
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 01 February 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1302001
This article is part of the Research TopicMultimodality Therapy for Older Cancer PatientsView all 10 articles
 Youssef Slama1*
Youssef Slama1* Gilles Baumont1
Gilles Baumont1 Angelique Arcambal1
Angelique Arcambal1 Mickael Begue1
Mickael Begue1 Olivier Maillot1
Olivier Maillot1 Rima Sayah1
Rima Sayah1 Romain Castanet1
Romain Castanet1 Raoul Caboche2
Raoul Caboche2 Pedro Liberati2
Pedro Liberati2 Hakim Slaoui2
Hakim Slaoui2 Medi Bouaziz2
Medi Bouaziz2 Olivier Borson3
Olivier Borson3 Nam P. Nguyen4
Nam P. Nguyen4 Fabien Dutheil1
Fabien Dutheil1Introduction: Prostate cancer is the fourth most commonly diagnosed cancer among men worldwide. Various tools are used to manage disease such as conventional radiotherapy. However, it has been demonstrated that large prostate volumes were often associated with higher rates of genitourinary and gastrointestinal toxicities. Currently, the improvements in radiotherapy technology have led to the development of stereotactic body radiotherapy, which delivers higher and much more accurate radiation doses. In order to complete literature data about short-term outcome and short-term toxic effects of stereotactic body radiotherapy, we aimed to share our experience about gastrointestinal and genitourinary toxicities associated with stereotactic body radiotherapy in prostate cancer in patients over 70 years old.
Methods: We retrospectively reviewed the medical records of elderly patients with prostate cancer treated between 2021 and 2022. The elderly patients were treated with a non-coplanar robotic stereotactic body radiotherapy platform using real-time tracking of implanted fiducials. The prostate, with or without part of the seminal vesicles, was treated with a total dose of 36.25 Gy delivered in five fractions, each fraction being administered every other day.
Results: We analyzed a total of 80 elderly patients, comprising 38 low-, 37 intermediate- and 5 high-risk patients. The median follow-up duration was 12 months. We did not observe biochemical/clinical recurrence, distant metastasis, or death. Grade 2 acute genitourinary toxicity was observed in 9 patients (11.25%) and Grade 2 acute gastrointestinal toxicity in 4 patients (5.0%). We did not observe any grade 3 or more acute or late toxicities.
Conclusion: Over the follow-up period, we noted a low frequency of gastrointestinal and genitourinary toxicities induced by stereotactic body radiotherapy in the context of prostate cancer in elderly patients. Therefore, stereotactic body radiotherapy seems to represent a promising treatment option for elderly patients, with acceptable acute toxicity.
Based on prostate cancer (PCa) statistics, PCa is the 2nd most commonly occurring cancer in men and the 4th most common cancer overall. There were more than 1.4 million new cases of prostate cancer in 2020 (1). Nowadays, the clinical localization of the disease determines the risk level of the disease through the National Comprehensive Cancer Network (NCCN) guidelines. As per these guidelines, most patients diagnosed for PCa have low-risk or intermediate-risk disease (2). The 5-year survival rate for people diagnosed with PCa is 84% for those with local- or regional-stage PCa. However, this rate drops to 31% for those diagnosed with distant-stage disease (3, 4). Despite an overall 10-year survival rate of 98% across all stages, which is attributed to the high cure rate of the disease in the United States, PCa treatment is still associated with a risk of disability and co-morbidities (5). Various disease management strategies can be considered for the treatment of PCa, including definitive external beam radiotherapy (EBRT) delivered in conventional fractions of 1.8 to 2.0 Gy for 8 or 9 weeks. Given that prostate cancer exhibits a high sensitivity to higher doses per fraction due to a low α to β ratio compared to organs at risk, hypofractionated treatment with a higher dose per fraction seems to be more appropriate and more effective. Over the past 25 years, radiotherapy procedures have significantly advanced, resulting in improved precision for locating and tracking the tumor, and decreased positioning error rates in the treatments (6). This improvement has led to the emergence of Stereotactic Body Radiation Therapy (SBRT) or extreme hypofractionation. In fact, the combination of multiple fields with image guidance and SBRT allows to deliver higher and more accurate radiation doses than in the past (7–13).
Nevertheless, hypofractionation may not be beneficial for all types of tumors. In case of PCa, it could help to balance the benefits and risks by improving cure rates and reducing risks of gastrointestinal and genitourinary toxicities (14–17).
Aging affects the individual’s tolerance to ionizing radiation due to physiological changes and comorbid illnesses. Indeed, geriatric conditions can influence the normal tissue response to radiation and affect the ability of patients to complete radiation treatment and tolerate radiotherapy-related side effects (18). Of note, radiation-induced toxicities are not directly proportional to age but are more associated with the severity of the comorbidities of patients (19). Moreover, it is well established that the probability of developing PCa increases with age and that men aged 70 and older may especially experience radiation-induced toxicities (4).
Even if over the past decade, SBRT technology has been extensively used worldwide and the data collected has proved that its effectiveness and acceptability are constantly increasing, there is a lack of literature data regarding elderly patients and the short-term outcomes of SBRT, particularly potential short-term toxic effects.
The aim of this study is to share our experience regarding the short-term outcomes of SBRT in elderly patients (70 years old and older) including acute toxicity associated with the treatment in a cohort of 80 patients with various PCa risk levels (low to high-risk) treated between 2021 and 2022.
