
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 April 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1295772
 Liliana Montella1*
Liliana Montella1* Luigi Di Marino2
Luigi Di Marino2 Maria Adele Marino3
Maria Adele Marino3 Vittorio Riccio4
Vittorio Riccio4 Nunzio Del Gaudio5
Nunzio Del Gaudio5 Lucia Altucci5,6†
Lucia Altucci5,6† Massimiliano Berretta7†
Massimiliano Berretta7† Gaetano Facchini1†
Gaetano Facchini1†Background: Hormone receptor-positive tumors are unlikely to exhibit a complete pathological tumor response. The association of CDK 4/6 inhibitor plus hormone therapy has changed this perspective.
Case presentation: In this study, we retrospectively reviewed the charts of patients with a diagnosis of luminal A/B advanced/metastatic tumors treated with a CDK 4/6 inhibitor-based therapy. In this part of the study, we present clinical and ultrasound evaluation. Eight female patients were considered eligible for the study aims. Three complete and five partial responses were reported, including a clinical tumor response of 50% or more in five out of nine assessed lesions (55%). All patients showed a response on ultrasound. The mean lesion size measured by ultrasound was 27.1 ± 15.02 mm (range, 6–47 mm) at the baseline; 16.08 ± 14.6 mm (range, 0–40 mm) after 4 months (T1); and 11.7 ± 12.9 mm (range, 0–30 mm) at the 6 months follow-up (T2). Two patients underwent surgery. The radiological complete response found confirmation in a pathological complete response, while the partial response matched a moderate residual disease.
Conclusion: The evaluation of breast cancer by ultrasound is basically informative of response and may be an easy and practical tool to monitor advanced tumors, especially in advanced/unfit patients who are reluctant to invasive exams.
Advanced breast cancer includes, according to the 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) (1), both inoperable locally advanced breast cancer (LABC) and metastatic breast cancer (MBC). LABC is often considered a candidate for therapies with the aim of tumor shrinkage before the surgery, decreasing the rate of mastectomy in favor of less demolition surgery, testing the in vivo drug sensitivity, and tailoring treatment according to the pathological response obtained.
The indication for neoadjuvant chemotherapy in hormone receptor-positive (HR+)/HER2-negative tumors is not straightforward (2). While recent trials reach 60% of pathological response rate (pCR) in HER2-positive and triple-negative tumors and are rapidly changing therapeutic algorithms (3, 4), standard chemotherapy produces an inadequate pCR (0%–18%) (1) and breast-conserving surgery (5) approximately 60% in advanced HR+ tumors with a low Ki-67 proliferation rate. A large study starting from 134,574 HR+ breast cancer patients extracted data on 29,250 patients undergoing neoadjuvant chemotherapy and reported approximately 8% of pCR (6). As in the HER-2 and TN tumors, pCR correlates with improved survival (7).
In 2013, the St. Gallen International Expert Consensus defined luminal A and luminal B tumors as ER-positive, HER2-negative tumors having respectively Ki-67 low and PR high, or Ki-67 high or PR (8). Almost uniformly, a Ki-67 <14% is associated with a luminal A profile, while above 14% corresponds to a luminal B tumor (9). Given the fluctuating Ki-67 value across the different studies, the definition of luminal A- or B-like is often adopted. The St. Gallen International Consensus Guidelines for the treatment of early breast cancer 2021 (10), acknowledging the conclusions from another working group (9), identified the groups of tumors with Ki-67 <5% and with Ki-67 >30% and recommended chemotherapy only in the latter group. The definition of the best treatment for the in-between band remains a controversial matter. Molecular characterization of the different histological subtypes has increasingly contributed to defining precision oncology-guided therapeutic algorithms (10). Some features such as luminal B subtype, high proliferation, and lack of progesterone receptor (2) may be predictive of increased pCR rates; however, information on efficacy outcomes is scarce, while surrogate endpoints such as Ki67 reduction are often adopted in clinical trials (11).
Alternative strategies have been explored including, very recently, the combination of chemo- and immunotherapy, which lead to an increased rate of pCR in luminal-B like BC defined as HR-positive/HER2-negative, Ki-67 ≥ 20%, and/or histological grade 3 (12). In other instances, a genomic-based approach was used to differentiate high- to low-risk luminal A BC patients and address the first to neoadjuvant chemotherapy and the others to endocrine therapy (13).
