- 1Beijing University of Chinese Medicine, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 3The Second School of Clinical Medicine, Beijing University of Chinese Medicine, Beijing, China
- 4Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 5Department of Acupuncture and Moxibustion, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
Background: Notwithstanding the acknowledged interplay between atrial fibrillation (AF) and the emergence of digestive system neoplasms, the intricacies of this relationship remain ambiguous. By capitalizing univariable Mendelian Randomization (MR) complemented by a mediated MR tactic, our pursuit was to elucidate the causative roles of AF in precipitating digestive system malignancies and potential intermediary pathways.
Method: This research endeavor seeks to scrutinize the causal clinical implications of whether genetic predispositions to AF correlate with an increased risk of digestive system malignancies, employing MR analytical techniques. Utilizing a dataset amalgamated from six studies related to AF, encompassing over 1,000,000 subjects, we performed univariable MR assessments, employing the random-effects inverse-variance weighted (IVW) methodology as our principal analytical paradigm. Subsequently, a mediated MR framework was employed to probe the potential mediating influence of AF on the nexus between hypertension (HT), heart failure (HF), ischemic stroke (IS), coronary artery disease (CAD), and digestive system neoplasms.
Result: The univariable MR evaluation unveiled a notable causal nexus between the genetic inclination toward AF and the genetic susceptibility to colon, esophageal, and small intestine malignancies. The mediated MR scrutiny ascertained that the genetic inclination for AF amplifies the risk profile for colon cancer via IS pathways and partially explains the susceptibility to esophageal and small intestine tumors through the HF pathway.
Conclusion: Our investigative endeavor has highlighted a definitive causative association between genetic inclination to AF and specific digestive system neoplasms, spotlighting IS and HF as instrumental mediators. Such revelations furnish pivotal perspectives on the complex genetic interconnections between cardiovascular anomalies and certain digestive tract tumors, emphasizing prospective therapeutic and diagnostic worthy of pursuit.
Introduction
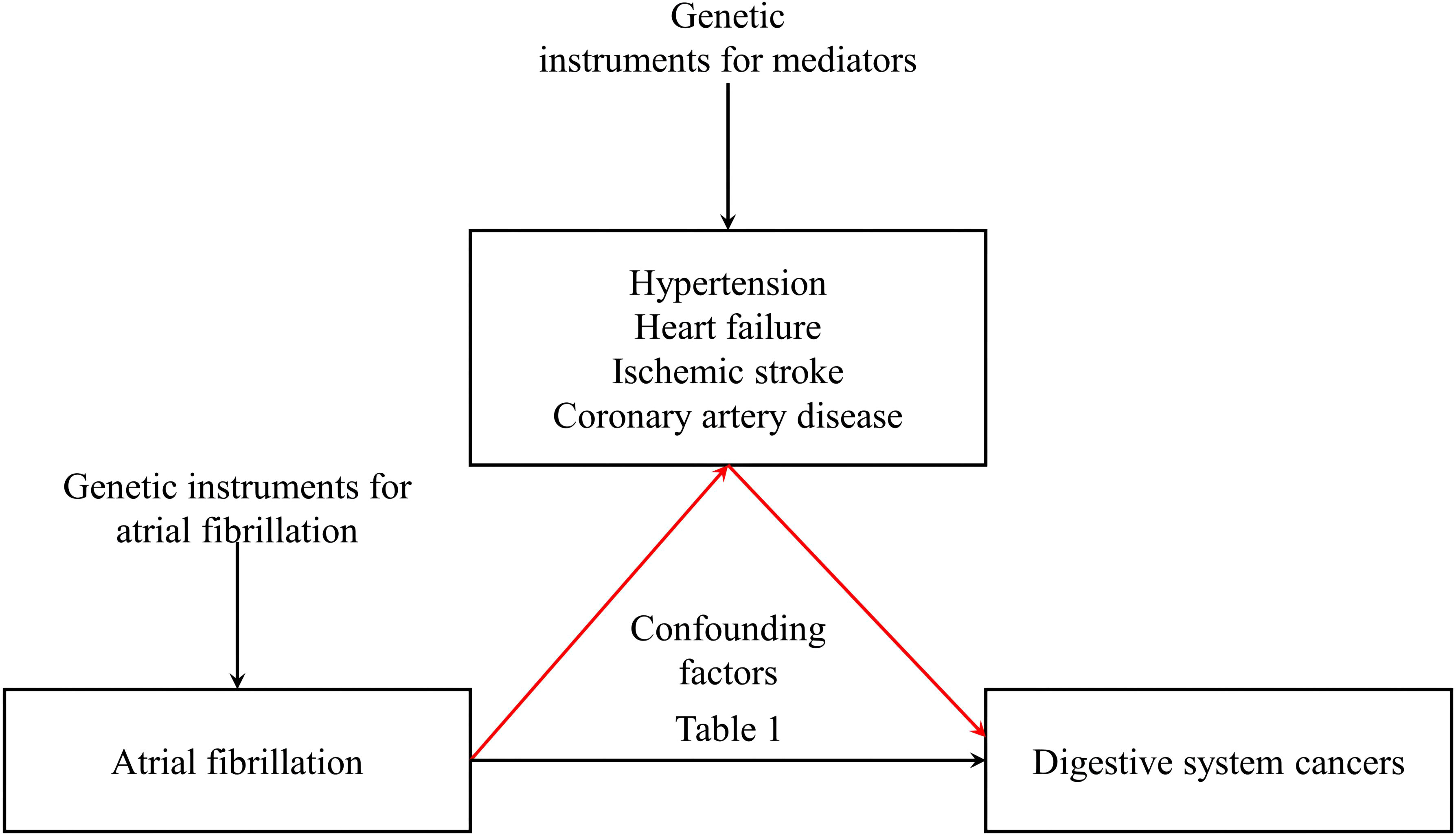
Atrial fibrillation (AF), the predominant cardiac dysrhythmia, fluctuates between symptomatic and asymptomatic manifestations. Given its escalating prevalence, there is a critical need for efficacious prophylactic interventions (1, 2). The sheer breadth of the afflicted demographic poses a substantial challenge to public health, underscored by the multifaceted sequela of AF—including ischemic stroke (IS), heart failure (HF), coronary artery disease (CAD), cognitive declination, and heightened mortality (3, 4). Intriguingly, an AF diagnosis heralds an augmented risk of malignancy (5). Cancer incidence in AF patients outstrips that of the broader populace by a staggering 30–40% (6). Numerous investigations posit that AF’s onset may intimate concealed neoplasms, especially pronounced within the initial trimester post-diagnosis. A Danish longitudinal study intimated that nascent AF diagnoses correlate with a magnified cancer proclivity within the inaugural three months (7). Scrutiny of the Women’s Health Study echoed this sentiment, identifying a surge in oncological susceptibility post-AF diagnosis, notably acute within its early stages. In alignment with these observations, an investigation based on the Danish Diet, Cancer, and Health study identified an elevated cancer risk—across genders—within the initial 90 days following AF diagnosis, notably with a prominence in colorectal malignancies that persists throughout prolonged observational periods (8, 9). While prior observational analyses corroborate AF’s association with heightened digestive system cancer risks, the causal nexus remains enigmatic. Concomitantly, AF frequently coexists with other cardiovascular pathologies, including HT, HF, IS, and CAD (10, 11). A retrospective analysis involving 332,555 AF patients without prior cancer histories unveiled notable rates of comorbidities: 68.5% with HT, 41.1% with HF, and 34.9% with a history of IS (6). These coexisting cardiovascular maladies are not mere bystanders; they are implicated as risk catalysts for diverse cancers. Consequently, we hypothesize that cardiovascular afflictions, spanning HT, HF, IS, and CAD, could potentially bridge the nexus between atrial fibrillation and digestive system malignancies.
