoing debate (43).
Findings from this study reveal that chemoimmunotherapy has promising intracranial activity and survival outcomes in the first-line setting. Individual treatment sequencing approaches across institutions reveal heterogeneity in the initial treatment paradigms for patients with brain metastases in the real-world setting. The results of future prospective studies are expected to answer questions about ideal response assessment criteria, optimal systemic therapy, and treatment sequencing to assist clinical decision making. Overall, the lack of survival benefit attributable to sequencing of surgery or radiotherapy before or after systemic therapy should give clinicians confidence that the outcomes may be similar when treating patients with NSCLC and brain metastases. However, prospective studies are required to answer this question definitively.
The raw data is not available but requests can be directed to the corresponding author for consideration.
The AUstralian Registry and biObank of thoracic cAncers (AURORA) has been approved by the PeterMacCallum Cancer Centre Human Research Ethics Committee and this study is in compliance with approved protocol number HREC/17/PMCC/42.
LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. VK: Data curation, Investigation, Writing – review & editing. CB: Data curation, Formal analysis, Investigation, Software, Visualization, Writing – review & editing. MA: Data curation, Investigation, Project administration, Writing – review & editing. DJ: Data curation, Investigation, Writing – review & editing. JW: Data curation, Investigation, Writing – review & editing. LG: Data curation, Investigation, Writing – review & editing. SS: Data curation, Investigation, Writing – review & editing. WC: Data curation, Investigation, Writing – review & editing. SH: Data curation, Investigation, Writing – review & editing. AM: Data curation, Investigation, Writing – review & editing. LW: Data curation, Investigation, Writing – review & editing. MI: Data curation, Investigation, Writing – review & editing. JL: Data curation, Investigation, Writing – review & editing. NP: Data curation, Investigation, Writing – review & editing. SC: Data curation, Investigation, Writing – review & editing. MB: Investigation, Writing – review & editing, Data curation. AN: Data curation, Investigation, Writing – review & editing. IP: Data curation, Investigation, Writing – review & editing, Supervision. EH: Data curation, Investigation, Writing – review & editing. SK: Data curation, Investigation, Writing – review & editing. BK: Data curation, Investigation, Writing – review & editing, Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1305720/full#supplementary-material
1. Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer (2018) 19(4):e373–9. doi: 10.1016/j.cllc.2018.01.007
2. Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol (1988) 6(9):1474–80. doi: 10.1200/JCO.1988.6.9.1474
3. Ge M, Zhuang Y, Zhou X, Huang R, Liang X, Zhan Q. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol (2017) 135(2):413–8. doi: 10.1007/s11060-017-2590-x
4. Erickson AW, Brastianos PK, Das S. Assessment of effectiveness and safety of osimertinib for patients with intracranial metastatic disease: A systematic review and meta-analysis. JAMA Netw Open (2020) 3(3):e201617. doi: 10.1001/jamanetworkopen.2020.1617
5. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. New Engl J Med (2020) 383(21):2018–29. doi: 10.1056/NEJMoa2027187
6. Hou X, Li M, Wu G, Feng W, Su J, Jiang H, et al. Gefitinib plus chemotherapy vs gefitinib alone in untreated EGFR-mutant non–small cell lung cancer in patients with brain metastases: the GAP BRAIN open-label, randomized, multicenter, phase 3 study. JAMA Network Open (2023) 6(2):e2255050. doi: 10.1001/jamanetworkopen.2022.55050
7. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34(4):358–76. doi: 10.1016/j.annonc.2022.12.013
8. Barlesi F, Gervais R, Lena H, Hureaux J, Berard H, Paillotin D, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol (2011) 22(11):2466–70. doi: 10.1093/annonc/mdr003
9. Bailon OK, Chouahnia K, Augier A, Bouillet T, Billot S, Coman I, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol (2012) 14(4):491–5. doi: 10.1093/neuonc/nos004
10. Lee DH, Han JY, Kim HT, Yoon SJ, Pyo HR, Cho KH, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first. Cancer (2008) 113(1):143–9. doi: 10.1002/cncr.23526
11. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3
12. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, Angelis De F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
13. Novello S, Kowalski DM, Luft A, Gumus M, Vicente D, Mazieres J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol (2023) 2023:JCO2201990. doi: 10.1200/JCO.22.01990
14. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol (2021) 39(21):2339–49. doi: 10.1200/JCO.21.00174
15. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. New Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
16. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
17. Powell SF, Rodríguez-Abreu D, Langer CJ, Tafreshi A, Paz-Ares L, Kopp HG, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: pooled analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol (2021) 16(11):1883–92. doi: 10.1016/j.jtho.2021.06.020
18. Nadal E, Rodríguez-Abreu D, Simó M, Massutí B, Juan O, Huidobro G, et al. Phase II trial of atezolizumab combined with carboplatin and pemetrexed for patients with advanced nonsquamous non–small-cell lung cancer with untreated brain metastases (Atezo-brain, GECP17/05). J Clin Oncol (2023) 41(28):JCO.22.02561. doi: 10.1200/JCO.22.02561