The interest in endocrine preoperative strategies for HR+ positive tumors has been progressively growing. Different CDKs have been investigated in BC. They have a prognostic role and are a pharmacological target for therapeutic intervention (14).
Seven studies have investigated CDK4/6 inhibitors in neoadjuvant HR+ positive, HER-2 negative BC (Supplementary Table S1). Most of the studies previously reported focused on the antiproliferative effect detected in vivo and showed moderate clinical activity. The need for periodical biopsies to assess tumor changes for study purposes has raised some ethical issues.
To date, many studies focus on pCR as a primary objective, which is quite disappointing (15), while there is a relative lack of information coming from radiological tools used during follow-up, which could be preliminary informative and maybe predictive of treatment response. The imaging of the breast is essential in the diagnosis, staging, and monitoring of breast cancer. In clinical practice, the response is mostly evaluated by ultrasound (US) or magnetic resonance imaging (MRI), the second one showing high sensitivity in the detection of a residual tumor. Additionally, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) provides functional information at baseline and after treatment and was shown to correlate with pCR (16, 17). Compared to MRI, US can be considered easier to perform, does not require the injection of exogenous contrast agents, and is less expensive. It is classically considered operator dependent, which is a limit that may be overcome by the selection of a unique and skilled operator. US is accurate in size evaluation, but less comprehensive than mammography in providing an overall view. This technique can simply be used to monitor treatment response. However, the US is not used for RECIST due to operator dependency.
We argue that an integrative approach including clinical and radiological information should best define the therapeutic algorithms of HR+, HER-negative breast cancer patients undergoing medical treatments. Therefore, we evaluate a series of advanced/metastatic HR+ patients treated with CDK 4/6 I+AI in a real-world setting, making the radiological assessment the cornerstone in comparison with clinical outcomes and pathological findings.
We retrospectively evaluated our archive of advanced and metastatic pre-and post-menopausal breast cancer patients with a pathologically confirmed ER+ and HER2− (0 or 1+ by IHC or FISH negative) invasive breast cancer treated with a combination of CDK 4/6 I+AI as per clinical practice. In detail, CDK 4/6 inhibitors used in this study were palbociclib and ribociclib. Palbociclib was used at a dose of 125 mg taken by mouth with food on 21 days and 7 days off schedule (on days 1–21 of each 28-day cycle). Ribociclib was administered at 600 mg/day: 3 weeks ON and 1 week OFF. Letrozole was the first-line endocrine treatment for all patients. Letrozole was administered every day of each 28-day cycle at a dose of 2.5 mg. Goserelin was administered to pre-menopausal subjects as a subcutaneous injection every 28 days at a dose of 3.6 mg.
No limit was formally defined for Ki-67. At baseline, an advanced stage was required related to the tumor (≥ T3 or surgically unresectable) or lymph nodes (≥ N2, upper arm edema due to breast and lymphnodes involvement). Metastatic patients at presentation with evaluable primary tumors were also considered eligible. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–2, adequate organ, and marrow function. Exclusion criteria included previous treatment with CDK4/6 inhibitors, organ failure, and treatment intolerance.
The study was approved by our Institutional Review Board (Campania Centro Ethical Committee approval n.391, N.Reg 22/2022) and followed the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was required before study entry.
The primary study aim was the evaluation of clinical and US tumor response.
Tumor and lymph nodes were clinically evaluated after 1 month of treatment and thereafter every 2 months/3 months with clinical and US examination. RX mammography, breast MRI, and PET/CT with FDG were performed after 4 months/6 months and in case of suspected progression. Computed tomography was performed as a staging procedure at study entry, after 4 months/6 months, and as clinically required.
Clinical response rate (cRR) was evaluated clinically using bi-dimensional clinical measurements and radiologically using the US before the start of treatment and every 4 months subsequently.
