
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 12 February 2024
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1294745
Introduction: The risk that a large polyp (≥10 mm) evolves into high-grade dysplasia (HGD) is relatively high compared with that of a small/diminutive polyp (<10 mm). Recently, the detection of small and diminutive polyps has been substantially improved with the advancement of endoscopy. However, further research is needed on the role of the incidence of HGD caused by the co-occurrence of small and diminutive polyps in the progression of HGD. In this study, we aim to investigate whether and how the small and diminutive polyps correlate with the incidence of HGD in the population.
Methods: The pooled data were deeply analyzed from four published randomized controlled trials (RCTs) regarding colon polyp detection. All polyps detected were examined and confirmed by pathologists. The primary outcome was the composition ratio of the HGD polyps in each polyp size category.
Results: Among a total of 3,179 patients with 2,730 polyps identified, there were 83 HGD polyps confirmed, and 68 patients had at least one polyp with HGD. The risk of development of HGD was lower for a single small and diminutive polyp than for one large polyp (2.18% vs. 22.22%, P < 0.0001). On the contrary, the composition ratio for HGD from small and diminutive polyps was significantly higher than that from the large ones (68.67% vs. 31.33%, P < 0.0001). The combined number of HGD presented a trend negatively correlated to size.
Conclusions: Our data demonstrated that the absolute number of HGD significantly derives more from small and diminutive polyps than from the large ones, and the collective number of small and diminutive polyps per patient is indicative of his/her HGD exposure. These findings positively provide novel perspectives on the management of polyps and may further optimize the prevention of colorectal cancer.
Systematic Review Registration: http://www.chictr.org.cn, identifier ChiCTR1900025235, ChiCTR1800017675, ChiCTR1800018058, and ChiCTR1900023086.
Considerable evidence demonstrates that there is a close correlation between polyps and colorectal cancer (CRC) (1–12). High-grade dysplasia (HGD), characterized by marked complex glandular crowding and irregularity of glands, cribriform architecture, and intraluminal necrosis as architectural features, is one of the characteristics of advanced adenomas and is associated with an increased risk of developing CRC (13). In addition, more than 90% of colon cancers are adenocarcinomas, with HGD being the main precursors (13–17). Thus, it is critically important to identify patients who are more prone to CRC among the endoscopic screening cohort (10, 18). It has been well-acknowledged that there is a strong correlation between the diameter of colorectal polyps and the development of CRC (19–23). For instance, a study has shown that large-size polyps account for 51.1% of cases diagnosed as malignancy or HGD via histopathology, versus 18.7% from small and diminutive polyps (24). Similarly, Vleugels J.L.A. et al. concluded that polyps <10 mm in diameter have a lower fraction of malignancy transformation than large ones (25), even if it is considered to be neglectable for sigmoid and rectum colon (15). More recently, the detection rate of colon polyps has increased as colonoscopy advances the sensitivity and accuracy of detecting small and diminutive polyps (26), especially with artificial intelligence (AI), i.e., computer-aided detection (CADe), in identifying small and diminutive polyps in a real-time manner (27, 28). Following extensive literature research, it is noted that there are substantially large numbers of small and diminutive polyps detected, yet the contribution of small and diminutive polyps to the incidence of HGD is scarcely reported (19, 24, 29).
In the current study, we retrospectively investigated the constitution of HGD cases according to different size categories of polyps, aiming to provide more perspective on the management of polyps of different sizes and HGD.
