
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 14 May 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1298786
 Chaithra N1†
Chaithra N1† Anisha Jain2†
Anisha Jain2† Sahana C1
Sahana C1 Bhargav Shreevatsa3,4
Bhargav Shreevatsa3,4 Saravanan Rajendrasozhan5
Saravanan Rajendrasozhan5 Chandan Dharmashekar3
Chandan Dharmashekar3 Kuralayanapalya Puttahonnappa Suresh6
Kuralayanapalya Puttahonnappa Suresh6 Sharanagouda S. Patil7
Sharanagouda S. Patil7 Pranav Singh8
Pranav Singh8 Prashant Vishwanath9
Prashant Vishwanath9 Chandrashekar Srinivasa10
Chandrashekar Srinivasa10 Shiva Prasad Kollur11*
Shiva Prasad Kollur11* Chandan Shivamallu3*
Chandan Shivamallu3*Background: Lung cancer is the foremost cause of cancer-related death globally, with non-small cell lung cancer (NSCLC) accounting for 85–90% of cases. Targeted therapy is the most essential therapeutic option for NSCLC, other common treatments include radiation therapy, surgery, chemotherapy, and immunotherapy.
Objective: Our study objective was to estimate whether progression-free survival (PFS) is an outcome of NSCLC extracted from 18 randomized control trials (RCTs) with docetaxel as experimental group and antineoplastic agent, kinase inhibitor, and monoclonal antibodies as a control group.
Methods: We selected relevant studies published between 2011 and 2022 using Google Scholar, PubMed, Scopus, Science Direct, and Cochrane Library. Advanced NSCLC, chemotherapy, RCT, docetaxel, and second-line treatment were the terms included in the search. A total of 9738 patients were evaluated from the 18 identified studies. We used the meta package of R Studio to perform the meta-analysis. Graphical funnel plots were used to evaluate publication bias visually.
Results: Patients who underwent docetaxel-based therapy had a considerably longer PFS than those who got antineoplastic agents, kinase inhibitors, or monoclonal antibodies-based treatment. Patients in the standard treatment arm had a slightly longer PFS than those in the experimental therapy arm in the overall meta-analysis.
Conclusion: Docetaxel outperformed monoclonal antibodies, antineoplastic agents, and kinase inhibitors in the second-line therapy of advanced NSCLC since PFS was extensively utilized.
Cancer results from a complex multistep system including the accumulation of several gene mutations, which comprises encoding microRNA (1). Heredity ionizing radiation, chemical substances, alcohol, nitrates, estrogens, viruses, stress, and age are the main risk factors (2). Carcinoma, sarcoma, leukemia, lymphoma, and myeloma are types of cancer (3). According to the World Health Organization (WHO), it is the first or second largest cause of mortality before the age of 70 in 112 (of 183) nations, ranks third or fourth in another 23 countries, and was a major impediment to improving life expectancy in every country on the planet in 2019 (4).It has an impact on the high incidence of stroke and coronary heart disease mortality in many nations (5). HPV, HBV, HIV, and bacteria like Helicobacter pylori (stomach cancers) are infectious agents increasing the risk of cancer (6). The number of cancer cases is expected to increase from 979 786 in 2010 to 1 148 757 cases in 2020 (7). Lung cancer is the most recurrently diagnosed and the leading cause of cancer mortality. The two most common types of lung cancer are NSCLC and small cell lung cancer (SCLC). NSCLC makes for 80 to 85% of lung cancer cases, with SCLC accounting for the rest. Patients with lung cancer may be eligible for various therapies, including surgery, radiation, chemotherapy, and targeted therapy, depending on their stage. Targeted therapy is the most essential therapeutic option for NSCLC, other common treatments include radiation therapy, surgery, chemotherapy, and immunotherapy. Targeted therapies include monoclonal antibodies and small-molecule inhibitors. Specific mutations have been detected thanks to advances in genetics and biomarker testing, allowing doctors to better target treatment for individual patients (8, 9). Cigarette smoking is considered a significant hazard factor with an 82% mortality rate in males compared to females (10). It is asymptomatic in its early-stage, and patients diagnosed at an advanced stage experience a poor prognosis (11). The objective of our study was to estimate whether the PFS is an outcome of NSCLC, using data from 18 RCTs (12). PFS, the time from therapeutic initiation to disease progression, may be used as a measure of clinical benefit for drug approvals, depending on the condition and response observed (13).