A single lesion, even in the case of multifocal, was chosen and evaluated at time 0, at time 1 (T1) after 4 months/6 months of therapy, and at time 2 (T2) after 8 months/12 months. B-mode US was performed in a supine and lateral position, with both upper limbs facing upwards. To study the breast lesion, a conventional B-mode US and Color-Doppler US were performed with a 4–14-MHz linear array transducer. In compliance with the 5th edition of the BI-RADS classification (18), the imaging characteristics have been recorded on a spreadsheet Excel database: size, position, shape, orientation to the skin, margins, echogenicity pattern, posterior characteristics, and vascularization. The secondary aims were the rate of response by MRI and PET, the rate of conservative surgery, the pCR obtained, changes in Ki-67, and immunohistochemistry from baseline in response to therapy and safety. The pathology exam of the resected specimen at the time of surgery was evaluated and assessed by a well-qualified pathologist. pCR was defined as the absence of invasive disease in the breast and sampled axillary lymph nodes (ypT0 ypN0). Residual tumor was defined according to the Residual Cancer Burden Index (19).
Clinically responder patients remained on treatment until disease progression if metastatic and without a fixed end-of-treatment point in case of locally advanced disease. A complete metabolic response was considered mandatory for surgical referral. If a surgical intervention was considered appropriate by the Multidisciplinary Teams, CDK 4/6 inhibitor was stopped at least 2 weeks before surgery, whereas letrozole was continued till the day of surgery.
The patients were regularly monitored with complete blood count and blood chemistry. All toxicities encountered during the study were evaluated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events, v3.0. Dosage reductions were performed according to the manufacturer’s datasheet.
From a screening of 24 patients treated with CDK 4/6 I+IA, we selected eight patients with in situ primary breast tumors and advanced or metastatic disease having complete clinical and radiological records. All but two patients were postmenopausal (age range, 48–84 years; mean, 64 years). Two patients had a bilateral BC, three patients had advanced BC, and five had metastatic BC at study entry. The comprehensive characteristics of the patients are reported in Table 1.
Two patients underwent surgery. The surgical intervention was in both cases radical mastectomy with axillary lymph node dissection.
The median number of CDK4/6 I + AI cycles before surgery was 8 (range, 6–10). The interval between the start-up of treatment and surgical procedure was 1 year and 6 months, respectively.
Before the treatment, the patients had clinically and radiologically large tumors [four out of 10 tumors were staged T2 (40%), three tumors were T3 (30%), and three were T4 (30%)]. All patients but two had clinical/radiological suspected nodal metastases.
Nine lesions having complete data were evaluated. Mean lesion size was 27.1 ± 15.02 mm [range, 6–47 mm) at the baseline; 16.08 ± 14.6 mm (range, 0–40 mm) after 4 months (T1), and 11.7 ± 12.9 mm (range, 0–30 mm) at 6 months follow-up (T2). In Table 2, the lesions’ size for each patient is listed. Imaging features were noted according to the Breast Imaging Report and Data System (BI-RADS) lexicon. Imaging features were noted according to the Breast Imaging Report and Data System (BI-RADS) lexicon as reported in Table 3. From baseline to T1, two out of seven (28%) lesions changed their shape from irregular to oval. None of the lesions changed their orientation with reference to the skin or margins. The only lesion described as complex at T1 had a combined posterior pattern at baseline. Furthermore, compared to the baseline, two lesions showed a better-defined duct infiltration after the first cycle of treatment. All lesions at T1 demonstrated absent vascularization. At T2, four lesions completely disappeared. Of the five visible lesions in the US, only 2/5 (40%) kept an irregular shape. Furthermore, all the lesions visible were hypoechoic with associated posterior shadowing. Five out of nine assessed lesions (55%) showed a clinical tumor response of 50% or more, including 4/5 (80%) complete responses and 1/5 (20%) partial responses. Changes in tumor sizes and shapes can be seen in Figure 1. Analysis of the area of cancer on bi-dimensional measurement before and after treatment showed that the mean values were 27.1 ± 15.02 mm at baseline, 16.08 ± 14.6 mm at T1, and 11.7 ± 12.9 mm at T2, respectively. All patients (100%%) showed a response in the US. In four cases, the lesion size halved as compared with basal.