Within the complex framework of genetic analyses, MR arises as a sophisticated methodology, grounded in genetic variants, providing a means to elucidate causal relationships under specified principles (12, 13). Traditionally, MR investigates the relationships between distinct exposures and their resulting outcomes in depth (14–16). The two-step MR employs an innovative strategy, grounded in the established MR framework, augmenting the accuracy of causal mediation understanding. Through this approach, one can delineate the causal impact of an exposure on an outcome, either in isolation (direct effect) or mediated via an intermediary (indirect effect) (17, 18). Notably, the two-step Mendelian randomization (MR) approach eliminates the necessity for detailed individual-level data, opting instead for the utilization of genome-wide association studies (GWAS) summary statistics. These statistics are frequently derived from extensive population samples, encompassing a diverse range of traits and phenotypes (19).
In our scholarly pursuit, human genetics were leveraged to hypothesize a causal association between AF and the susceptibility to a septet of digestive system malignancies. This investigation initiated an exhaustive exploration of traits associated with AF. Subsequently, causal mediation analyses, supported by the two-step MR approach, were rigorously employed to elucidate the potential intermediary roles of HT, HF, IS, and CAD in the causative linkage with these digestive system malignancies.
Materials and methods
Study design
This investigation commenced with summary-level data obtained from GWAS focusing on traits associated with AF (20). Extending previous epidemiological inquiries, we performed a two-sample MR analysis concerning atrial fibrillation and malignancies of the digestive system. Subsequently, through the robust framework of two-step MR, causal mediation analyses were utilized to shed light on the potential intermediary roles of HT, HF, IS, and CAD in establishing a causative connection with these gastrointestinal neoplasms. (refer to Figure 1) This MR investigation adheres to the subsequent three foundational assumptions: 1. Pertinence: Demonstrating a noteworthy association with the exposure in question at a significance level of p < 5 × 10−8, the genetic variant maintains pertinence. 2. Independence: The genetic variant persists independently, showing no association with any confounding factors that could potentially distort the exposure-outcome relationship, as evidenced by the horizontal pleiotropy test. 3. Restriction of Exclusion: Exerting influence on the outcome exclusively through its potential ramifications on the exposure under examination, the genetic variant has been verified by consulting PhenoScanner V2.
Data sources
(1) Exposure GWAS
We utilized genome-wide summary statistics from a published meta-analysis on AF, which included six study cohorts (20). The Nord-Trøndelag Health Study (HUNT) is an enduring, population-based health survey, steadfastly conducted in Norway since 1984 (21). The deCODE study encompasses all patients diagnosed with atrial fibrillation at Landspitali from 1987 to 2015, encapsulating the entirety of the Icelandic atrial fibrillation populace. The Michigan Genomics Initiative represents a hospital-based cohort accrued in the United States. The DiscovEHR incorporates a hospital-based assemblage of European lineage in the United States (22). The UKBB is a population-based cohort in the United Kingdom (23). The AFGen Consortium proffers atrial fibrillation association summary statistics derived from 31 cohorts. In total, these studies represent a significant cohort of 60,620 cases and 970,216 controls (24).
(2) Mediator GWAS
The CAD and HF data were from the archives of the UK Biobank (UKBB), including 34,541 cases and 261,984 controls for CAD (25), 1,405 cases, and 359,789 controls for HF. The IS data was from a genome-wide association study comprising 7,193 cases and 406,111 controls (26) and HT data was from the International Consortium of Blood Pressure comprising 757,601 individuals (27).
(3) Outcome GWAS
The FinnGen consortium constitutes a research initiative that aggregates health and genetic data derived from Finnish health registries (28). It operates as a public–private partnership research project, amalgamating imputed genotype data obtained from both newly collected and legacy samples sourced from Finnish biobanks, alongside digital health record information sourced from Finnish health registries (https://www.finngen.fi/en). The project harnesses data from the nationwide longitudinal health register, spanning data collection since 1969 and encompassing every resident in Finland (29). These 7 digestive system cancers were from cancer register (ICD-O-3) of FinnGen studies (29): the case group comprised 126 cases of cancers in the lip, oral cavity, and pharynx, 232 cases of esophageal cancers, 633 cases of stomach cancers, 605 cases of pancreas cancers, 252 cases of small intestinal cancers, 1803 cases of colorectal cancers, and 1078 cases of rectal cancers. The control group for all the aforementioned cancers consisted of 174,006 individuals. It is imperative to note that the current analysis harnesses exclusively publicly accessible summary statistics, thus circumventing the necessity for additional ethical consent.
Selection of IVs for MR analyses
In this investigation, we scrutinized the following traits: AF, CAD, HF, IS, and HT. MR analyses were conducted among individuals of European descent, employing genome-wide significance (p < 5 × 10−8) and linkage disequilibrium (R2 <0.1) subsequent to standard exclusions (20). These exclusions encompass instances of withdrawn consent, suspected sex chromosome aneuploidy, and discrepancies between genetically inferred and self-reported gender (30). Appropriate instrumental variables (IVs) for the MR evaluations were culled from disparate GWAS summary findings. Single-nucleotide polymorphisms (SNPs) that met the rigorous criterion of genome-wide significance (p < 5 × 10−8) were selected during the initial phase. Subsequently, relevant SNPs were retained based on the linkage disequilibrium criterion, stipulated by an R² < 0.1 according to the Genome reference panel (31). SNPs exhibiting an association with the outcome variables at a significance level of p < 5 × 10−8 were methodically excluded from consideration. IVs signifying correlations with AF, CAD, HF, IS, and HT-associated characteristics, meeting conventional GWAS thresholds (P < 5 × 10−8), were selected for each respective phenotype, refer to Figure 2. A genetic instrument embodies one or numerous genetic variances imbued with attributes conducive to their utilization as an IV within the purview of MR (12). Throughout the harmonization process encompassing both exposure and outcome data sets, palindromic SNPs and those devoid of requisite information were removed. The robustness of the IVs was evaluated via the computation of F-statistics, with values beneath the threshold of 10 indicating an inherently weak instrument strength, thereby necessitating their removal from the analysis (32, 33).