We selected relevant studies published between 2011 and 2022 using Google Scholar, PubMed, Scopus, Science Direct, and Cochrane Library. Advanced NSCLC, chemotherapy, RCT, docetaxel, and second-line treatment were the terms included in the search.
Randomized trials including patients diagnosed with NSCLC that evaluated docetaxel with a kinase inhibitor, antineoplastic agents, and monoclonal antibodies for NSCLC were included. Docetaxel compared with other therapeutic agents except for kinase inhibitors, monoclonal antibodies and antineoplastic agents was considered exclusion criterion. Similarly, studies that compared docetaxel to other drugs were excluded, as well as early studies published as a series of articles by the same author with overlapping data, editorials, case reports, conference articles, experimental studies, and related studies that failed to provide significant findings. Authorship, publication bias, clinical trials, demographic attributes, histology characteristics, smoking status, treatment for each group, and adverse events were all extracted using a fixed standardized procedure. The conventional treatment in this trial was docetaxel, while the experimental arm was a kinase inhibitor, antineoplastic drug, or monoclonal antibody.
A comprehensive search approach was devised to reduce the risk of publishing bias. Graphical funnel plots were used to visually evaluate publication bias and the quality of RCTs.
Pooled HR was calculated with 95% CI. We used forest plots and inconsistency statistic [I2] to determine heterogeneity. The OR was the summary measure used for pooling the studies. Hedge’s method evaluates the effect size calculated by standard mean difference (SMD) given as Hedge’s g-value. The meta-analysis was summarized graphically using a forest plot. Meta package of R Studio (v2021.09.0) was used to perform the meta-analysis.
The details of study selection criteria followed for the meta-analysis of drug intervention prevalence are given in Figure 1. The number of published articles was 1240, of which 256 were rejected for duplication in one or the other form, 68 were excluded since reviews or meta-analysis and 429 were excluded as non-randomized control trials, 65 were excluded due to Irrelevant or Insufficient information and 50 excluded because of not NSCLC. Then after filtering 350 randomized control trials were selected for detailed evaluation, in which 180 were excluded which were treatment arms without docetaxel, and 152 were excluded which were without monoclonal antibodies, kinase inhibitors, and antineoplastic agents. Hence, finally 18 Randomized control trials were selected for the study.
The characteristics of selected RCTs of meta-analysis are given in Table 1. Six RCTs phase 3 data for the antineoplastic agent classes of intervention were analyzed, with 850 the maximum number of patients recorded with a median age of 64 and PFS as the primary endpoint. Data from seven phase 2 and 3 RCTs were analyzed for the kinase inhibitor class of intervention, with 1314 the highest number of patients having a primary endpoint of PFS with a median age of 60. The remaining five RCTs were phase 2 and 3 monoclonal antibody class intervention data analyzed with PFS as the main endpoint. The highest number of patients recorded was 1253 with a median age of 61.5 years.
Figures 2-4 show forest plots comparing the PFS of docetaxel to antineoplastic agents, kinase inhibitors, and monoclonal antibodies-based treatment. The six studies reported the PFS of antineoplastic agents compared with docetaxel with 2160 patients involved. The meta-analysis of all involved studies revealed significant statistical heterogeneity (I2 = 96%, τ2 = 0.2502, p < 0.01), and Hedge’s corrected SMD was -0.36 (95% CI: -1.01–0.29). There was a moderate effect because it was a negative value smaller than -0.20, which implies the result favored the antineoplastic agents-based treatment.