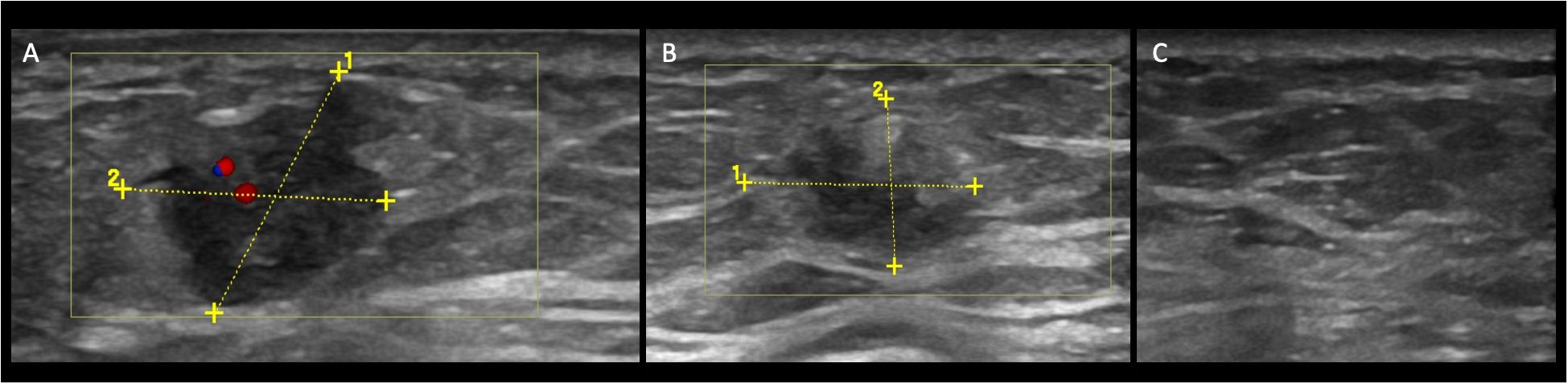
Figure 1 Case patient no. 4. (A) Color-Doppler US demonstrates a hypoechoic mass with an irregular shape and not-circumscribed margin, vertical orientation posterior, and internal vascularization at baseline. (B) At T1, the lesion changed the size (14 mm to 11 mm), and the vascularization at Color Doppler is now absent. The echo pattern remained hypoechoic. At T2 (C), the lesion is not visible anymore.
The two patients that underwent surgery were selected because of a metabolic complete response by PET/CT. In detail, one of them had a complete radiological response, the other reported a partial response. The radiological complete response found confirmation in a pCR, while the partial response matched an RCB-II (moderate residual disease). In this latter case, Ki-67 lowered from 40% to 6%.
Treatment with the CDK 4/6 inhibitors was generally well tolerated. In detail, only two patients aged more than 65 required a lower dose, and only one patient aged 84 years needed both dose reduction and prolonged drug rest to allow hematological recovery.
Approximately 80% of patients with breast cancer are diagnosed at an age >50, and luminal A BC is most diagnosed in over 70 years population (20). This epidemiological landscape requires customized management in real-world practice that regards the frequent old age of our patients’ population and acceptance of treatment/diagnostic procedures.
HR+ breast cancer larger than 2 cm, with involved lymph nodes and high Ki-67 index, have been considered candidates for preoperative treatments. Assessment of breast cancer treatment response is challenging, with the potential of underestimation or overestimation of residual cancer for the available different imaging techniques. Both morphological and functional imaging methods can be used to assess the response to treatments. Morphological techniques, such as full-field digital mammography (FFDM), digital breast tomosynthesis (DBT), and the US, and advanced techniques, such as breast MRI, contrast-enhanced spectral mammography (CESM), 18F-FDG PET/CT, and MRI, are nowadays pivotal in breast cancer. The selection of the imaging methods depends on the availability and should always be established in the multidisciplinary tumor board (21–25).
Ultrasound may be useful to predict the molecular subtype before pathological diagnosis (26) as reported by Zhu et al., having evaluated ultrasound features concerning more than 80 patients. This report emphasizes the value of the US in breast cancer that should not be neglected within the array of more complex radiological techniques.
In our series, the number of each histotype (Table 1) limited an extensive description of US reports correlated to histology.