Elderly patients with PCa, for whom radiation therapy was selected as the preferred treatment in a multidisciplinary consultation meeting and who opted for SBRT over EBRT, were included in this study. The 80 patients were exclusively treated with SBRT at La Clinique Sainte-Clotilde (Reunion Island, France) for the first time and all toxicity data were collected. To enable tracking of the prostate and improve the accuracy of the dose delivery during SBRT, 3 or 4 gold seeds were inserted into the prostate transperineally or transrectally. In case of transrectal insertion, prophylactic antibiotics were administrated to the patient before and after the procedure. The fiducial markers inserted were gold anchor (0.4x10 mm) delivered through a thin needle (G22) (20, 21). Patients underwent planning computed tomography with a slice thickness of 1.5 mm at least 7 days after fiducial markers insertion. The computed tomography scan extended at least 15 cm above and below the prostate to ensure the inclusion of the testicles in the scanned volume. Additionally, a magnetic resonance imaging of the prostate was performed, specifically to delineate the urethra (22, 23). For low-risk patients, the clinical target volume (CTV) included only the prostate. However, for intermediate or high-risk patients, the CTV included the prostate and a proximal 1 cm of the seminal vesicles (24). The organs at risk (OAR) were delineated according to the recommendations of the Radiation Therapy Oncology Group (RTOG) (25). The bladder was contoured as a solid organ from base to dome. The rectum was contoured from recto-sigmoid flexure to anal verge. and the urethra from bladder to 2 cm below the prostatic apex. The bowel was countered as a “bowel bag” i.e in the space within the peritoneal cavity that could contain the bowel.
The following instructions were given to all patients to ensure an appropriate bladder and rectum preparation:
● Empty the bladder one hour before the dosimetric scanner and the radiotherapy sessions then drink 50 cl of water and avoid urinating.
● Low residue diet during the treatment phase (from the medical consultation).
● Prescription of daily laxative (from the medical consultation).
● Fasting 4 hours before the dosimetric scanner and the treatment sessions.
● Prescription of Enema 2 hours before the dosimetric scanner and the treatment sessions.
The radiotherapy planning target volume (PTV) is created by adding appropriate margins to the CTV. To create the PTV, the CTV is expanded by 5 mm in all directions, except 3 mm posteriorly. This volume likely includes 1-2 mm of microscopic extracapsular spread, which helps mitigate delineation uncertainties and treatment delivery inaccuracies as reported in literature trials (26, 27). However, optimal margins for high-risk patients needed to be defined. The primary planning objective was to deliver 36.25 Gy in 5 fractions to the PTV. The plans were normalized such that 95% of the PTV volume receives at least 36.25 Gy. The dosimetric objectives to the OAR are summarized in Table 1.
All patient were treated with a non-isocentric robotic radiation therapy platform (CyberKnife; Accuray, Sunnyvale, CA) capable of producing rapid fall-off dose gradients with submillimeter accuracy in dose delivery (28, 29). Three or four prostate fiducials were tracked in real time, with automatic correction for translational and rotational target motion. Treatment was completed over a period of 10 to 14 days. Retrospective assessment of genitourinary and gastrointestinal functions was performed using the CTCAE V.5 scale systems at regular intervals during the first 12 months following the beginning of the treatment (end of treatment, 1, 3, 6 month and 1 year).
The blood prostate specific antigen (PSA) levels were measured before SBRT treatment and after the completion of SBRT. The PSA bounce was defined as a PSA circulating level increase of 0.2 ng/mL from the previous level measured, followed by an important decrease.
Results were expressed as median ± standard deviation (SD), mean ± SD or median ± interquartile range when appropriate. One-way analysis of variance (ANOVA) followed by Sidak tests was assessed. Multiple comparison between groups was performed using Graph-Pad Prism 8 program (GraphPad Software, Inc.). A p values ≤ 0.05 was considered statistically significant.
Eighty elderly patients were treated between September 2021 and December 2022 with SBRT. All characteristics of patients and tumors are listed in Table 2. The majority of elderly patients had a prostate cancer classified as T2a and T2b stages and 72.5% of them had a Gleason score established at 3 + 3 and 3 + 4. Furthermore, this study included a majority of low and intermediate-risk patients with 5 patients considered as being at high-risk disease and 15% of patients with a PSA>20 ng/mL. It is worth noting that 58.8% of patients underwent a hormone therapy.
Dosimetric data were collected and listed in Table 3. Results show selected dose-volume histogram parameters for the rectum, the bladder and target volumes. This table also indicates the CTV volume.
The typical dose distribution for radiotherapy treatment of prostate patients are represented in Figure 1 with the axial (Figure 1A), sagittal (Figure 1B) and coronal (Figure 1C) views. The typical Dose-Volume Histogram is represented on Figure 1D as well as the corresponding dosimetric validation table (Figure 1E).