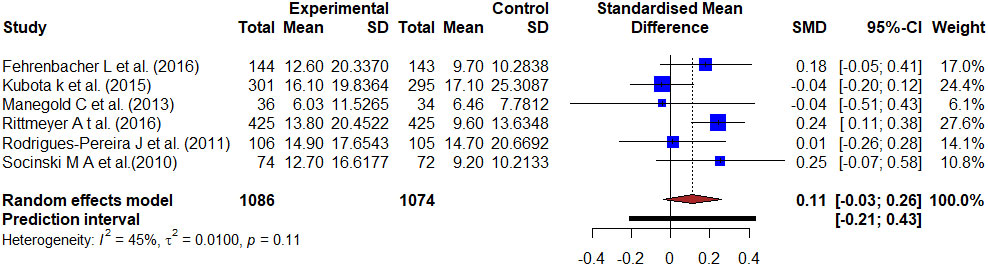
Figure 2 Forest plot representing the PFS of docetaxel versus antineoplastic agents treatment. Hedge’s corrected SMD is -0.36, and Higgin’s and Thompson’s I2 statistic is 96%.
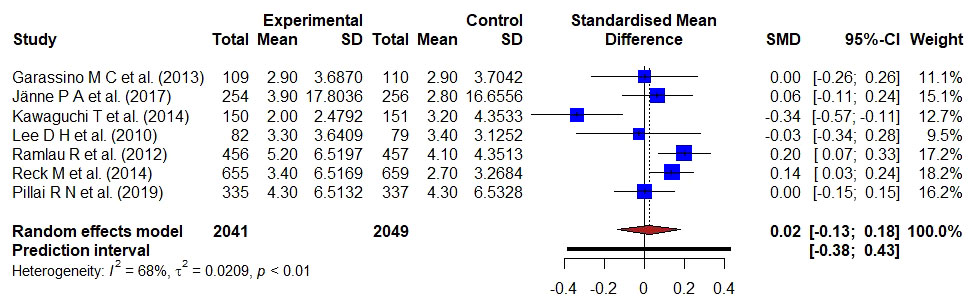
Figure 3 Forest plot representing the PFS of docetaxel- versus kinase inhibitors- treatment; the Hedge’s corrected SMD is 0.02, and Higgin’s and Thompson’s I2 statistic is 68%.
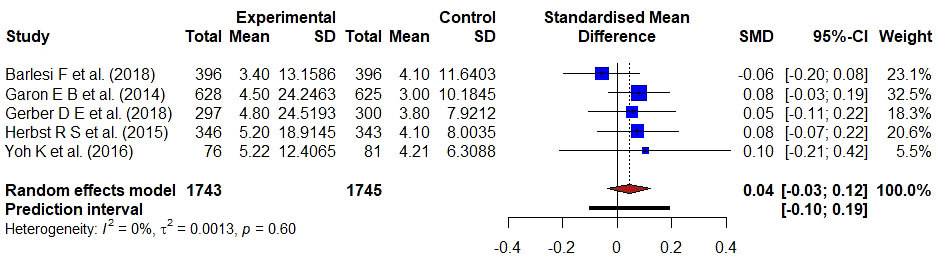
Figure 4 Forest plot representing the PFS of docetaxel versus monoclonal antibodies treatment. Hedge’s corrected SMD is 0.04, and Higgin’s and Thompson’s I2 statistic is 0%.
A total of 4090 patient data from seven studies reported the PFS of kinase inhibitor compared with docetaxel-based treatment. A bias-corrected SMD; Hedge’s g-value was 0.02 (95% CI: -0.13–0.18), implying the result favored the docetaxel-based standard treatment. A significant statistical heterogeneity (I2 = 68%, τ2 = 0.0209, p < 0.01) was found in the pooled analysis of all included studies.