US is superior to FFDM, DBT, and clinical breast examination in monitoring the response to NAC and is overall well tolerated by the patients. Sonographic evaluation of the residual tumor includes not only the size but also changes in tumor echogenicity and a decreased mass stiffness, and both could be considered good predictors of a pCR (27, 28). US is not used for RECIST due to operator dependency. However, US is easily accepted by patients and may be routinary and repeatedly performed. Moreover, the percentage of US diameter reduction after three cycles of treatment showed acceptable sensitivity and specificity response in a study on 64 patients (27).
In our cases, US demonstrated how the lesions changed not only in size but also in echogenicity and, by using the BI-RADS lexicon, the associated features described at the baseline. The most salient changes were the absence of vascularization after the first cycle of treatment and the changes in echogenicity after the second cycle of treatment.
Several factors may influence US breast evaluation. Body fat-driven obesity and breast fat density and their changes according to menopausal status are known factors influencing radiological assessment (29).
Efforts have been devoted to unraveling the impact of body mass index (BMI) on the therapeutic response among breast cancer patients, yet controversy persists. A recent meta-analysis disclosed that overweight/obese patients exhibited a lower pCR rate compared to under/normal weight counterparts (30).
In detail, also the assessment of the axilla may change with varying patient BMI, thus possibly conditioning the selection of a given radiological technique (31).
Defining a precise sensitivity for ultrasound alone proves challenging, with variations strongly dependent on lesion size, breast tissue type, and, as with all methods, patient selection.
While breast density stands as an independent risk factor for breast cancer, the widely acknowledged association between overweight/obesity and breast cancer development, particularly impacting the prognosis of HR-positive postmenopausal breast cancer, prompts further exploration. Various studies suggest that factors such as the chronic inflammatory state, circulating adipokines, insulin, insulin-like growth factor (IGF), and sex hormones may play pivotal roles in mediating the link between overweight/obesity and breast cancer (32).
The histological BC subtypes notoriously correlate with a different response to medical treatment. A different sensitivity in detecting pCR after NAC was found to be related to parameters such as estrogen receptor expression, on the one hand and HER-2 overexpression, luminal B, or Ki-67 proliferation >14%, on the other hand, respectively favoring MRI and PET-CT (33). Therefore, the need for radiological studies set by subtypes is progressively acknowledged (34).
While luminal A tumors are poorly chemoresponsive and better candidates for the association of CDK4/6 and aromatase inhibitors (CDK 4/6 I+AI), luminal B tumors represent an area of investigation. The NCT04137640 (35) is investigating the efficacy of the CDK 4/6 inhibitor palbociclib plus letrozole in comparison to chemotherapy in locally advanced breast cancer with ≤30% Ki-67 with group allocation according to Ki-67. The PREDIX LumB (NCT02603679) (35) is a phase II study that enrolled luminal B tumors and compares two sequences of treatments: weekly paclitaxel versus the combination of the CDK 4/6 inhibitor palbociclib and standard endocrine treatment for an initial phase of 12 weeks with following cross-over for further 12 weeks of treatment (neoadjuvant phase: 24 weeks) and adjuvant chemotherapy for both groups.
RIBOLARIS (NCT05296746) (35) used ribociclib as neoadjuvant treatment for clinically high-risk ER+ and HER2− breast cancer with a choice of CDK 4/6 inhibitor prosecution or alternately treatment with chemotherapy in the adjuvant phase according to the pathological and biological response assessed by Prosigna.
CDK 4/6 I+AI combination shows distinctive features as compared to other therapies. First, from a biological point of view, the inhibition of growth arrest may be reversible. CDK4/6 inhibition induces cytostatic effect in cell cultures and solid tumor models with potential regression associated with the intrinsic tumor cell turnover (36). Variably, according to the studied models, growth arrest may be irreversible or not (36). This aspect translates into Ki-67 changes during and at treatment, stopping with a rapid rise while off-therapy. From a clinical point of view, further consequences are hypothetical prolonged treatments to achieve the best response and redefinition of the best time for surgical intervention, which, in clinical studies, canonically falls 16–20 weeks after the start of treatment. This window may be functional for chemo- but not for CDK 4/6 inhibitor-based treatment. In line with this concept, NEOLETRIB trial (NCT05163106) (35), RIBOLARIS, and FELINE pre-specified a study treatment of 24 weeks or at least 6 months.