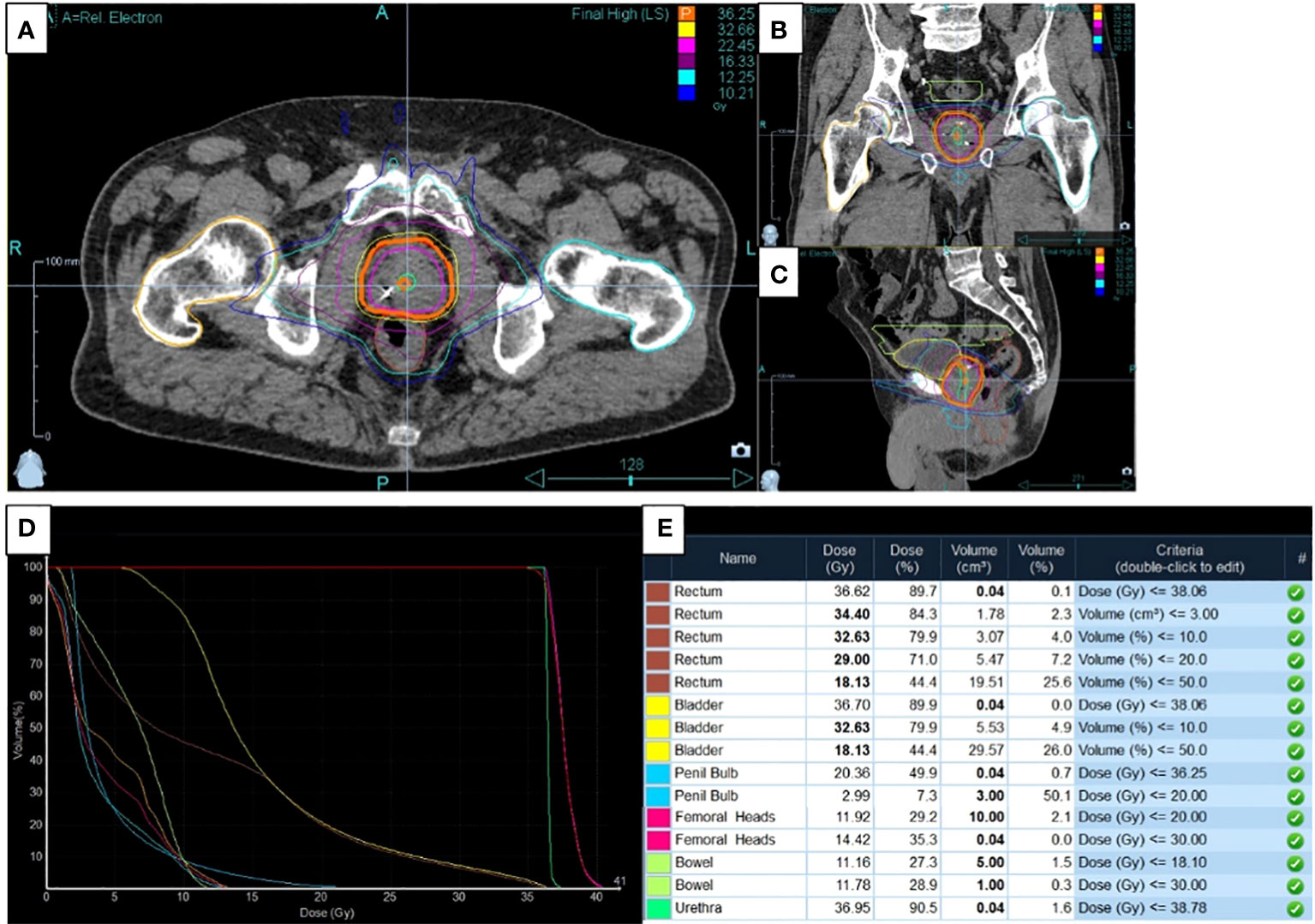
Figure 1 Typical dose distribution, dose-volume and dosimetric data for application of radiotherapy treatment on prostate cancer. (A) The axial, (B) sagittal and (C) coronal views were presented concerning dose distribution as well as (D) the dose-Volume Histogram and (E) the corresponding dosimetric validation table.
Toxicity induced by SBRT was assessed by gathering patients’ feedback (Figure 2). The reported toxicity, as measured on the CTCAE V.5 scale, was low; the gastrointestinal and the genitourinary grade 2 toxicity occurrence after treatment was 5% (Figure 2A) and 11.25%, respectively (Figure 2B). Data indicated that genitourinary toxicity became more significant over time than gastrointestinal toxicity. Moreover, two patients reported a grade 3 genitourinary toxicity at the vesical globe level.
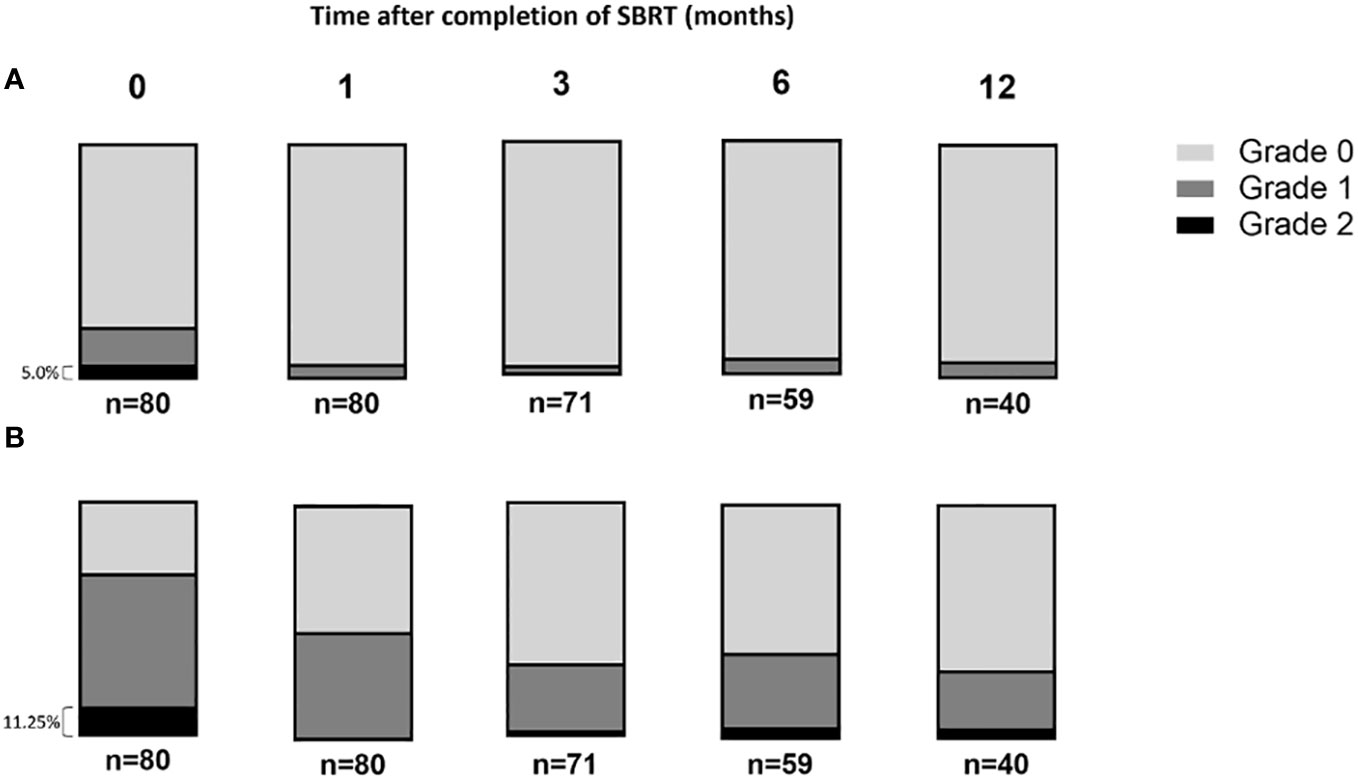
Figure 2 The rate of acute gastrointestinal and genitourinary toxicities after prostate stereotactic body radiotherapy scored following Radiation Therapy Oncology Group score. (A) The gastrointestinal and (B) genitourinary toxicities were presented overtime after the completion of SBRT protocol.