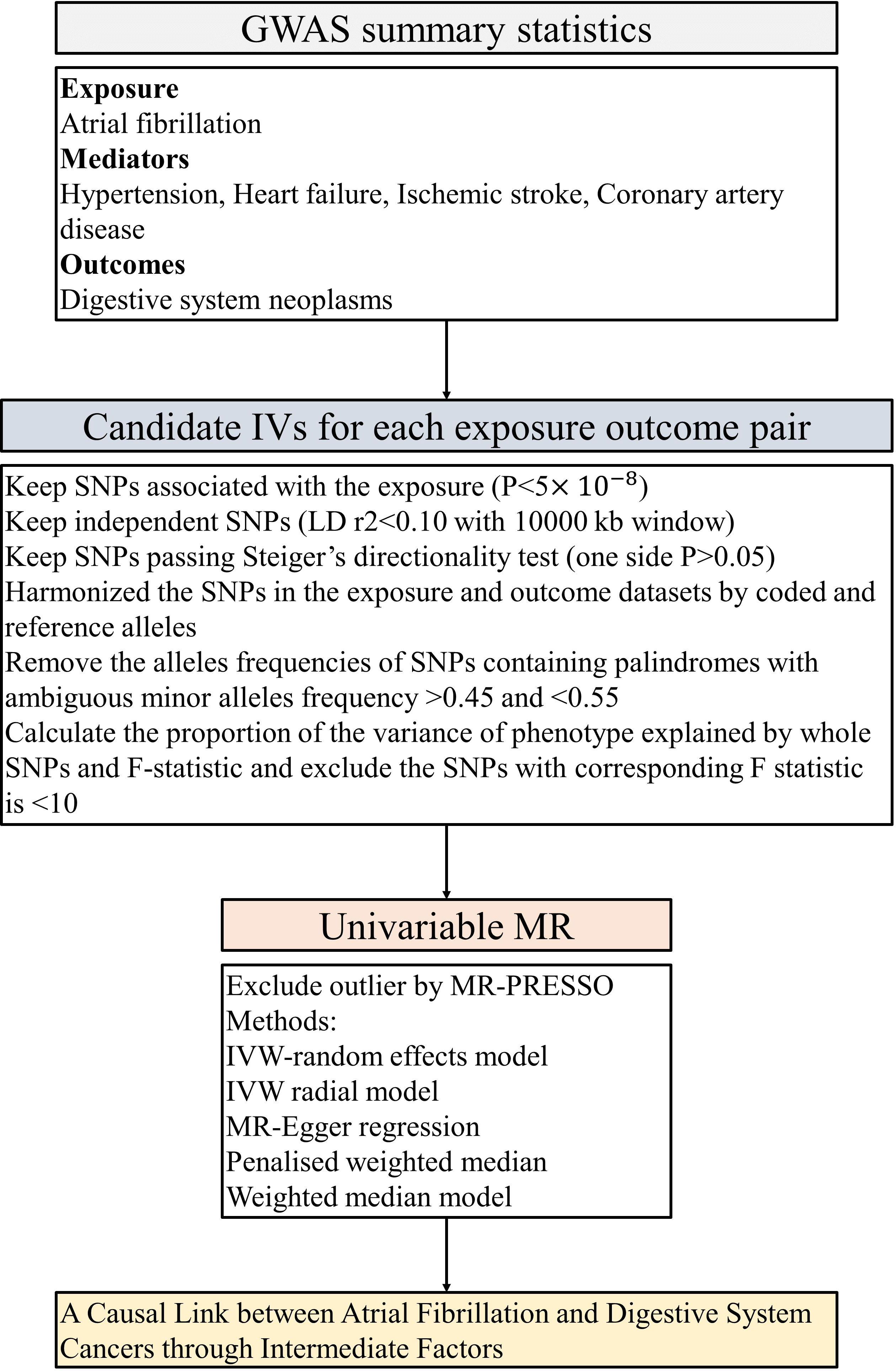
Figure 2 The flow chart of the inclusion and exclusion criterion of candidate SNPs for each exposure-outcome pair. GWAS, genome-wide association studies; LID, Linkage disequilibrium; IVW, inverse-variance weighted; PRESSO, Pleiotropy Residual Sum and Outlier; MR, Mendelian randomization.
MR analysis
Initially, we executed univariable MR analyses for AF on 7 digestive system cancers. To amalgamate an aggregate effect estimate across a multitude of genetic instruments, we employed an array of four distinct methodologies: inverse-variance weighted (IVW), the weighted median method, MR-Egger, and weighted mode (34). After this, we sought to tackle the exclusion restriction assumption by employing the MR-Egger regression intercept technique. This method assessed potential biases in causal estimations arising from the complex issue of horizontal pleiotropy, considering its associated 95% confidence interval (CI) (35, 36). Furthermore, we assessed the presence of horizontal pleiotropy using the MR pleiotropy residual sum. Simultaneously, outlier SNPs were identified and removed using the MR-PRESSO outlier test (p < 0.05), thereby ensuring the robustness and integrity of the analysis (37). We conducted an investigation of the pleiotropic implications of each genetic instrument by consulting PhenoScanner V2 to identify secondary phenotypes associated (p < 5 × 10−8) with the IVs or their proxies (r² > 0.8) (38). It is crucial to explicate the importance of incorporating multiple testing as an essential statistical methodology. However, in the context of this article, structured as an exploratory study with the objective of unveiling a diverse range of potential positive associations, and considering the utilization of non-identical SNPs in each two-sample MR study, we have temporarily abstained from implementing multiple testing (39). Acknowledging the significant roles of HT, HF, IS, and CAD as consequential outcomes of AF, we aimed to determine whether the causative impacts of AF on these malignancies pass through these mediators (40). The proportionality of this mediated effect was ascertained by comparing the indirect effect to the overall effect. The mediating proportion was epitomized as the quotient of the indirect to the comprehensive effect, and was deemed “non-causal” should the total and indirect effects diametrically oppose.