Ki-67 determination at 2–4 weeks of treatment has been considered the most accurate surrogate endpoint in neoadjuvant endocrine treatments. However, the recognized limits are represented by heterogeneous Ki-67 within the tumor, which required more than one biopsy to best collect comprehensive information and the invasive nature of repeated biopsies (37). From a biological approach, we then moved to a radiological-centered approach. This pattern has been typically described by MRI but is also detected by US and results to be predictive of worse outcomes compared to concentric shrinkage (38).
Similar to MRI, US is a technique that does not expose the woman to radiation and its related risks. However, opposite to MRI, there are no contraindications to the breast US. Furthermore, breast US is a valid tool that is widely available, and it is not prone to time slots and shortage of equipment as much as MRI. Furthermore, breast US is cheap, its cost being comparable to that of mammography and much lower than that of breast MRI REF. In Italy, the cost of a single breast MRI (250€) is up to seven times higher than that of one breast US (35.89 €). Yet, these ways are less expensive compared to the costs of a breast MRI in the US (>500 $).
Therefore, the US preserves its role as an easy and practical tool to monitor local response to medical treatments, especially in unfit and elderly patients, reserving a delayed time for more demanding and expansive exams. Further prospective studies including a larger number of patients should define the value of each tool and best guide diagnostic guidelines drafting in NAC. At present, we highlight the role of a basic breast US in the follow-up of advanced/metastatic BC with in situ tumors. In a time of limited economic resources, the appropriate use of each radiological technique should be encouraged.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Campania Centro Ethical Committee. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
LM: Writing – review & editing, Writing – original draft. LDM: Writing – review & editing, Resources, Investigation, Conceptualization. MM: Writing – review & editing, Writing – original draft, Supervision, Project administration, Formal analysis, Data curation. VR: Writing – review & editing, Visualization, Supervision. NG: Writing – review & editing, Validation, Supervision. LA: Writing – review & editing, Visualization, Validation, Supervision. MB: Writing – review & editing, Visualization, Validation, Supervision. GF: Writing – review & editing, Visualization, Validation, Supervision.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank the following: Campania Regional Government Lotta alle Patologie Oncolog-iche iCURE (CUP B21C17000030007); MIUR Proof of Concept (POC01_00043); and MISE2020: Nabucco 1682; VALERE: Vanvitelli per la Ricerca Program: EPInhibitDRUGre (CUP B66J20000680005); and Bando giovani ricercatori D.R. n.834 del 30/09/2022 Università Vanvitelli: IDEA (CUP: B63C22001470005) for supporting NG and LA team.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1295772/full#supplementary-material.
1. Cardoso F, Paluch–Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. (2020) 31:1623–49. doi: 10.1016/j.annonc.2020.09.010
2. Torrisi R, Marrazzo E, Agostinetto E, De Sanctis R, Losurdo A, Masci G, et al. Neoadjuvant chemotherapy in hormone receptor–positive/HER2–negative early breast cancer: When, why, and what. Crit Rev Oncol Hematol. (2021) 160:103280. doi: 10.1016/j.critrevonc.2021.103280
3. van der Voort A, Liefaard MC, van Ramshorst MS, van Werkhoven E, Sanders J, Wesseling J, et al. Efficacy of neoadjuvant treatment with or without pertuzumab in patients with stage II and III HER2–positive breast cancer: a nationwide cohort analysis of pathologic response and 5–year survival. Breast. (2022) 65:110–5. doi: 10.1016/j.breast.2022.07.005
4. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. KEYNOTE–522 investigators. Pembrolizumab for early triple–negative breast cancer. N Engl J Med. (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
5. Gu J, Groot G, Holtslander L, Engler–Stringer R. Understanding women’s choice of mastectomy versus breast conserving therapy in early–stage breast cancer. Clin Med Insights Oncol. (2017) 11:1179554917691266. doi: 10.1177/1179554917691266
6. Zeidman M, Alberty–Oller J, Ru M, Pisapati KV, Moshier E, Soojin Ahn S, et al. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor–positive breast cancer: a National Cancer Database (NCDB) study. Breast Cancer Res Treat. (2020) 184:203–12. doi: 10.1007/s10549-020-05809-w
7. Haque W, Verma V, Hatch S, Klimberg VS, Butler EB, Teh BS. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat. (2018) 170:559–67. doi: 10.1007/s10549–018–4801–3
8. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart–Gebhart M, Thürlimann B, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
9. Feeley LP, Mulligan AM, Pinnaduwage D, Bull SB, Andrulis IL. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Mod Pathol. (2014) 27:554–61. doi: 10.1038/modpathol.2013.153
10. Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. (2021) 32:1216–35. doi: 10.1016/j.annonc.2021.06.023
11. Cabrera–Galeana P, Muñoz–Montaño W, Lara–Medina F, Alvarado–Miranda A, Pérez–Sánchez V, Villarreal–Garza C, et al. Ki67 changes identify worse outcomes in residual breast cancer tumors after neoadjuvant chemotherapy. Oncologist. (2018) 23:670–8. doi: 10.1634/theoncologist.2017–0396
12. Dieci MV, Guarneri V, Tosi A, Bisagni G, Musolino A, Spazzapan S, et al. Neoadjuvant chemotherapy and immunotherapy in luminal B–like breast cancer: results of the phase II GIADA trial. Clin Cancer Res. (2022) 28:308–17. doi: 10.1158/1078–0432.CCR–21–2260
13. Han W. Personalized neoadjuvant strategy in luminal A breast cancer to increase BCS rate (PLATO study). J Clin Oncol. (2020) 38:vi17. doi: 10.1093/annonc/mdy318.030
14. Bao X.–J, Wenli L, Sun T, Wang J–W, Liu X–F, Li M–X, et al. Clinical data analysis of CDKs expression and prognosis in breast cancer. WCRJ. (2023) 10:e2475. doi: 10.32113/wcrj_20231_2475
15. Hong K, Yao L, Sheng X, Ye D, Guo Y. Neoadjuvant therapy of cyclin–dependent kinase 4/6 inhibitors combined with endocrine therapy in HR+/HER2– breast cancer: A systematic review and meta–analysis. Oncol Res Treat. (2021) 44:557–67. doi: 10.1159/000518573
16. Groheux D. Predicting pathological complete response in breast cancer early. Lancet Oncol. (2014) 15:1415–6. doi: 10.1016/S1470–2045(14)71020–4
17. Li P, Wang X, Xu C, Liu C, Zheng C, Fulham MJ, et al. 18F–FDG PET/CT radiomic predictors of pathologic complete response (pCR) to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imaging. (2020) 47:116–1126. doi: 10.1007/s00259–020–04684–3
18. American College of Radiology. ACR BI–RADS atlas: breast imaging reporting and data system. 5th ed. Virginia, Reston: American College of Radiology (2013).
19. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. (2007) 25:4414–22. doi: 10.1200/JCO.2007.10.6823
20. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers (Basel). (2021) 13:4287. doi: 10.3390/cancers13174287
21. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. NCCN guidelines® Insights: breast cancer, version 4.2021. J Natl Compr Cancer Netw. (2021) 19. doi: 10.6004/jnccn.2021.0023
22. Park J, Chae EY, Cha JH, Shin HJ, Choi WJ, Choi Y–W, et al. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur J Radiol. (2018) 108:261–8. doi: 10.1016/j.ejrad.2018.09.032
23. Vinnicombe SJ, MacVicar AD, Guy RL, Sloane JP, Powles TJ, Knee G, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. (1996) 198:333–40. doi: 10.1148/radiology.198.2.8596827
24. Goldberg H, Zandbank J, Kent V, Leonov–Polak M, Livoff A, Chernihovsky A, et al. Chemotherapy may eradicate ductal carcinoma in situ (DCIS) but not the associated microcalcifications. Eur J Surg Oncol. (2017) 43:1415–20. doi: 10.1016/j.ejso.2017.04.011