The genitourinary toxicity grades were determined by gathering patients’ feedback over time following SBRT treatment. As shown in Figure 2, most of the toxicities reported by the patients were genitourinary. Therefore, we analyzed the dose-volume data to investigate whether these toxicities could be predicted and correlated with CTV, PTV, and bladder volume, as well as the doses received by the bladder (Figure 3). No difference was observed between groups of grades 0, 1 or 2 regarding the prostate CTV, PTV (Figures 3A, B) and bladder volume (Figure 3C), one week after the end of SBRT.
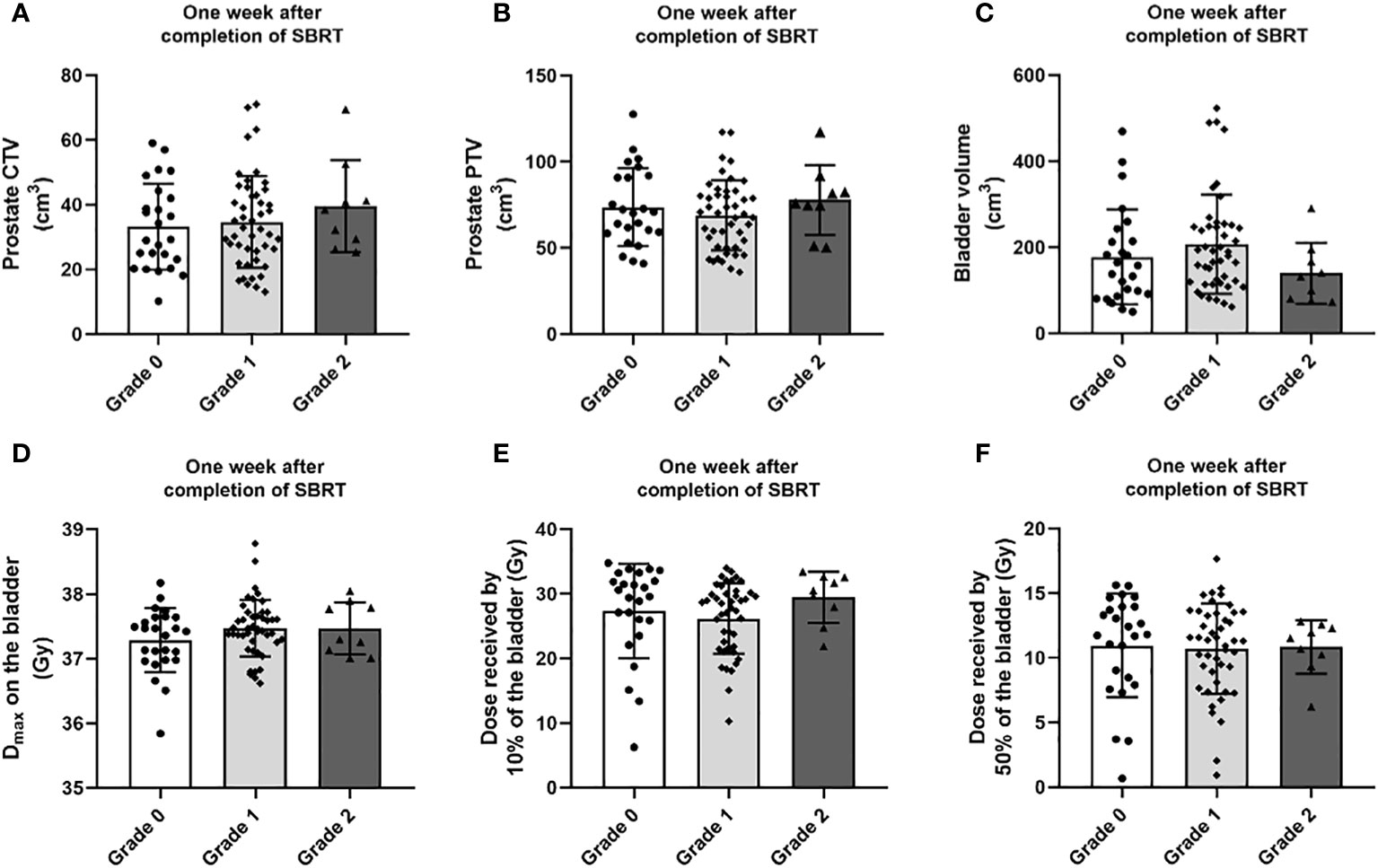
Figure 3 Distribution of (A) CTV, (B) PTV, (C) bladder volume, (D) maximum dose (dose in a volume of 0.035 cc) to the bladder, (E) the dose received by 10% of the bladder volume and (F) the dose received by 50% of the bladder volume by genitourinary toxicity grade occurring one week after the end of the SBRT treatment. Data are expressed as mean ± SD.
On this cohort, we did not observe any correlation between the toxicity recorded 1 week after the end of SBRT and the doses received by the bladder (Figures 3D-F).