We incorporated all GWAS-associated SNPs for each trait into the model and calculated them in four ways. We demarcated causal estimates for binary outcomes, complemented by p-values for each distinct methodology, β coefficients, and their corresponding standard errors. It is quintessential to acknowledge that all p-values were ascertained by employing a two-tailed paradigm.
Sensitivity analyses
In the field of sensitivity analyses, we performed univariable MR assessments that were robust to specific manifestations of potential unbalanced horizontal pleiotropy. This involved the utilization of MR-Egger methodologies. In the realm of sensitivity analyses, we engaged in univariable MR evaluations resilient to certain manifestations of potential unbalanced horizontal pleiotropy (41), utilizing weighted median (42), weighted mode (43), and MR-Egger methodologies (35). We conducted a variety of sensitivity analyses, encompassing leave-one-out assessment and single SNP analysis, aimed at determining if an individual SNP was exerting a disproportionate sway on the primary causal relationship under scrutiny (36).
To conduct these analyses, we harnessed the MendelianRandomization and TwoSampleMR packages within the R programming milieu (version 4.2.0, available at www.r-project.org/).
Results
Univariable MR analysis of AF on seven digestive system cancers
The univariable MR analysis elucidated that a genetically deduced susceptibility to AF bears a robust causal correlation with the genetic inclinations for colon cancer (IVW approach: Odds Ratio (OR) [95% CI] = 1.12 [1.00, 1.25], p = 0.043), esophageal cancer (IVW approach: OR [95% CI] = 1.40 [1.07, 1.83], p = 0.013), and small intestine cancer (IVW approach: OR [95% CI] = 1.34 [1.04, 1.74], p = 0.025). However, MR evaluations of the ensuing tetrad of malignancies revealed no pronounced causal ties: neoplasms of the lip, oral cavity, and pharynx (IVW approach: OR [95% CI] = 1.00 [0.70, 1.44], p = 0.981), pancreatic cancer (IVW approach: OR [95% CI] = 1.03 [10.87, 1.23], p = 0.730), rectal cancer (IVW approach: OR [95% CI] = 0.91 [0.78, 1.05], p = 0.185), and gastric cancer (IVW approach: OR [95% CI] = 1.11 [0.93, 1.32], p = 0.263). (refer to Figure 3) Complementarily, insights derived from the weighted median technique, MR-Egger, and weighted mode resonated harmoniously with the IVW determinations, accentuating the rigor of our analytical paradigm (refer to Supplementary Table 2). A comprehensive delineation of the SNPs associated with AF traits is furnished in Supplementary Table 1.
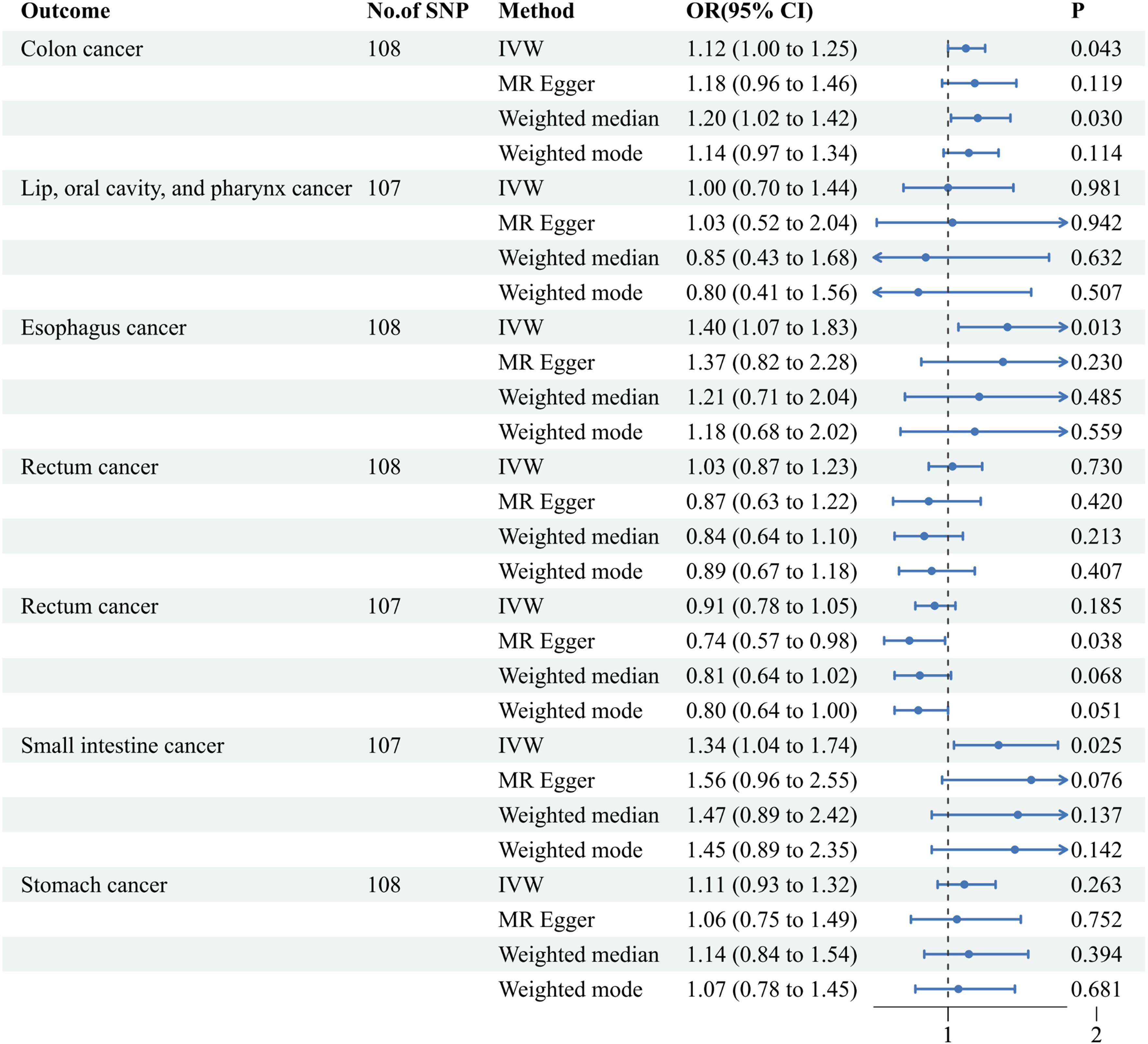
Figure 3 Associations of genetically predicted atrial fibrillation with seven digestive system cancers. IVW, inverse-variance weighted; MR, Mendelian Randomization; Cl, confidence interval; OR, Odds ratio; SNP, single nucleotide polymorphism.