The PFS of monoclonal antibodies was compared to docetaxel in five studies involving 3488 individuals. There was no substantial statistical heterogeneity in a pooled analysis of all included trials (I2 = 0, τ2 = 0.0013, p = 0.60), and Hedge’s g-value was 0.04 (95% CI: -0.03–0.12), indicating that the result favored docetaxel-based treatment. The SMD value was less than 0.20, indicating that docetaxel had a minor effect.
The p-values for the meta-analyses of PFS of 18 RCTs are > 0.05, indicating that formal statistical testing revealed no indication of significant publication bias (PFS: Egger’s test, p = 0.947) (Figure 5).
A meta-analysis was conducted for 18 RCTs (14–31) with docetaxel as the experimental group and antineoplastic agent, and kinase inhibitor and monoclonal antibodies as a control group including 9738 patients with stage III–IV NSCLC. The objective of this study was to see if the PFS of patients improved. Platinum-based two-drug combinatorial chemotherapy has been the standard of care for advanced NSCLC patients (22–24). Our study’s main aim was to compare the two treatment regimens in terms of PFS in patients with advanced NSCLC (24). A total of 2160 cases with six RCTs were used to compare the docetaxel with antineoplastic agents. Six studies compared the improvement of PFS between docetaxel and atezolizumab, S-1 plus cisplatin, cilengitide, pemetrexed/carboplatin (15, 22, 24, 27–29). The period from randomization to either progressing illness or death was referred to as PFS. The different randomization methods are used to receive either 60 mg/m2 docetaxel plus cisplatin, 75 mg/m2 docetaxel, docetaxel 75 mg/m2/3W + carboplatin 5 mg/ml/min or oral S-1 80 mg/m2/day plus cisplatin 60 mg/m2, cilengitide 600 mg/m2, pemetrexed 500mg/m2/3W + carboplatin 5mg/ml/min, atezolizumab 1200 mg to see the improvement of PFS between these groups (15, 22, 24, 27–29). The PFS was similar between each control and treatment group. The median PFS was 2.7 months with atezolizumab and 3.0 months with docetaxel with a HR of 0.94 (95% CI 0.72–1.23) (15). The median PFS was 2.8 months with atezolizumab and 4.0 months with docetaxel. The HR was 0.63 [95% CI 0.43–0.91] (27). The median PFS was 4.9 months in the SP group and 5.2 months in the DP group with a HR of 1.113; 95% CI, 0.945 to 1.311 (22). There were no statistically significant differences in PFS between the treatment groups with a HR of 0.91 (0.67–1.23) (24). Therefore, there was no improvement in PFS between the groups.
In patients with metastatic NSCLC, antibodies targeting the immune checkpoint molecules PD-L1 or PD-1 enhance PFS compared to standard of care chemotherapy treatment (14). A total of 3488 patients in five trials have been used to compare docetaxel-based treatment with monoclonal antibody-based therapy (14, 17–19, 30). The meta-analysis of avelumab versus docetaxel in advanced NSCLC patients and progression of disease following platinum-based treatment was described by Barlesi et al. (14). A block randomized method was used to acquire either docetaxel 75 mg/m2 or avelumab 10 mg/kg and PFS was a secondary endpoint. The median PFS in the avelumab group was 2.8 months (95% CI 2.7–3.5) and 4.2 months (3.3–5.2) in the docetaxel group with HR 1.16 [95% CI 0.97–1.40]. As a result, with avelumab, PFS was substantially longer, and objective responses were more likely than with docetaxel. Garon et al., compared the effectiveness and safety of docetaxel with ramucirumab versus placebo as second-line therapy for stage IV NSCLC patients (17). A randomized method was used to assign the patients either ramucirumab 10 mg/kg or docetaxel 75 mg/m2. The median PFS for the ramucirumab group was 45 months, compared to 30 months for the control group with a HR of 