All elderly patients selected for the present study were included in the post-treatment analysis of prostate specific antigen. The data presented in Table 2 shows that 58.8% of patients had concomitant androgen deprivation therapy. The PSA levels quantified before the start of treatment were 19.03 ± 39.69 within a range of [0.230 – 266.00] for 80 patients.
The median follow-up time was 12 months and we observed a gradual decline of the median PSA level over time (Figure 4). Indeed, the 6 months post-treatment median PSA has dropped to 0.33 ng/ml. At 6 months after treatment, 20% of patients exhibited a temporary rise in PSA levels, followed by a subsequent decrease to the previous levels. However, PSA outcomes with such a short follow-up period should be interpreted with caution and will need to be reassessed when the median follow-up period approaches 5 years.
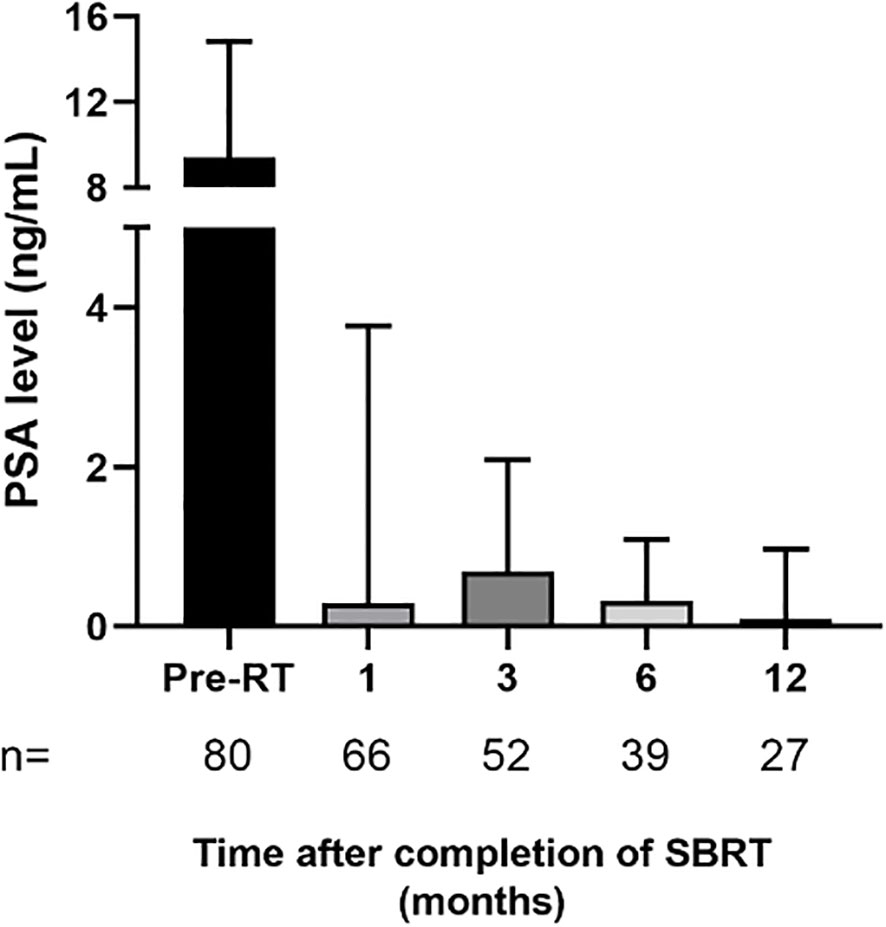
Figure 4 Patient level of prostate-specific antigen (PSA) after completion of prostate stereotactic body radiotherapy. Data are expressed as median ± interquartile range. The number of patients corresponding to PSA levels quantified at each period is specified on figure histogram. Abbreviations: pre-RT = pre-radiotherapy..
The aim of this retrospective study was to report the toxicity data collected from 80 elderly patients with PCa and treated with the SBRT technique using the Cyberknife system. No instances of biochemical or clinical recurrence, distant metastasis occurrence or death were observed during the follow-up period. Acute gastrointestinal and genitourinary toxicities observed in this study were not correlated with the calculated dose levels received by the bladder or the rectum. Two patients experienced grade 3 toxicity level during the SBRT treatment, which led to the interruption of their treatment.
Importantly, geriatric conditions may affect response to radiation and studies reported that comprehensive geriatric assessment (CGA) can help to predict the occurrence of acute radiation-induced toxicities for patients treated for PCa or Head and neck cancers (30–32).
In the present study, we did not perform a CGA before SBRT, which represents a limitation of our study. Recently, studies tried to predict tolerance of radiotherapy by proceeding to CGA to identify predictors of reduced Quality of Life (QoL) and occurrence of toxicities. Nevertheless, some studies on cohorts of prostate cancer elderly patients using conventional or hypofractionated radiotherapy demonstrated the lack of sensitivity of CGA outcome and did not find predictive factors to determine toxicities or impaired QoL following radiotherapy (33–35). Indeed, screening tools need to be more experienced.
PCa incidence risk increases with age and it seems crucial to pay attention to acute and or late radiation-induced toxicities for elderly patients after the completion of their radiotherapy protocol. However, literature data about the side effects of radiotherapy are more associated with protocols using EBRT than those using SBRT and the level of evidence in older patients is limited. Thus, we chose to focus on SBRT-induced toxicities.
Currently, SBRT technology is a new technique that presents a significant benefit for PCa treatment. As demonstrated in literature, results of SBRT are encouraging, supporting its use for PCa treatment (36, 37).