Assessing the potential mediation of digestive system cancers by AF via HT, HF, IS, or CAD
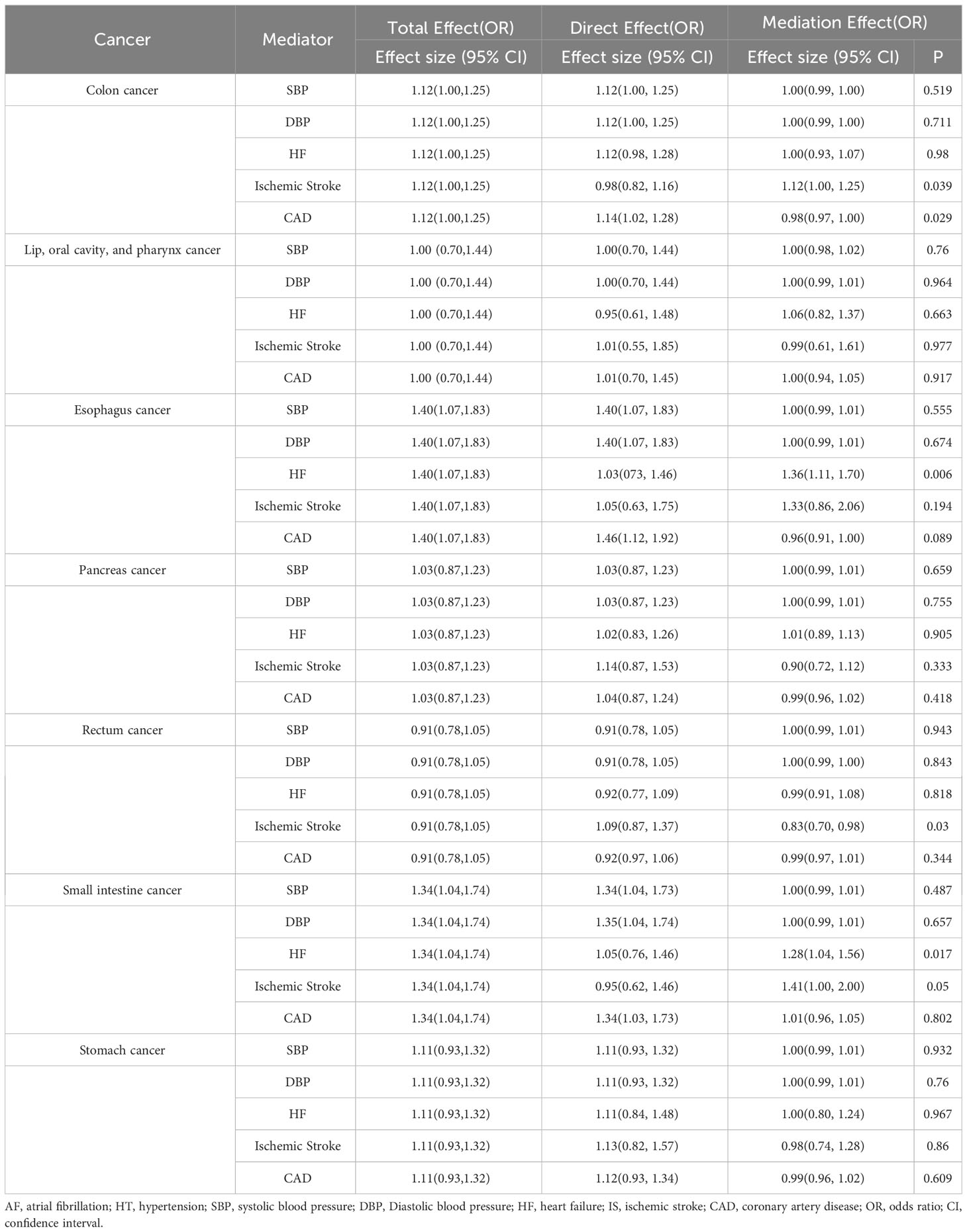
In light of the substantiated nexus between AF and ailments such as HT, HF, IS, and CAD, we endeavored to ascertain whether the interrelation of digestive system neoplasms with AF is orchestrated via these cardiovascular afflictions (40). Firstly, we validated the relationship between atrial fibrillation and these mediating factors, establishing the existence of varying degrees of causal linkage among them (refer to Supplementary Figure 1). Through MR mediation analyses, we inferred that the genetic predilection toward AF amplifies the peril of colon cancer through ischemic stroke mechanisms, manifesting an effect magnitude of 1.12 (95% CI: 1.00-1.25, p = 0.039). Furthermore, MR mediation analyses revealed that genetic predispositions to AF partially contribute to the development of esophageal and small intestine neoplasms through HF pathways. The observed effect sizes were 1.36 (95% CI: 1.11-1.70, p = 0.006) with 92% of the attributable risk of genetically predicted AF for esophageal cancer and 1.28 (95% CI: 1.04-1.56, p = 0.017) with 83% of the attributable risk for small intestine cancer. In contrast, other examined outcomes exhibited no discernible divergences (refer to Table 1). Given prior evidence demonstrating bidirectional causation between AF and HF (44), we supplemented our analysis with a two-sample MR study investigating the causal relationship between HF and digestive system cancers. The results indicate a lack of significant causal association (refer to Supplementary Figure 1).
Sensitivity analyses
In the context of the univariable MR evaluation, the outcomes gleaned from sensitivity analyses proffered no tangible evidence suggestive of latent horizontal pleiotropy as discerned through the MR-Egger intercept regression evaluations (Intercept < 0.01, p > 0.05, Supplementary Table 2). However, the p-value resulting from Cochran’s Q test, which integrates information from both the IVW and MR-Egger methodologies (p < 0.05, Supplementary Table 2), revealed significant heterogeneity in certain univariable MR analyses. Scatter diagrams vividly illustrated the potential causal relationships connecting AF and these digestive malignancies, as interpreted through the four MR methodologies employed (refer to Supplementary File 1). The steadfastness of our discoveries was further buttressed by the unwavering nature of the delineated associations, even upon the excision of any singular SNP in ‘leave-one-out’ explorations (refer to Supplementary File 2) or in isolated SNP assessments (refer to Supplementary File S3).