0.76 (0.68–0.86). The PFS is improved in ramucirumab compared to docetaxel in patients with stage IV NSCLC. The efficacy of bavituximab in combination with docetaxel in patients with advanced NSCLC who have already been treated was investigated by Gerber et al. (18). The authors used a stratified randomized technique to provide docetaxel plus placebo or docetaxel plus bavituximab 3 mg/kg to the patients. With HR 1.00; 95% CI, 0.82–1.22, there was no alteration in PFS. The addition of bavituximab to docetaxel did not improve PFS. Herbst et al. compare pembrolizumab’s effectiveness and safety to those of docetaxel (19). A randomized method was used to acquire either pembrolizumab 10 mg/kg or docetaxel 60 mg/m to the selected participants. The median PFS was 3.9 months with pembrolizumab, 4.0 months with docetaxel (HR 0.88, 95% CI 0.74–1.05). Therefore, PFS was significantly longer with pembrolizumab than docetaxel. Yoh et al., explain how a phase II, double-blind, randomized, placebo-controlled trial in Japanese patients with NSCLC examined the safety and effectiveness of second-line ramucirumab-docetaxel (30). The median PFS was 5.22 months for ramucirumab-docetaxel and 4.21 months for placebo-docetaxel with HR of 0.83 (95% CI 0.59–1.16). Hence, PFS was longer with ramucirumab-docetaxel than with placebo-docetaxel. Seven clinical studies, including 4090 participants, were conducted to compare the docetaxel-based therapy with kinase inhibitor for patients with advanced NSCLC. The authors of seven studies compared the efficacy and safety of Gefitinib, erlotinib, aflibercept (Ziv-aflibercept), docetaxel plus nintedanib, mitogen-activated protein kinase (MEK) inhibitor, selumetinib + docetaxel and combination of ganetespib-docetaxel with the treatment group of docetaxel in patients with advance NSCLC to check the improvement of PFS between the groups. A randomized clinical method was used and patients received either docetaxel (75 mg/m2), IV placebo plus docetaxel (75 mg/m2), placebo + docetaxel (75 mg/m2/3W) or gefitinib (250 mg/d), erlotinib orally 150mg/day, (Ziv-) aflibercept 6 mg/kg intravenous plus docetaxel 75 mg/m2 erlotinib 150 mg/D, nintedanib 200 mg orally, selumetinib 75mg/0.5D plus docetaxel 75mg/m2/3W, ganetespib 150 mg/m until unacceptable side effects or disease progression based on previous bevacizumab treatment, histology, ECOG performance status, and presence of brain metastases (15, 19, 20, 22, 24, 25, 30). PFS was estimated as a primary and secondary endpoint in these studies. The median PFS was 3.9 months with selumetinib + docetaxel and 2.8 months with placebo + docetaxel with HR, 0.93 [95% CI, 0.77–1.12] (19). The median PFS in the ganetespib and docetaxel arm was 4.2 months, and 4.3 months in the docetaxel arm, with an HR of 1.16 (95% CI, 0.96–1.403) (31). Gefitinib had a better PFS than docetaxel, with a HR of 0.729; 90% CI, 0.533–0.998. The PFS was longer with gefitinib than docetaxel. As a result, gefitinib was a crucial and effective second-line treatment option for Korean NSCLC patients (23). Gefitinib had a longer PFS than docetaxel. The median PFS was 2.9 months with docetaxel versus 2.4 months with erlotinib with HR 0.71, 95% CI 0.53–0.95 (16). Median PFS was significantly longer in the (Ziv-)aflibercept arm of 5.2 months than in the placebo arm of 4.1 months with HR was 0.82 (95% CI 0.72–0.94). Erlotinib had a median PFS of 2.0 months against 3.2 months when compared to docetaxel with an HR of 1.22; 95% CI, 0.97 to 1.55. In an EGFR-unselected patient sample, erlotinib failed to improve PFS compared to docetaxel (21). The median PFS in the docetaxel plus nintedanib group was 3·4 months compared to 2.7 months in the docetaxel plus placebo group (HR 0.79, 95% CI 0.68–0.92) (26).