In fact, several phase III randomized non-inferiority trials have mentioned that SBRT allowed tumor control without providing serious toxicities (13, 38). Our study demonstrated that SBRT was well tolerated in elderly patients; however, a longer follow-up period would be necessary to assess the real effect of the treatment. Acute grade 2 genitourinary toxicity was reported for 21 patients (13.6%). The frequency of acute genitourinary toxicity reported in previous Phase II or III studies was within a range of 20.2 to 35.3%. Thus, the frequency of these toxicities found out in our study may seem low compared to literature data (13, 38, 39). This difference could be explained by our strict adherence to the bladder dose constraints recommended by RTOG, in our treatment plans. Similarly, our study found a lower incidence of gastrointestinal toxicities compared to the levels commonly reported in the literature for prostate cancer patients undergoing SBRT. In fact, the 1-year cumulative incidence rate of grade 2 gastrointestinal toxicities reached 4% compared to 14% in other studies (40–42).
Interestingly, it has been demonstrated that moderate hypofractionated RT by helical tomotherapy used to treat patients aged ≥ 75 years with localized prostate cancer, induced acceptable acute and late toxicity. As observed in our study using extreme hypofractionated RT, Cuccia et al. did not observe a significant difference in urinary and bowel function of patients being treated by moderate hypofractionated RT (43).
Moreover, we did not observe any post-treatment grade 3 toxicity in our patients, unlike other studies which reported a toxicity of grade ≥ 3 for 1 to 2% of patients (37, 44). This low frequency of gastrointestinal toxicities could be also explained by the strict compliance with the dose constraints to the organs at risk. The low levels of toxicity in our study may be also attributed to other factors. First of all, several publications have demonstrated that image guided radiotherapy is associated with a lower level of genitourinary and gastrointestinal toxicities compared to non-image-guided radiotherapy. This may be attributed to the smaller positioning errors, thereby avoiding unnecessary irradiation of the healthy surrounding organs. Moreover, the image guidance allows for the reduction of radiotherapy planning margins, resulting in delivering lower doses to the normal tissue (45–47). In addition, the dose fall-off resulting from the use of multiple noncoplanar beams produced by the Cyberknife is sharper than in conventional techniques. Besides, the difference in the alpha/beta ratios between prostate and rectum may have helped to improve the therapeutic balance in our favor. In fact, we may be able to achieve the same cure rates with lower toxicity to the rectum which has a lower fractionation sensitivity compared to prostate cancer cells.
Biochemical response rates for prostate SBRT have been published in several trials, the largest being a trial with a cohort of 1100 patients treated within eight independent US institutions using similar protocols with doses ranging from 35 to 40 Gy delivered in 5 fractions (40).
The biochemical response rate at 5-year follow-up was 95.2% for low risk and 84.1% for intermediate risk patients (including Gleason 4 + 3) and 86% of patients did not receive androgen deprivation therapy. The authors noted that out of a total cohort of 49 patients who met the criteria for biochemical failure, 9 patients experienced a mild PSA rebound but remained biochemically controlled. A PSA rebound is a recognized but poorly understood phenomenon occurring after prostate irradiation, and is observed in 20% of the patients in our study. It can persist for several years after SBRT treatment (48).
In this context, it is important for radiation oncologists to be aware of this phenomenon to avoid subjecting patients to unnecessary examinations.
Our study was also limited by its retrospective design and low sampling. Few patients had associated comorbidities, such as diabetes or a history of cardiovascular disease requiring supplemental medication. So, we could not include the confounding factors in our study. It should be noted that this study only presents preliminary results. In particular, an important aspect to consider would be the evaluation of late toxicities, such as hematuria and rectal hemorrhage which are commonly observed within 2 years following SBRT treatment. Therefore, the short-term findings reported in our study should be interpreted with caution. A longer follow-up is necessary, especially to assess treatment effectiveness and late toxicities.
The retrospective results obtained from this cohort showed that SBRT treatment for PCa in elderly patients is well tolerated and provides an early biochemical response and good efficacy over a period of one year for patients from Reunion Island. This is in line with the data from randomized trials such as PACE B and HYPO-RT trials, which showed the benefit of SBRT for men with low- and intermediate-risk prostate cancer. Although the treatment is generally well-tolerated by the patients, the occurrence of gastrointestinal and genitourinary toxicities remains a significant problem.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This retrospective clinical study was evaluated and approved bythe Ethics Committee of Research at the University of Montpellier on 09 May 2023 (UM 2023-013). All data collected were obtained during routine clinical practice and all authors conducted this study by respecting the ethical standards of their respective institutions as laid down in the 1964 Declaration of Helsinki. All patients were informed about the use of their individual personal data and gave their consent to participate in the present study.
YS: Writing – original draft, Data curation, Methodology, Supervision. GB: Validation, Writing – review & editing. AA: Methodology, Writing – original draft, Formal analysis, Resources. MiB: Methodology, Writing – original draft. OM: Validation, Writing – review & editing. RS: Methodology, Writing – review & editing. RoC: Conceptualization, Writing – original draft. RaC: Data curation, Writing – review & editing. PL: Writing – original draft, Data curation. HS: Methodology, Writing – review & editing. MeB: Data curation, Writing – review & editing. OB: Data curation, Writing – original draft. NN: Writing – original draft. FD: Writing – original draft, Data curation, Methodology, Supervision.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CGA, comprehensive geriatric assessment; CTCAE, Common Terminology Criteria for Adverse Events; CTV, Clinical Target Volume; EBRT, External Beam Radiation Therapy; NCCN, National Comprehensive Cancer Network; OAR, Organ at risk; PCa, Prostate Cancer; PSA, Prostate Specific Antigen; PTV, Planning Target Volume; QoL, Quality of Life; RTOG, Radiation Therapy Oncology Group; SBRT, Stereotactic Body Radiation Therapy.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Mohler JL, Higano CS, Pugh TJ. Prostate Cancer, Version 4.2019, NCCN Clinical Practice Guidelines in Oncology. NCCN (2019). Available at: https://www.nccn.org.
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
5. Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975-2018. Natl Cancer Inst (2021).