Discussion
In this study, we performed an MR investigation to clarify the potential causal nexus between AF and the susceptibilities of seven digestive malignancies. Simultaneously, we explored the potential mediating roles of HT, HF, IS, and CAD within a large AF cohort comprising over a million individuals from six distinct studies. The evidence suggests that AF is positively correlated with the likelihood of esophageal, colon, and small intestine malignancies. Importantly, ischemic stroke acts as a mediator for colon cancer, while heart failure plays a similar role for both esophageal and small intestine cancers (see Figure 3; Supplementary Table 2). These findings confirm a tangible causal link between a genetic predisposition for AF and specific digestive neoplasms, emphasizing the importance of preventing ischemic strokes and heart failures in individuals with AF. Meanwhile, it is well-established that specific digestive malignancies heighten the risk of AF (45). Moreover, AF is unequivocally identified as a pronounced risk factor for certain digestive cancers. Previous research reveals that one in four individuals diagnosed with atrial fibrillation has a preceding cancer diagnosis (46), and extensive cohort studies demonstrate an increased likelihood of cancer detection within the initial three months following the onset of the inaugural atrial fibrillation episode (5, 7).
To our current comprehension, extant MR investigations have not scrupulously delineated the interplay between AF and digestive malignancies, including the potential intermediary pathways. Nevertheless, numerous intricate biological processes are hypothesized to explain the association, highlighting the significance of AF in the onset of susceptibility to digestive cancer. Research indicates a notable prevalence of digestive malignancies in individuals with AF (47–49). One plausible conjecture suggests that anticoagulant administration in AF therapy may reveal latent malignancies by provoking hemorrhagic events within the neoplasm (50). Furthermore, the interconnection between inflammation in the genesis of cardiovascular ailments and oncogenesis is a dynamic area of investigation, recurring across these maladies. Increased synthesis of chemotactic agents and cytokines, specifically interleukins 1 and 6, along with systemic acute-phase reactants like C-reactive protein (CRP), has been delineated in individuals afflicted with digestive malignancies (51). Concurrently, inflammatory indices, including elevated leukocyte counts, and CRP, correlate with an amplified propensity to manifest incipient (52) and pronounced AF, potentially via instigating structural and electrical atrial alterations (53). In an encompassing survey involving 5,806 participants, an ascended CRP index correlated with prevalent AF and prognosticated forthcoming episodes of this dysrhythmia (54). Intriguingly, inflammation may potentiate AF and oncogenesis via reactive oxygen species (ROS) — by-products of metabolic processes and oxygen utilization. ROS are intrinsically linked to elevated oncogenic risk due to DNA impairment and genetic flux (55, 56). In AF, ROS genesis, mediated by leukocyte-sourced myeloperoxidase, may lead to atrial fibrogenesis and extracellular matrix metamorphosis through matrix metalloproteinases (57, 58). With the rapid aging of the population, the confluence of atrial fibrillation and cancer-related complications is becoming increasingly significant and prevalent in clinical settings, particularly in low- and middle-income countries with weaker healthcare and economic infrastructures (59). Given the escalating incidence of thromboembolic events in atrial fibrillation patients, there is an associated poorer prognosis for newly diagnosed atrial fibrillation in the context of cancer (60). On the other hand, initiating anticoagulant therapy after the diagnosis of atrial fibrillation may serve as an indicator of occult cancer, especially in gastrointestinal sites, as manifested by bleeding warning signs (59). Henceforth, it may be warranted to implement more intensive surveillance for potential malignancies within the digestive system among patients with AF, aiming to optimize prognostication, assessment, and prevention of cardiovascular complications.
Contemporary epidemiological inquiries and case-control examinations indicate that individuals with extant HF and roughly 4% of those enduring IS exhibit a heightened likelihood for subsequent malignancy onset (61, 62). In a distinct community-oriented cohort, HF-afflicted subjects bore an amplified oncogenic risk, notwithstanding age or gender considerations (63). One analytical pursuit projected a 20% surge in age-normalized oncogenic onset a year post-IS (64). The physiological intricacies anchoring the ties between acute ischemic stroke and oncogenesis remain nebulous (65). Importantly, the aforementioned investigations predominantly proffer associative insights, lacking a causative underpinning. In contrast, our analysis evidences HF as a mediating agent between AF and malignancies of the esophagus and small intestine, and IS as an intermediary between AF and colon cancer. On the flip side, preliminary indications suggest that AF might intrinsically heighten oncogenic diagnosis susceptibility (5, 66). Yet, the contention of whether this correlation is a manifestation of detection skewness or a genuine causal liaison remains a matter of ongoing discourse (8). The results and mechanistic interpretations from the previously referenced observational investigations align consistently with our findings.
Our insights are predicated upon a confluence of advancements: initially, the availability of extensive phenotyping of GWAS confers a sufficiently voluminous array of genetic instruments to facilitate the identification of robust genetic variants conducive to MR scrutiny of each disease. These bedrock initiatives capacitate a more stringent comparative appraisal of traits within univariate and mediator MR frameworks. Additionally, utilizing summary statistics from extensive GWAS for exposures, mediators, and outcomes enhances the statistical analysis power, supporting our hypothesis. Techniques such as MR-Egger and weighted median MR can furnish credible evidence of causality (67), notwithstanding the presence of confounding due to unbalanced pleiotropic influences. Within the purview of our investigation, the univariate MR outcomes bolster the thesis that AF plays a cardinal role among the cancers mentioned above, and mediated MR results suggested a unique reciprocal risk factor relationship with HF and IS.
Parallel to the inherent constraints of our undertaking, several pivotal facets warrant underscoring. We employed an MR model to contrast outcomes across genotypes, analogous to the dichotomy seen in randomized controlled trials between intervention and control groups. Yet, caution is warranted in drawing inferences, as genetically driven alterations in risk differ from those induced by interventions such as dietary changes (68). Our study pioneers the use of two-step MR to elucidate the relationship between AF and susceptibility to digestive cancers. Additionally, HF and IS emerge as potential mechanisms bridging the gap between atrial fibrillation and digestive malignancies. While the European ancestry of our participant cohort contributes to ancestral homogeneity, the applicability of our findings to wider ethnographic spectrums is limited. Further investigations involving diverse ethnic populations are imperative to confirm these findings and uncover additional clinical implications. Although horizontal pleiotropy can be investigated or corrected using methods such as MR-Egger or the IVW method of Cochran’s Q test, these methods typically require a substantial number of instrumental SNPs. Due to the limited quantity and potency of the genetic instruments’ SNPs, caution should be exercised in the interpretation of certain conclusions drawn in this study. In future research, acknowledging the critical role of sample size constraints is pivotal. It is imperative to explore collaborations and acquire larger datasets to fortify the robustness and generalizability of upcoming studies. In culmination, our analytical profundity rests upon substantial cohorts extracted from repositories like UKBB and FinnGen, buttressed by supplementary research endeavors (20, 29).