We collected data from four prospective randomized controlled trials (RCTs) conducted at the Endoscopy Center of Sichuan Provincial People’s Hospital, China, from September 2017 to September 2019. Three of the studies were two-arm trials aiming to analyze the adenoma detection rate (ADR) (27, 30, 31), while the fourth was a two-arm tandem trial focusing on the adenoma miss rate (AMR) (32). The trials employed the same CADe System (EndoScreener, Wision A.I., China) as the AI intervention. In the four trials, all detected polyps were taken cold forceps biopsy or resected by snare polypectomy and pathologically diagnosed according to WHO standards (13). All colonoscopy procedures were performed by experienced endoscopists, and the diameter of the polyps was measured based on the endoscopists’ judgment by comparing fully opened biopsy forceps during colonoscopy procedures. All pathological diagnoses in four studies were performed by two pathologists, first diagnosed by one registrar and then reviewed by one staff specialist. Pathological slides containing HGD were then selected and confirmatory checked by the third pathologist according to WHO standards. Precancerous polyps and non-neoplastic polyps detected in these four RCTs were included, such as sessile serrated lesions (SSLs) and traditional serrated adenomas (TSAs), and polyps with invasive cancer were all excluded. The HGD diagnostic criteria for conventional adenomas were based on the definition of the WHO (2019, 5Th). In SSL, cases with serrated dysplasia, high-grade intestinal dysplasia, were regarded as SSLD and were included in the HGD group in the study, due to the high and rapid progression to carcinoma (33).
In this study, diminutive polyps refer to polyps with a diameter ≤5 mm, small polyps refer to those with a diameter of 6–9 mm, and large polyps refer to those ≥10 mm. The primary outcome was the composition ratio of the HGD polyps in each polyp size category, defined as the number of polyps containing HGD within each size category divided by the total number of polyps containing HGD. Notably, if a patient is found to have both large and small/diminutive polyps with HGD, this patient will be categorized under the group of patients with large-sized polyps with HGD at the patient-level analysis. Our secondary outcome is the absolute proportion of HGD between small/diminutive and large polyps, which was determined by dividing the number of polyps with HGD by the total number of polyps in each respective size category.
The two-sample proportion test was used as the primary endpoint is the compositional ratio between the HGDs found in small/diminutive polyps and large polyps, and it fitted the assumptions of the two-sample proportion test. The null hypothesis of the two-sample proportion test was that two proportions were equal, and we could reject the null hypothesis and say that the two compared proportions were significantly different when the P-value was less than 0.05.
Statistical analysis was performed with R studio V.3.4.0. Regarding the composition ratio of HGD in screening and diagnostic populations, the two-sample proportion test was conducted for statistical difference. Comparison of the HGD ratios between different size categories was performed using the χ2 test for categorical variables. A two-sided P-value of 0.05 was used as the threshold for statistical significance. Linear analysis was applied in this study.
All procedures performed in this study involving human participants were under the ethical standards of the institutional IRB and with the Helsinki declaration.
Due to the retrospective nature of the study, the Institutional Review Board (IRB) of the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital waived the need to obtain informed consent.
The protocols of the stated four trials were approved by the IRB of the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital. The trials were registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn) (Registration Nos.: ChiCTR1900025235, ChiCTR1800017675, ChiCTR1800018058, and ChiCTR1900023086, respectively). Written informed consent was obtained from all participants before the colonoscopy procedure in the four trials.
The four well-designed RCTs were two-arm designed and performed with large prospective cohorts in China, describing the improvement of ADR after deploying a novel CADe system during colonoscopy.
There were 3,179 patients included from four trials, and 2,730 polyps were detected, biopsied, and pathologically diagnosed, among which 83 (83/2,730, 3.04%) were found to have pathological evidence of HGD and 68 patients were found to have at least one polyp with HGD (Table 1). The classification of the polyp and the patient baseline details are shown in Supplementary Figures S1, S2 and Supplementary Table S1.
From a single polyp point of view, large polyps are more likely to harbor HGD compared with small and diminutive polyps. The risk of HGD for a single polyp is positively correlated with polyp size (r2 = 0.5396, P = 0.0002), and the positive correlation also holds on the sigmoid colon and rectum (r2 = 0.4098, P = 0.0024) (Figure 1).