25. Mun HS, Kim HH, Shin HJ, Cha JH, Ruppel PL, Oh HY, et al. Assessment of extent of breast cancer: comparison between digital breast tomosynthesis and full–field digital mammography. Clin Radiol. (2013) 68:1254–9. doi: 10.1016/j.crad.2013.07.006
26. Zhu J–Y, He H–L, Jiiang X–C, Bao H–W, Chen F. Multimodal ultrasound features of breast cancers: correlation with molecular subtypes. BMC Med Imaging. (2023) 23:57. doi: 10.1186/s12880–023–00999–3
27. Evans A, Whelehan P, Thompson A, Purdie C, Jordan L, Macaskill J, et al. Prediction of pathological complete response to neoadjuvant chemotherapy for primary breast cancer comparing interim ultrasound, shear wave elastography and MRI. Ultraschall Med. (2018) 39:422–31. doi: 10.1055/s–0043–111589
28. Dobruch–Sobczak K, Piotrzkowska–Wróblewska H, Klimonda Z, Roszkowska–Purska K, Litniewski J. Ultrasound echogenicity reveals the response of breast cancer to chemotherapy. Clin Imaging. (2019) 55:41–6. doi: 10.1016/j.clinimag.2019.01.021
29. Chu A, Sung P, Lee J, Cheun J–H, Hwang K–T, Lee K, et al. Association of body composition fat parameters and breast density in mammography by menopausal status. Sci Rep. (2022) 12:22224. doi: 10.1038/s41598-022-26839-y
30. Wang H, Zhang S, Yee D, Basu S, Beckwith H, Potter D, et al. Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: a meta–analysis. Breast Cancer. (2021) 28:618–29. doi: 10.1007/s12282-020-01194-w
31. Macaione I, Galvano A, Graceffa G, Lupo S, Latteri M, Russo A, et al. Impact of BMI on preoperative axillary ultrasound assessment in patients with early breast cancer. Anticancer Res. (2020) 40:7083–8. doi: 10.21873/anticanres.14736
32. Zhao C, Hu W, Xu Y, Wang D, Wang Y, Lv W, et al. Current landscape: the mechanism and therapeutic impact of obesity for breast cancer. Front Oncol. (2021) 11:704893. doi: 10.3389/fonc.2021.704893
33. Baysal H, Serdaroglu AY, Ozemir IA, Baysal B, Gungor S, Erol CI, et al. Comparison of magnetic resonance imaging with positron emission tomography/computed tomography in the evaluation of response to neoadjuvant therapy of breast cancer. J Surg Res. (2022) 78:223–32. doi: 10.1016/j.jss.2022.04.063
34. Jones EF, Hathi DK, Freimanis R, Mukhtar RA, Chien AJ, Esserman LJ, et al. Current landscape of breast cancer imaging and potential quantitative imaging markers of response in ER–positive breast cancers treated with neoadjuvant therapy. Cancers (Basel). (2020) 12:1511. doi: 10.3390/cancers12061511
36. Knudsen ES, Witkiewicz AK. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer. (2017) 3:39–55. doi: 10.1016/j.trecan.2016.11.006
37. Barchiesi G, Mazzotta M, Krasniqi E, Pizzuti L, Marinelli D, Capomolla E, et al. Neoadjuvant endocrine therapy in breast cancer: current knowledge and future perspectives. Int J Mol Sci. (2020) 21:3528. doi: 10.3390/ijms21103528
Keywords: breast cancer, hormone-responsive, radiology, ultrasound, CDK4/6 inhibitors
Citation: Montella L, Di Marino L, Marino MA, Riccio V, Del Gaudio N, Altucci L, Berretta M and Facchini G (2024) Case report: An ultrasound-based approach as an easy tool to evaluate hormone receptor-positive HER-2-negative breast cancer in advanced/metastatic settings: preliminary data of the Plus-ENDO study. Front. Oncol. 14:1295772. doi: 10.3389/fonc.2024.1295772
Received: 17 September 2023; Accepted: 11 March 2024;
Published: 16 April 2024.
Edited by:
Dirk Geerts, University of Amsterdam, NetherlandsReviewed by:
Anca Maria Cimpean, Victor Babes University of Medicine and Pharmacy, RomaniaCopyright © 2024 Montella, Di Marino, Marino, Riccio, Del Gaudio, Altucci, Berretta and Facchini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liliana Montella, bGlsaWFuYS5tb250ZWxsYUBhc2xuYXBvbGkybm9yZC5pdA==
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.