Conclusion
In summary, our findings indicate that AF is a pivotal causal factor in the predisposition to esophageal, colon, and small intestine neoplasms, with IS serving as a mediator in the etiology of colon cancer and HF coordinating similar processes for both esophageal and small intestine malignancies; other associations did not reach statistical significance. This research enhances understanding of the complex relationship in cardiovascular oncology, offering a potential prophylactic direction to address the rising global burden of AF and digestive malignancies, championing a shift toward salubrious living.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Access to genetic and phenotypic data from UK Biobank and FinnGen is granted through a formal application and approval process.
Author contributions
ZX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YX: Data curation, Writing – review & editing. ZZ: Data curation, Formal analysis, Writing – review & editing. LY: Formal analysis, Writing – review & editing. JH: Writing – review & editing. JZ: Conceptualization, Supervision, Writing – review & editing. RZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (81904287).
Acknowledgments
We are grateful to the participants of the UKBB and FinnGen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1301327/full#supplementary-material
Supplementary File 1 | Scatter diagrams.
Supplementary File 2 | ‘leave-one-out’ explorations.
Supplementary File 3 | isolated SNP assessments.
Abbreviations
AF, atrial fibrillation; MR, Mendelian Randomization; GWAS, Genome-Wide Association Study; SNPs, single nucleotide polymorphisms; IVW, inverse-variance weighted; HT, hypertension; HF, heart failure; IS, ischemic stroke; CAD, coronary artery disease; CRP, C-reactive protein; OR, odds ratio; CI, confidence interval.
References
1. Zhao M, Song L, Zhao Q, Chen Y, Li B, Xie Z, et al. Elevated levels of body mass index and waist circumference, but not high variability, are associated with an increased risk of atrial fibrillation. BMC Med (2022) 20(1):215. doi: 10.1186/s12916-022-02413-1
2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: A report from the American heart association. Circulation (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
3. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet Lond Engl (2015) 386(9989):154–62. doi: 10.1016/S0140-6736(14)61774-8
4. Kahwati LC, Asher GN, Kadro ZO, Keen S, Ali R, Coker-Schwimmer E, et al. Screening for atrial fibrillation: updated evidence report and systematic review for the US preventive services task force. JAMA (2022) 327(4):368–83. doi: 10.1001/jama.2021.21811
5. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, et al. Risk of Malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol (2016) 1(4):389–96. doi: 10.1001/jamacardio.2016.0280
6. Hung YP, Hu YW, Liu CJ, Lin YJ, Chang SL, Lo LW, et al. Risk and predictors of subsequent cancers of patients with newly-diagnosed atrial fibrillation - A nationwide population-based study. Int J Cardiol (2019) 296:81–6. doi: 10.1016/j.ijcard.2019.08.021
7. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sørensen HT. Atrial fibrillation as a marker of occult cancer. PloS One (2014) 9(8):e102861. doi: 10.1371/journal.pone.0102861
8. Saliba W, Rennert HS, Gronich N, Gruber SB, Rennert G. Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies. PloS One (2018) 13(1):e0190324. doi: 10.1371/journal.pone.0190324
9. Vinter N, Christesen AMS, Fenger-Grøn M, Tjønneland A, Frost L. Atrial fibrillation and risk of cancer: A Danish population-based cohort study. J Am Heart Assoc (2018) 7(17):e009543. doi: 10.1161/JAHA.118.009543
10. Mekhael M, Marrouche N, Hajjar AHE, Donnellan E. The relationship between atrial fibrillation and coronary artery disease: Understanding common denominators. Trends Cardiovasc Med (2022) S1050-1738(22)00123-2. doi: 10.1016/j.tcm.2022.09.006
11. Schnabel RB, Witt H, Walker J, Ludwig M, Geelhoed B, Kossack N, et al. Machine learning-based identification of risk-factor signatures for undiagnosed atrial fibrillation in primary prevention and post-stroke in clinical practice. Eur Heart J Qual Care Clin Outcomes (2022) 9(1):16–23. doi: 10.1093/ehjqcco/qcac013
12. Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
13. Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
14. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
15. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
16. Ziegler A, Mwambi H, König IR. Mendelian randomization versus path models: making causal inferences in genetic epidemiology. Hum Hered (2015) 79(3-4):194–204. doi: 10.1159/000381338
17. Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol (2012) 41(1):161–76. doi: 10.1093/ije/dyr233
18. Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol (2021) 36(5):465–78. doi: 10.1007/s10654-021-00757-1
19. Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet (2019) 20(8):467–84. doi: 10.1038/s41576-019-0127-1
20. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet (2018) 50(9):1234–9. doi: 10.1038/s41588-018-0171-3
21. Åsvold BO, Langhammer A, Rehn TA, Kjelvik G, Grøntvedt TV, Sørgjerd EP, et al. Cohort profile update: the HUNT study, Norway. Int J Epidemiol (2023) 52(1):e80–91. doi: 10.1093/ije/dyac095
22. Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, et al. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med Off J Am Coll Med Genet (2016) 18(9):906–13. doi: 10.1038/gim.2015.187
23. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
24. Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet (2017) 49(6):946–52. doi: 10.1038/ng.3843
25. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res (2018) 122(3):433–43. doi: 10.1161/CIRCRESAHA.117.312086
26. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet (2018) 50(4):524–37. doi: 10.1038/s41588-018-0058-3
27. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet (2018) 50(10):1412–25. doi: 10.1038/s41588-018-0205-x
28. Chen J, Yuan S, Fu T, Ruan X, Qiao J, Wang X, et al. Gastrointestinal consequences of type 2 diabetes mellitus and impaired glycemic homeostasis: A Mendelian randomization study. Diabetes Care (2023) 46(4):828–35. doi: 10.2337/dc22-1385
29. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature (2023) 613(7944):508–18. doi: 10.1038/s41586-022-05473-8
30. Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc (2010) 5(9):1564–73. doi: 10.1038/nprot.2010.116
31. 1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature (2012) 491(7422):56–65. doi: 10.1038/nature11632
32. Burgess S, Thompson SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med (2011) 30(11):1312–23. doi: 10.1002/sim.4197
33. Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
34. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37(7):658–65. doi: 10.1002/gepi.21758
35. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
36. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
37. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
38. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinforma Oxf Engl (2019) 35(22):4851–3. doi: 10.1093/bioinformatics/btz469
39. Zhang Y, Li D, Zhu Z, Chen S, Lu M, Cao P, et al. Evaluating the impact of metformin targets on the risk of osteoarthritis: a mendelian randomization study. Osteoarthritis Cartilage (2022) 30(11):1506–14. doi: 10.1016/j.joca.2022.06.010
40. Hu M, Tan J, Yang J, Gao X, Yang Y. Use of Mendelian randomization to evaluate the effect of atrial fibrillation on cardiovascular diseases and cardiac death. ESC Heart Fail (2023) 10(1):628–36. doi: 10.1002/ehf2.14237
41. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.3
42. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
43. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
44. Causal effects between atrial fibrillation and heart failure: evidence from a bidirectional Mendelian randomization study . PubMed. (Accessed December 3, 2023).
45. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol (2020) 17(8):474–502. doi: 10.1038/s41569-020-0348-1
46. Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes (2017) 3(3):192–7. doi: 10.1093/ehjqcco/qcx004
47. Chang TY, Chan YH, Chiang CE, Lin YJ, Chang SL, Lo LW, et al. Risks and outcomes of gastrointestinal Malignancies in anticoagulated atrial fibrillation patients experiencing gastrointestinal bleeding: A nationwide cohort study. Heart Rhythm (2020) 17(10):1745–51. doi: 10.1016/j.hrthm.2020.05.026
48. Raposeiras Roubín S, Abu Assi E, Barreiro Pardal C, Cespón Fernandez M, Muñoz Pousa I, Cobas Paz R, et al. New cancer diagnosis after bleeding in anticoagulated patients with atrial fibrillation. J Am Heart Assoc (2020) 9(22):e016836. doi: 10.1161/JAHA.120.016836
49. Ay C, Grilz E, Nopp S, Moik F, Königsbrügge O, Klimek P, et al. Atrial fibrillation and cancer: prevalence and relative risk from a nationwide study. Res Pract Thromb Haemost (2023) 7(1):100026. doi: 10.1016/j.rpth.2022.100026
50. Wallis CJD, Juvet T, Lee Y, Matta R, Herschorn S, Kodama R, et al. Association between use of antithrombotic medication and hematuria-related complications. JAMA (2017) 318(13):1260–71. doi: 10.1001/jama.2017.13890
51. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
52. Pastori D, Miyazawa K, Li Y, Shahid F, Hado H, Lip GYH. Inflammation and the risk of atrial high-rate episodes (AHREs) in patients with cardiac implantable electronic devices. Clin Res Cardiol Off J Ger Card Soc (2018) 107(9):772–7. doi: 10.1007/s00392-018-1244-0
53. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
54. Menichelli D, Vicario T, Ameri P, Toma M, Violi F, Pignatelli P, et al. Cancer and atrial fibrillation: Epidemiology, mechanisms, and anticoagulation treatment. Prog Cardiovasc Dis (2021) 66:28–36. doi: 10.1016/j.pcad.2021.04.004
55. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol (2018) 80:50–64. doi: 10.1016/j.semcdb.2017.05.023
56. Stanicka J, Russell EG, Woolley JF, Cotter TG. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J Biol Chem (2015) 290(15):9348–61. doi: 10.1074/jbc.M113.510495
57. Rudolph V, Andrié RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med (2010) 16(4):470–4. doi: 10.1038/nm.2124
58. Friedrichs K, Baldus S, Klinke A. Fibrosis in atrial fibrillation - role of reactive species and MPO. Front Physiol (2012) 3:214. doi: 10.3389/fphys.2012.00214
59. Chen M, Li C, Zhang J, Cui X, Tian W, Liao P, et al. Cancer and atrial fibrillation comorbidities among 25 million citizens in Shanghai, China: medical insurance database study. JMIR Public Health Surveill (2023) 9:e40149. doi: 10.2196/40149
60. Guha A, Fradley MG, Dent SF, et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J (2022) 43(4):300–12. doi: 10.1093/eurheartj/ehab745
61. Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F, et al. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail (2016) 18(3):260–6. doi: 10.1002/ejhf.472
62. Selvik HA, Thomassen L, Bjerkreim AT, Næss H. Cancer-associated stroke: the Bergen NORSTROKE study. Cerebrovasc Dis Extra (2015) 5(3):107–13. doi: 10.1159/000440730
63. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, et al. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol (2016) 68(3):265–71. doi: 10.1016/j.jacc.2016.04.053
64. Qureshi AI, Malik AA, Saeed O, Adil MM, Rodriguez GJ, Suri MFK. Incident cancer in a cohort of 3,247 cancer diagnosis free ischemic stroke patients. Cerebrovasc Dis Basel Switz (2015) 39(5-6):262–8. doi: 10.1159/000375154
65. Rioux B, Keezer MR, Gioia LC. Occult cancer diagnosed following acute ischemic stroke. CMAJ Can Med Assoc J J Assoc Medicale Can (2020) 192(36):E1037–9. doi: 10.1503/cmaj.200725
66. D’Souza M, Smedegaard L, Madelaire C, Nielsen D, Torp-Pedersen C, Gislason G, et al. Incidence of atrial fibrillation in conjunction with breast cancer. Heart Rhythm (2019) 16(3):343–8. doi: 10.1016/j.hrthm.2018.10.017
67. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med (2017) 36(11):1783–802. doi: 10.1002/sim.7221
Keywords: atrial fibrillation, hypertension, heart failure, ischemic stroke, coronary artery disease, Mendelian randomization, digestive system cancers
Citation: Xu Z, Rao X, Xing Y, Zhu Z, Yan L, Huang J, Zhang J and Zheng R (2024) Connecting atrial fibrillation to digestive neoplasms: exploring mediation via ischemic stroke and heart failure in Mendelian randomization studies. Front. Oncol. 14:1301327. doi: 10.3389/fonc.2024.1301327
Received: 24 September 2023; Accepted: 22 January 2024;
Published: 20 February 2024.
Edited by:
Benjamin YQ Tan, National University Hospital, SingaporeReviewed by:
Shaun Loong, National University of Singapore, SingaporeMarco Manfrini, University of Ferrara, Italy
Copyright © 2024 Xu, Rao, Xing, Zhu, Yan, Huang, Zhang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingchun Zhang, emhhbmdqaW5nY2h1bjI3NkAxMjYuY29t; Ruwen Zheng, WnJ3MTIzQHNpbmEuY29t
†These authors have contributed equally to this work
 Zhijie Xu
Zhijie Xu Xuezhi Rao
Xuezhi Rao Yaxuan Xing
Yaxuan Xing Zhiwei Zhu1,2
Zhiwei Zhu1,2 Longmei Yan
Longmei Yan