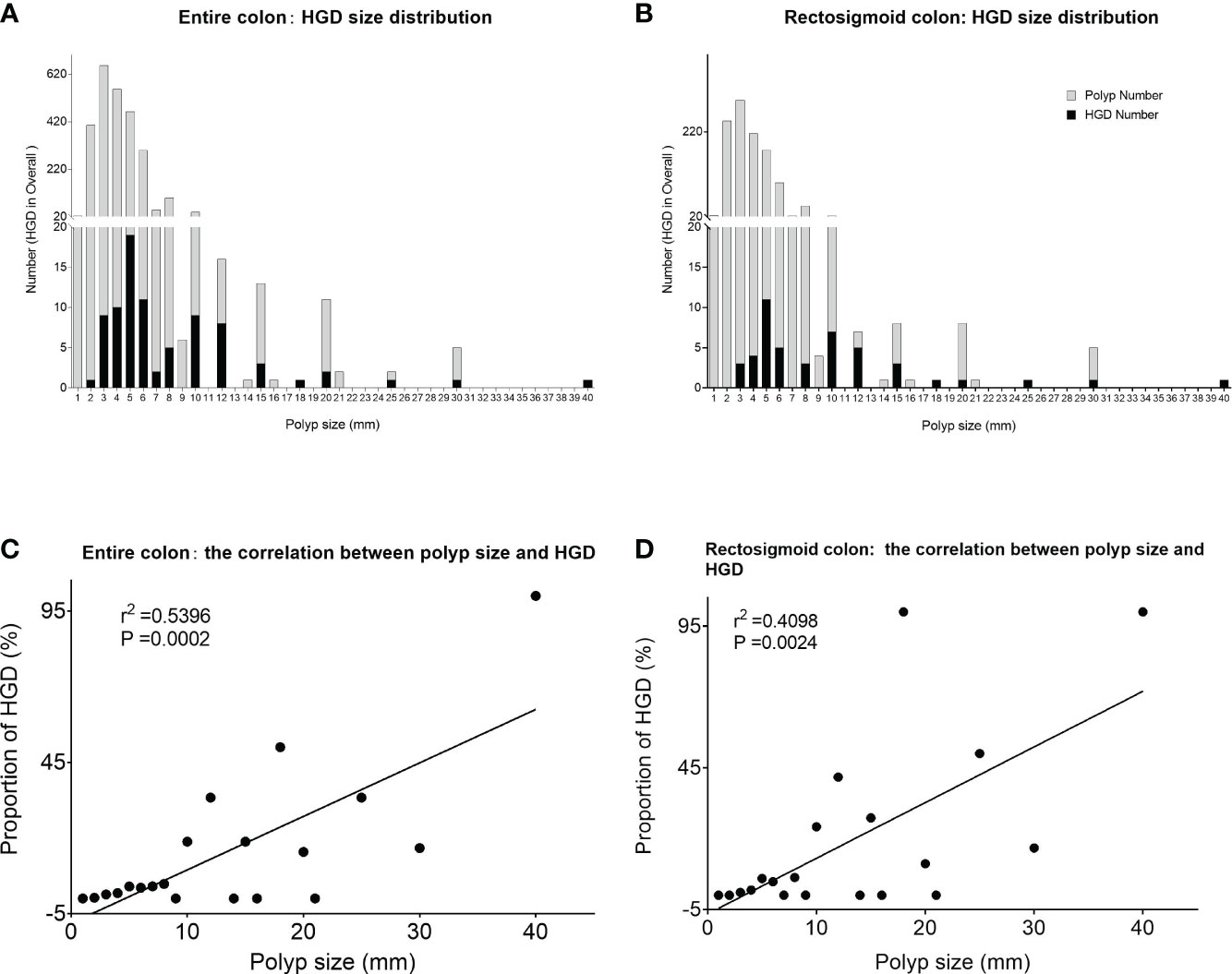
Figure 1 Size distribution and proportion of HGD polyps at the per-polyp level. (A) The distribution of HGD among all confirmed polyps of different sizes in the entire colon. (B) The distribution of HGD among all confirmed polyps of different sizes within the rectosigmoid colon. (C) Significant positive correlation between polyp size and HGD in the entire colon. (D) The significant positive correlation between polyp size and HGD within the rectosigmoid colon. *HGD, high-grade dysplasia.