6. P. Weiner J, Schwartz D, Shao M, Osborn V, Choi K, Schreiber D. Stereotactic radiotherapy of the prostate: fractionation and utilization in the United States. Radiat Oncol J (2017) 35:137–43. doi: 10.3857/roj.2017.02026
7. Kole TP, Tong M, Wu B, Lei S, Obayomi-Davies O, Chen LN, et al. Late urinary toxicity modeling after stereotactic body radiotherapy (SBRT) in the definitive treatment of localized prostate cancer. Acta Oncol (2016) 55:52–8. doi: 10.3109/0284186X.2015.1037011
8. Helou J, D’Alimonte L, Quon H, Deabreu A, Commisso K, Cheung P, et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: Is there an optimal dose? Radiother Oncol (2017) 123:478–82. doi: 10.1016/j.radonc.2017.03.006
9. Zhang L, Johnson J, Gottschalk AR, Chang AJ, Hsu I-C, Roach M, et al. Receiver operating curves and dose-volume analysis of late toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol (2017) 7:e109–16. doi: 10.1016/j.prro.2016.07.004
10. Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, et al. Stereotactic body radiation therapy for localized prostate cancer: A systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol (2019) 104:778–89. doi: 10.1016/j.ijrobp.2019.03.051
11. Miszczyk L, Napieralska A, Namysł-Kaletka A, Głowacki G, Grabińska K, Woźniak G, et al. CyberKnife-based prostate cancer patient radioablation – early results of irradiation in 200 patients. Cent Eur J Urol (2015) 68:289–95. doi: 10.5173/ceju.2015.582
12. Ito M, Yoshioka Y, Takase Y, Suzuki J, Matsunaga T, Takahashi H, et al. Stereotactic body radiation therapy for Japanese patients with localized prostate cancer: 2-year results and predictive factors for acute genitourinary toxicities. Jpn J Clin Oncol (2021) 51:1253–60. doi: 10.1093/jjco/hyab094
13. Tree AC, Ostler P, van der Voet H, Chu W, Loblaw A, Ford D, et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol (2022) 23:1308–20. doi: 10.1016/S1470-2045(22)00517-4
14. Proust-Lima C, Taylor JMG, Sécher S, Sandler H, Kestin L, Pickles T, et al. Confirmation of a low α/β Ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol (2011) 79:195–201. doi: 10.1016/j.ijrobp.2009.10.008
15. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) gy. Int J Radiat Oncol (2012) 82:e17–24. doi: 10.1016/j.ijrobp.2010.10.075
16. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited – an analysis of clinical results from 14 168 patients. Acta Oncol (2012) 51:963–74. doi: 10.3109/0284186X.2012.719635
17. Cosset J-M, Chargari C, Créhange G. Quel rapport alpha/bêta pour le cancer prostatique en 2019? Cancer/Radiothérapie (2019) 23:342–5. doi: 10.1016/j.canrad.2019.01.004
18. Gomez-Millan J. Radiation therapy in the elderly: More side effects and complications? Crit Rev Oncol Hematol (2009) 71:70–8. doi: 10.1016/j.critrevonc.2008.11.004
19. Pragnahitha N, Rao AS. Radiation tolerance in geriatric patients: A retrospective observational study. J Radiat Cancer Res (2023) 14:76. doi: 10.4103/jrcr.jrcr_92_22
20. Suzuki T, Saito M, Onishi H, Mochizuki K, Satani K, Yamazaki A, et al. Comparison of CT artifacts and image recognition of various fiducial markers including two types of thinner fiducial markers for CyberKnife treatment. Rep Pract Oncol Radiother (2020) 25:117–24. doi: 10.1016/j.rpor.2019.12.005
21. Marsico M, Gabbani T, Livi L, Biagini MR, Galli A. Therapeutic usability of two different fiducial gold markers for robotic stereotactic radiosurgery of liver Malignancies: A pilot study. World J Hepatol (2016) 8:731. doi: 10.4254/wjh.v8.i17.731
22. Zakian KL, Wibmer A, Vargas HA, Alberts E, Kadbi M, Mychalczak B, et al. Comparison of motion-insensitive T2-weighted MRI pulse sequences for visualization of the prostatic urethra during MR simulation. Pract Radiat Oncol (2019) 9:e534–40. doi: 10.1016/j.prro.2019.06.009
23. Kataria T, Gupta D, Goyal S, Bisht SS, Chaudhary R, Narang K, et al. Simple diagrammatic method to delineate male urethra in prostate cancer radiotherapy: an MRI based approach. Br J Radiol (2016) 89:20160348. doi: 10.1259/bjr.20160348
24. Salembier C, Villeirs G, De Bari B, Hoskin P, Pieters BR, Van Vulpen M, et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol (2018) 127:49–61. doi: 10.1016/j.radonc.2018.01.014
25. Gay HA, Barthold HJ, O’Meara E, Bosch WR, El Naqa I, Al-Lozi R, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol (2012) 83:e353–62. doi: 10.1016/j.ijrobp.2012.01.023
26. Song C, Kang T, Lee M, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: A multi-institutional update. Korean J Urol (2007) 48:125. doi: 10.4111/kju.2007.48.2.125
27. Goldsmith C, Green MM, Middleton B, Cowley I, Robinson A, Plowman NP, et al. Evaluation of cyberKnife® Fiducial tracking limitations to assist targeting accuracy: A phantom study with fiducial displacement. Cureus (2018) 10:e3523. doi: 10.7759/cureus.3523
28. Xie Y, Djajaputra D, King CR, Hossain S, Ma L, Xing L. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol (2008) 72:236–46. doi: 10.1016/j.ijrobp.2008.04.051
29. Gao J, Xu B, Lin Y, Xu Z, Huang M, Li X, et al. Stereotactic body radiotherapy boost with the cyberKnife for locally advanced cervical cancer: dosimetric analysis and potential clinical benefits. Cancers (2022) 14:5166. doi: 10.3390/cancers14205166