There are limits to our analysis that should be considered while evaluating the results. First, the different treatment regimens add to the meta-analysis’ clinical heterogeneity, which makes meta-analysis interpretation more difficult. In three studies, docetaxel was used in conjunction with other medicines, either cisplatin or carboplatin, in the control arm. The quality of the results was influenced by the quality of each study’s results. Finally, because the research included in this study was all conducted in the West, the findings must be confirmed in Asia. Docetaxel was revealed to be more effective in the second-line therapy of advanced NSCLC than antineoplastic drugs, kinase inhibitors, and monoclonal antibodies, according to the findings.
The phase 2 and 3 study of antineoplastic agents demonstrate a clinically significant survival benefit over docetaxel in patients with NSCLC. Compared to docetaxel, monoclonal antibodies and kinase inhibitors did not affect PFS in NSCLC patients. From the results of 18 trials involving 9738 patients, those who received docetaxel-based therapy had a significantly longer PFS than those who received kinase inhibitors or monoclonal antibodies. In the overall meta-analysis, patients in the standard treatment arm had a slightly longer PFS than those in the experimental therapy arm. Biological behavior subgroups such as those entirely refractory, those with partial and incomplete responses, and those with short and extended disease-free intervals will be examined in future meta-analysis investigations.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
CN: Methodology, Software, Writing – original draft, Writing – review & editing. AJ: Validation, Writing – original draft, Writing – review & editing. SC: Methodology, Validation, Writing – original draft. BS: Resources, Writing – review & editing. SR: Funding acquisition, Writing – review & editing. CD: Data curation, Writing – review & editing. KS: Validation, Writing – review & editing. SP: Resources, Supervision, Writing – review & editing. PS: Supervision, Writing – review & editing. PV: Supervision, Validation, Writing – review & editing. CSr: Data curation, Writing – review & editing. SK: Supervision, Validation, Writing – original draft. CSh: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The manuscript received funding from JSS Academy of Higher Education & Research (JSS AHER), Mysuru Karnataka, India.
The Authors acknowledge the support, infrastructure and funding offered by the JSS Academy of Higher Education and Research (JSSAHER), Mysuru, Manipal Academy of Higher Education, Manipal and Amrita Vishwa Vidyapeetham, Mysuru campus, University of South Carolina, Columbia, SC, USA University of Hail, Hail, Saudi Arabia and the ICAR-National Institute of Veterinary Epidemiology and Disease Informatics, Bengaluru for the support regarding the research work. The authors acknowledge JSS Academy of Higher Education & Research, Mysuru for the support extended towards the funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ali Syeda Z, Langden SS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci. (2020) 21:1723. doi: 10.3390/ijms21051723
2. Cicero G, De Luca R, Dieli F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco Targets Ther. (2018) 11:3059–63. doi: 10.2147/OTT.S151276
3. Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. (2005) 115:1503–21. doi: 10.1172/JCI23412
4. Sundar S, Wu J, Hillaby K, Yap J, Lilford R. A systematic review evaluating the relationship between progression free survival and post progression survival in advanced ovarian cancer. Gynecol Oncol. (2012) 125:493–9. doi: 10.1016/j.ygyno.2011.12.420
5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
6. Masrour-Roudsari J, Ebrahimpour S. Causal role of infectious agents in cancer: An overview. Caspian J Intern Med. (2017) 8:153–8. doi: 10.22088/cjim.8.3.153
7. Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010-2020) by cancer groups. Asian Pac J Cancer Prev. (2010) 11:1045–9.