Moreover, polyps with villous or tubulovillous features have a higher risk of HGD, 33.33% and 44.44%, respectively, compared with the other histology features (Table 2A). Within villous-structured polyps, larger ones are more likely to contain HGD in total. These data are consistent with the traditional view of assessing risk from the size of each polyp. On the contrary, from the perspective of composition ratio, which means the absolute number of HGD contributors, far more HGDs came from non-villous polyps (Table 2B). Due to the significantly higher quantity of small and diminutive polyps compared with large polyps, the total number of HGD contained within the small polyps far exceeds those within the large polyps. Similarly, the composition ratio of HGD derived from small and diminutive polyps was significantly higher compared with that from larger polyps (68.67% vs. 31.33%, p < 0.0001). Considering only rectosigmoid, there is also a trend that small/diminutive polyp groups contribute more HGD than large polyp groups (56.52% vs. 43.48%, p = 0.297) (Table 3). The composition ratio and individual risk of small/diminutive HGD polyps are detailed in Supplementary Table S2.
Interestingly, from the collective point of view, a trend was seen in the number of HGD polyps that were negatively correlated with the polyp size category (r2 = 0.1901, P = 0.0547, Figure 2).
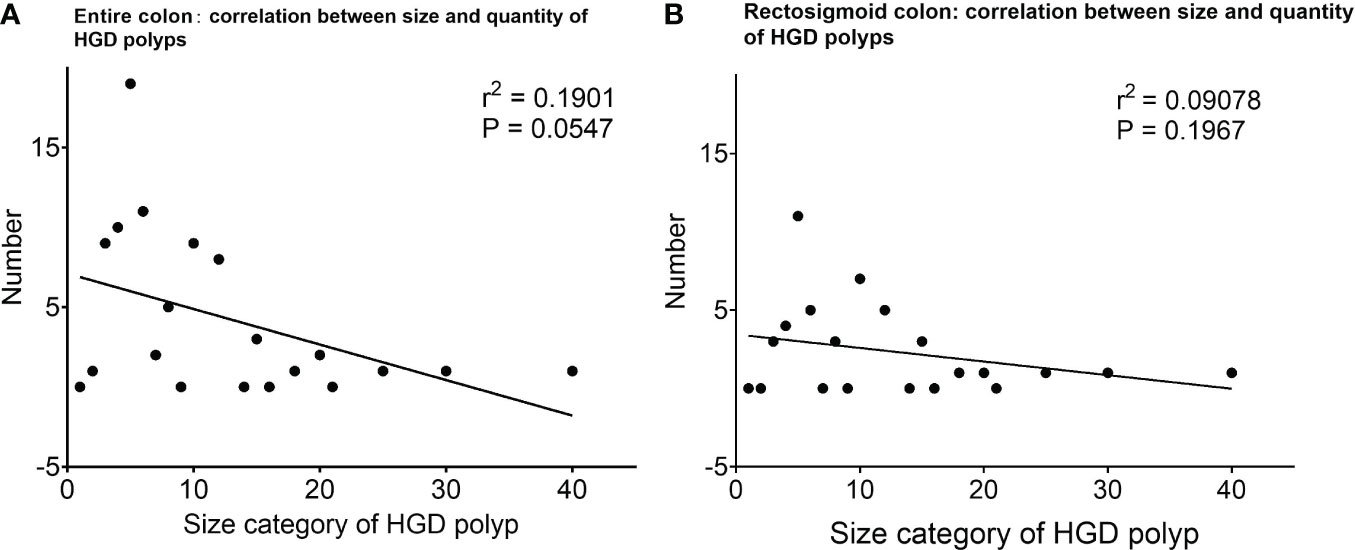
Figure 2 The number of HGD polyps at different size categories and their correlation. (A) The number of HGD polyps of different sizes, and the correlation analysis shows a trend that the number of HGD polyps is more related to small/diminutive polyps. (B) The number of HGD polyps of different sizes, and the correlation analysis shows a trend that the number of HGD polyps is more related to small/diminutive polyps within the rectosigmoid colon. *HGD, high-grade dysplasia.
The composition ratio of patients with large HGD polyps is far lower than that of patients with only small and diminutive polyps (36.76% vs. 63.24%, P = 0.0036). Meanwhile, in the rectosigmoid colon, the composition ratio of patients with large HGD polyps did not show a significant difference from patients with only small and diminutive polyps (52.63% vs. 47.37%, P = 0.8185) (Table 4).
Our data show that the majority of patients with HGD polyp only have one small HGD polyp, which indicates that the abundance of small and diminutive polyps might be the stronger related factor for patients to develop HGD (Figure 3).