19. Hou X, Zhou C, Wu G, Lin W, Xie Z, Zhang H, et al. Efficacy, safety, and health-related quality of life with camrelizumab plus pemetrexed and carboplatin as first-line treatment for advanced nonsquamous NSCLC with brain metastases (CAP-BRAIN): A multicenter, open-label, single-arm, phase 2 study. J Thorac Oncol (2023) 18(6):769–79. doi: 10.1016/j.jtho.2023.01.083
20. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer (2016) 62:132–7. doi: 10.1016/j.ejca.2016.03.081
21. Nigen B, Goronflot T, Herbreteau G, Mathiot L, Sagan C, Raimbourg J, et al. Impact of first-line immunotherapy on survival and intracranial outcomes in a cohort of non-small cell lung cancer patients with brain metastases at diagnosis. Lung Cancer (2023) 184:107321. doi: 10.1016/j.lungcan.2023.107321
22. Carbone D, Ciuleanu T, Cobo M, Schenker M, Zurawski B, Menezes J, et al. OA09.01 first-line nivolumab + Ipilimumab + Chemo in patients with advanced NSCLC and brain metastases: results from checkMate 9LA. J Thorac Oncol (2021) 16(10). doi: 10.1016/j.jtho.2021.08.061
23. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21(5):655–63. doi: 10.1016/S1470-2045(20)30111-X
24. Reck M, Ciuleanu TE, Lee JS, Schenker M, Zurawski B, Kim SW, et al. Systemic and intracranial outcomes with first-line nivolumab plus ipilimumab in patients with metastatic NSCLC and baseline brain metastases from checkMate 227 part 1. J Thorac Oncol (2023) 18(8):P1055–69. doi: 10.1016/j.jtho.2023.04.021
25. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol (2018) 19(5):672–81. doi: 10.1016/S1470-2045(18)30139-6
26. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/JCO.20.03579
27. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) 520(7547):373–7. doi: 10.1038/nature14292
28. Deng L, Liang H, Burnette B, Weicheslbaum RR, Fu YX. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology (2014) 3:e28499. doi: 10.4161/onci.28499
29. Shaverdian N, Lisberg A, Bornazyan K, Veruttipong D, Goldman J, Formenti S, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7
30. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1–mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res (2015) 3(4):345–55. doi: 10.1158/2326-6066.Cir-14-0196
31. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity (2016) 44(2):343–54. doi: 10.1016/j.immuni.2015.11.024
32. Aggarwal C, Abreu DR, Felip E, Carcereny E, Gottfried M, Wehler T, et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann Oncol (2016) 27:vi363. doi: 10.1093/annonc/mdw378.14
33. Wang S-y, Ye X, Ou W, Lin Y-b, Zhang B-b, Yang H, et al. Risk of cerebral metastases for postoperative locally advanced non-small-cell lung cancer. Lung Cancer (2009) 64(2):238–43. doi: 10.1016/j.lungcan.2008.08.012
34. Yokoi K, Kamiya N, Matsuguma H, Machida S, Hirose T, Mori K, et al. Detection of brain metastasis in potentially operable non-small cell lung cancer: A comparison of CT and MRI. CHEST (1999) 115(3):714–9. doi: 10.1378/chest.115.3.714
35. Li S, Zhang H, Liu T, Chen J, Dang J. The effect of asymptomatic and/or treated brain metastases on efficacy of immune checkpoint inhibitors in metastatic non–small cell lung cancer: a meta-analysis. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.702924
36. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
37. Vecht CJ, Hovestadt A, Verbiest HB, Vliet van JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology (1994) 44(4):675–80. doi: 10.1212/WNL.44.4.675
38. Skribek M, Rounis K, Afshar S, Grundberg O, Friesland S, Tsakonas G, et al. Effect of corticosteroids on the outcome of patients with advanced non–small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer (2021) 145:245–54. doi: 10.1016/j.ejca.2020.12.012
39. Diana VM, Karine T, Madhav KC, Victoria S, Helen Y, Cameron P, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J ImmunoTher Cancer (2021) 9(7):e002261. doi: 10.1136/jitc-2020-002261
40. Nelson BE, Greiner B, Hong A, Tipton S, Lee M, Dixon A, et al. Corticosteroid use and its impact on the efficacy of immunotherapy in multiple tumor types. J Clin Oncol (2021) 39(15_suppl):e14583. doi: 10.1200/JCO.2021.39.15_suppl.e14583
41. Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology (2006) 66(8):1258–60. doi: 10.1212/01.wnl.0000208958.29600.87
42. Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent Malignant gliomas. Efficacy toxic patterns recurrence (2008) 70(10):779–87. doi: 10.1212/01.wnl.0000304121.57857.38
43. Wen PY, Bent Den Van MJ, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: Proposal for an update to the Response Assessment in Neuro-Oncology (RANO) criteria for high- and low-grade gliomas in adults. J Clin Oncol (2023) 41(Suppl 16):2017–7. doi: 10.1200/JCO.2023.41.16_suppl.2017
Keywords: non-small cell lung cancer, brain metastases, immune checkpoint inhibitor, chemoimmunotherapy, stereotactic radiosurgery, whole brain radiotherapy, intracranial therapy
Citation: Brown LJ, Khou V, Brown C, Alexander M, Jayamanne D, Wei J, Gray L, Chan WY, Smith S, Harden S, Mersiades A, Warburton L, Itchins M, Lee JH, Pavlakis N, Clarke SJ, Boyer M, Nagrial A, Hau E, Pires da Silva I, Kao S and Kong BY (2024) First-line chemoimmunotherapy and immunotherapy in patients with non-small cell lung cancer and brain metastases: a registry study. Front. Oncol. 14:1305720. doi: 10.3389/fonc.2024.1305720
Received: 02 October 2023; Accepted: 08 January 2024;
Published: 07 February 2024.
Edited by:
Jianxin Xue, Sichuan University, ChinaReviewed by:
Elizabeth Gaughan, University of Virginia, United StatesCopyright © 2024 Brown, Khou, Brown, Alexander, Jayamanne, Wei, Gray, Chan, Smith, Harden, Mersiades, Warburton, Itchins, Lee, Pavlakis, Clarke, Boyer, Nagrial, Hau, Pires da Silva, Kao and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Y. Kong, YmVuLmtvbmdAaGVhbHRoLm5zdy5nb3YuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.