8. Jain AS, Prasad A, Pradeep S, Dharmashekar C, Achar RR, Silina E, et al. Everything old is new again: drug repurposing approach for non-small cell lung cancer targeting MAPK signaling pathway. Front Oncol. (2021) 11:741326. doi: 10.3389/fonc.2021.822865
9. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. (2008) 83:584–94. doi: 10.4065/83.5.584
10. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. (2021) 149(4):778–89. doi: 10.1002/ijc.33588
11. Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. (2008) 26:3213–21. doi: 10.1200/JCO.2007.15.8923
12. Gutman SI, Piper M, Grant MD, Basch E, Oliansky DM, Aronson N. Progression-Free Survival: What Does It Mean for Psychological Well-Being or Quality of Life? [Internet] Rockville (MD: Agency for Healthcare Research and Quality (US). (2013).
13. Hess LM, Brnabic A, Mason O, Lee P, Barker S. Relationship between progression-free survival and overall survival in randomized clinical trials of targeted and biologic agents in oncology. J Cancer. (2019) 10:3717–27. doi: 10.7150/jca.32205
14. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. (2018) 19:1468–79. doi: 10.1016/S1470-2045(18)30673-9
15. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
16. Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. (2013) 14:981–8. doi: 10.1016/S1470-2045(13)70310-3
17. Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. (9944) 2014:384. doi: 10.1016/S0140-6736(14)60845-X
18. Gerber DE, Horn L, Boyer M, Sanborn R, Natale R, Palmero R, et al. Randomized phase III study of docetaxel plus bavituximab in previously treated advanced non-squamous non-small-cell lung cancer. Ann Oncol. (2018) 29:1548–53. doi: 10.1093/annonc/mdy177
19. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
20. Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non–small cell lung cancer: The SELECT-1 randomized clinical trial. JAMA. (2017) 317:1844–53. doi: 10.1001/jama.2017.3438
21. Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol. (2014) 32:1902–8. doi: 10.1200/JCO.2013.52.4694
22. Kubota K, Sakai H, Katakami N, Nishio M, Inoue A, Okamoto H, et al. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol. (2015) 26:1401–8. doi: 10.1093/annonc/mdv190
23. Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. (2010) 16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903
24. Manegold C, Vansteenkiste J, Cardenal F, Schuette W, Woll PJ, Ulsperger E, et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs. (2013) 31:175–82. doi: 10.1007/s10637-012-9842-6
25. Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. (2012) 30:3640–7. doi: 10.1200/JCO.2012.42.6932
26. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. (2014) 15:143–55. doi: 10.1016/S1470-2045(13)70586-2
27. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
28. Rodrigues-Pereira J, Kim J-H, Magallanes M, Lee DH, Wang J, Ganju V, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. (2011) 6:1907–14. doi: 10.1097/JTO.0b013e318226b5fa
29. Socinski MA, Manikhas GM, Stroyakovsky DL, Makhson AN, Cheporov SV, Orlov SV, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. (2010) 5:852–61. doi: 10.1097/JTO.0b013e3181d5e39e
30. Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer. (2016) 99:186–93. doi: 10.1016/j.lungcan.2016.07.019
Keywords: non-small cell lung carcinoma, lung neoplasms, progression-free survival, randomized control trials, meta analysis
Citation: N C, Jain A, C S, Shreevatsa B, Rajendrasozhan S, Dharmashekar C, Suresh KP, Patil SS, Singh P, Vishwanath P, Srinivasa C, Kollur SP and Shivamallu C (2024) Progression-free survival estimation of docetaxel-based second-line treatment for advanced non-small cell lung cancer: a pooled analysis from 18 randomized control trials. Front. Oncol. 14:1298786. doi: 10.3389/fonc.2024.1298786
Received: 22 September 2023; Accepted: 02 April 2024;
Published: 14 May 2024.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
Theodoros Karampitsakos, University of South Florida, United StatesCopyright © 2024 N, Jain, C, Shreevatsa, Rajendrasozhan, Dharmashekar, Suresh, Patil, Singh, Vishwanath, Srinivasa, Kollur and Shivamallu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chandan Shivamallu, Y2hhbmRhbnNAanNzdW5pLmVkdS5pbg==; Shiva Prasad Kollur, c2hpdmFjaGVtaXN0QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.