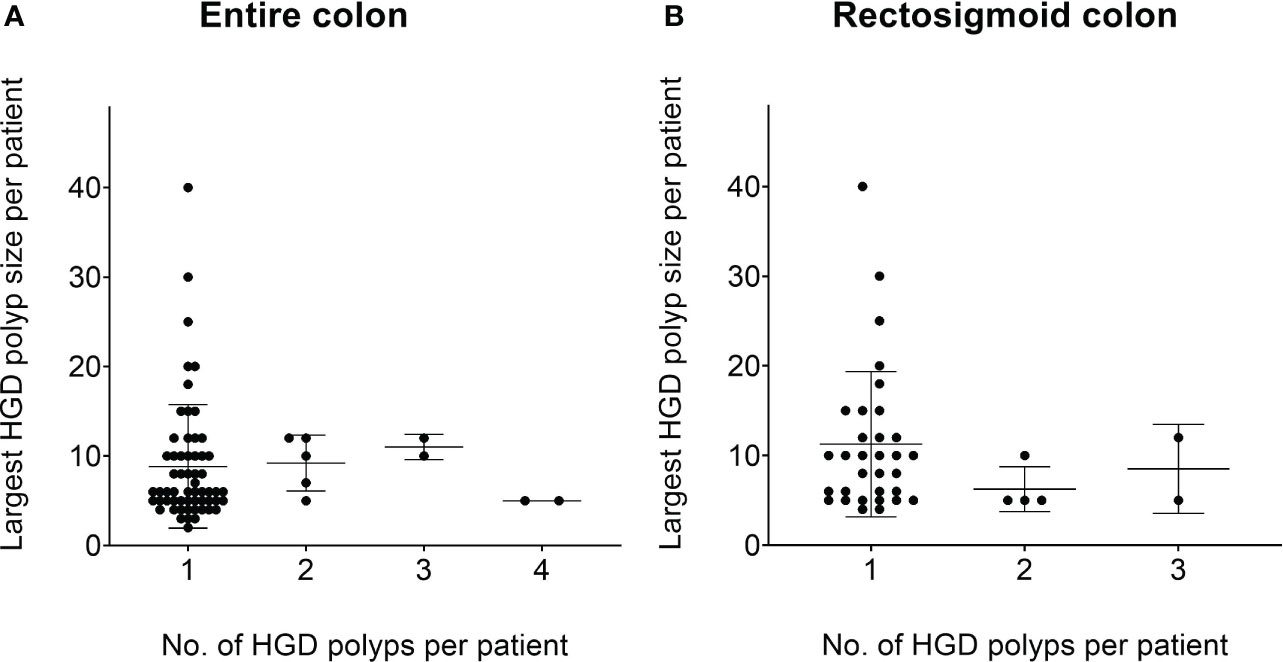
Figure 3 The number of HGD polyps at different size categories at the per-patient level. (A) The relationship between the average number of HGD polyps per patient and the size of HGD polyps per patient. It can be seen that the vast majority of patients with HGD polyps originate from a small/diminutive polyp. (B) A similar trend within the rectosigmoid colon. *HGD, high-grade dysplasia.
Our study revealed that, when considering the collective perspective, the majority of HGD incidences originated from small/diminutive and non-villous polyps. In addition, the combined number of HGD exhibited a trend of being negatively correlated with its size category. It is important to note that this does not conflict with the conventional viewpoint that large polyps are more likely to harbor HGD compared with small or diminutive ones (34). The conventional perception typically focuses on a single lesion, and our findings provide valuable insights into the cumulative risk associated with the abundance of small and diminutive polyps.
As well-documented in the literature among all adenomatous polyps (25), the ones with a diameter>=10mm, or containing villous histology, were generally considered equivalent indicators to the presence of HGD in determining a high-risk polyp (35). It is worth noting that the pathological nature of cancer is the high atypia of glands and cells (36), which is exactly what HGD represents. Therefore, we take HGD as the research object rather than subordinate features such as villous structure and polyp size. In addition, the concept of advanced adenoma is only a way to assess the risk of one single polyp and does not help to assess the overall risk of cancer in one patient, which leaves us to question the optimal approach to identifying high-risk patients. From the result of the study, a patient’s overall number of small and diminutive polyps emerges as a potential indicator of their risk for HGD presence and even the risk of CRC development, which is worthy of further investigation.