30. Paal K, Stranz B, Thurner E-M, Langsenlehner U, Renner W, Brunner TB, et al. Comprehensive geriatric assessment predicts radiation-induced acute toxicity in prostate cancer patients. Strahlenther Onkol (2023). doi: 10.1007/s00066-023-02132-3
31. de Vries J, Poelman A, Sidorenkov G, Festen S, de Bock GH, Langendijk JA, et al. The association of frailty and outcomes of geriatric assessment with acute radiation-induced toxicity in patients with head and neck cancer. Oral Oncol (2022) 130:105933. doi: 10.1016/j.oraloncology.2022.105933
32. VanderWalde NA, Deal AM, Comitz E, Stravers L, Muss H, Reeve B, et al. Comprehensive geriatric assessment as a predictor of tolerance, quality of life, and toxicity in older patients receiving radiation. Int J Radiat Oncol Biol Phys (2016) 94:885. doi: 10.1016/j.ijrobp.2015.12.070
33. Osborne GEC, Appleyard SA, Gilbert DC, Jones CI, Lorimer C, Villanueva M, et al. Comprehensive geriatric assessment in men aged 70 years or older with localised prostate cancer undergoing radical radiotherapy. Clin Oncol (2017) 29:609–16. doi: 10.1016/j.clon.2017.05.003
34. Goineau A, Campion L, Commer J-M, Vié B, Ghesquière A, Béra G, et al. Can comprehensive geriatric assessment predict tolerance of radiotherapy for localized prostate cancer in men aged 75 years or older? Cancers (2020) 12:635. doi: 10.3390/cancers12030635
35. Goineau A, Campion L, d’Aillières B, Vié B, Ghesquière A, Béra G, et al. Comprehensive Geriatric Assessment and quality of life after localized prostate cancer radiotherapy in elderly patients. PloS One (2018) 13:e0194173. doi: 10.1371/journal.pone.0194173
36. King CR, Brooks JD, Gill H, Presti JC. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol (2012) 82:877–82. doi: 10.1016/j.ijrobp.2010.11.054
37. Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open (2019) 2:e188006. doi: 10.1001/jamanetworkopen.2018.8006
38. Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet (2019) 394:385–95. doi: 10.1016/S0140-6736(19)31131-6
39. Fuller DB, Falchook AD, Crabtree T, Kane BL, Medbery CA, Underhill K, et al. Phase 2 multicenter trial of heterogeneous-dosing stereotactic body radiotherapy for low- and intermediate-risk prostate cancer: 5-year outcomes. Eur Urol Oncol (2018) 1:540–7. doi: 10.1016/j.euo.2018.06.013
40. King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol (2013) 109:217–21. doi: 10.1016/j.radonc.2013.08.030
41. Thomas TO, Hasan S, Small W, Herman JM, Lock M, Kim EY, et al. The tolerance of gastrointestinal organs to stereotactic body radiation therapy: what do we know so far? J Gastrointest Oncol (2014) 5:236–46. doi: 10.3978/j.issn.2078-6891.2014.024
42. Tree AC, Ostler P, Hoskin P, Dankulchai P, Nariyangadu P, Hughes RJ, et al. Prostate stereotactic body radiotherapy — First UK experience. Clin Oncol (2014) 26:757–61. doi: 10.1016/j.clon.2014.08.007
43. Cuccia F, Fiorentino A, Corrao S, Mortellaro G, Valenti V, Tripoli A, et al. Moderate hypofractionated helical tomotherapy for prostate cancer in a cohort of older patients: a mono-institutional report of toxicity and clinical outcomes. Aging Clin Exp Res (2020) 32:747–53. doi: 10.1007/s40520-019-01243-1
44. Musunuru HB, Quon H, Davidson M, Cheung P, Zhang L, D’Alimonte L, et al. Dose-escalation of five-fraction SABR in prostate cancer: Toxicity comparison of two prospective trials. Radiother Oncol (2016) 118:112–7. doi: 10.1016/j.radonc.2015.12.020
45. Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol (2012) 84:125–9. doi: 10.1016/j.ijrobp.2011.11.047
46. Park SS, Yan D, McGrath S, Dilworth JT, Liang J, Ye H, et al. Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: clinical evidence. Int J Radiat Oncol (2012) 83:947–52. doi: 10.1016/j.ijrobp.2011.08.025
47. Kok D, Gill S, Bressel M, Byrne K, Kron T, Fox C, et al. Late toxicity and biochemical control in 554 prostate cancer patients treated with and without dose escalated image guided radiotherapy. Radiother Oncol (2013) 107:140–6. doi: 10.1016/j.radonc.2013.04.007
Keywords: prostate cancer, elderly patients, stereotactic body radiotherapy, gastrointestinal toxicity, genitourinary toxicity
Citation: Slama Y, Baumont G, Arcambal A, Begue M, Maillot O, Sayah R, Castanet R, Caboche R, Liberati P, Slaoui H, Bouaziz M, Borson O, Nguyen NP and Dutheil F (2024) Retrospective study on the toxicity induced by stereotactic body radiotherapy: overview of the reunion experience on prostate cancer in elderly patients. Front. Oncol. 14:1302001. doi: 10.3389/fonc.2024.1302001
Received: 25 September 2023; Accepted: 17 January 2024;
Published: 01 February 2024.
Edited by:
Benyi Li, University of Kansas Medical Center, United StatesReviewed by:
Siyuan Cheng, Louisiana State University Health Shreveport, United StatesCopyright © 2024 Slama, Baumont, Arcambal, Begue, Maillot, Sayah, Castanet, Caboche, Liberati, Slaoui, Bouaziz, Borson, Nguyen and Dutheil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssef Slama, eW91c3NlZi5zbGFtYUBjbGluaWZ1dHVyLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.