The small proportions of HGD within small and diminutive polyps have the potential to create a deceptive impression of safety, which could result in polyps being disregarded (35). Consequently, those polyps missed or untreated at the screening colonoscopy may be the cause of interval cancers, which account for approximately 8.2% of all CRC cases (1). In addition, patients whose polyps were removed during the screening colonoscopy but not pathologically evaluated have been advised a prolonged surveillance interval due to the unawareness of any possible HGD, which may also be the source of interval cancers due to the occurrence of metachronous advanced neoplasia during the prolonged surveillance interval (37–41). Our results might partially explain the interval CRC incidence in those days when small and diminutive polyps could not be effectively identified or treated.
The latest research further proves that with the application of high-definition imaging and CADe in colonoscopy, the number of small and diminutive polyps detected correctly has increased dramatically, and one issue was raised that the absolute number of HGD evolved from small and diminutive polyps is likely to be not lower than or even higher than large polyps both at the polyp and patient levels (16, 36, 42–44), which is very consistent with our results. Meanwhile, the neglect of small polyps is not only due to false sense induced by traditional statistical data focused on individual lesion risk but also due to the increasing application of optical biopsy (i.e., magnified narrow-band imaging) which allows endoscopists to predict pathological features via image enhanced endoscopy. Optical biopsy, recognized for its efficiency and cost-effectiveness, has gained increasing favor. In practice, endoscopists may often overlook small and diminutive polyps with a smooth surface, JNET 2A, III-L pit patterns, or lower classifications, as these clues usually indicate a low risk of malignant transformation (45–49). It is important to note that features on a plane such as mucosal microsurface structure and microvascular patterns have not been theoretically demonstrated to have the potential to achieve 100% one-to-one correspondence with stereoscopic histological features. Various studies have reported the accuracy of classification systems in the colon, such as Pit-Pattern, NICE, or JNET, ranging from 80% to 95%, with a notably low sensitivity of 40%–50% for advanced lesions (e.g., type 2B in the JNET system) (45–49). Consequently, optical diagnosis tends to underestimate the pathological characteristics of lesions (50–52), and any underestimation of pathological diagnosis may lead to the occurrence of interval colon cancer. In addition, there has been no universally recognized standard for the accuracy of predictions to be considered high enough in the long term. As a result of these limitations, these classification systems are currently employed as supporting tools to aid endoscopists in enhancing detection rather than serving as definitive diagnostic criteria to replace pathological diagnosis. Therefore, there is still controversy over whether to promote optical biopsy on a larger scale worldwide.
In current PIVI guidelines of ASGE, “diagnose-and-leave” and “resect-and-discard” strategies (53, 54) were recommended, from a population-based cost–benefit perspective, to treat optically diagnosed benign and diminutive polyps in the rectum and sigmoid colon, because these polyps are recognized to be less correlated with cancer development and metachronous cancer (34, 55, 56). However, the subgroup analysis in our study revealed a trend of a higher proportion of small-sized HGD not only in the entire colon but also in the sigmoid colon plus rectum. This finding suggests that an excessive disregard for small and diminutive polyps might pose a significant risk of interval cancer, even in the “safest colon segments”.
Furthermore, even from a cost-effectiveness and health economics perspective, the economic implications of these strategies differ across countries. In regions with higher healthcare costs or limited resources for colonoscopy and pathologists, adopting such strategies that prioritize optical diagnosis may reduce the financial burden on the healthcare system. Nevertheless, each patient should still reserve the right to make informed choices on the treatment of their colon polyps. In regions where colonoscopy is more available and affordable (57), the “detect more, resect all and evaluate more” might be a more suitable strategy.
We acknowledge several limitations in our study. Firstly, being a single-center study with a patient population limited to Asians, the generalizability of our findings may be constrained by the relatively confined genetic and population background. Secondly, a more comprehensive understanding could be achieved through subgroup analysis of different segments of the colon, thereby enhancing clinical relevance. Lastly, future research endeavors will explore potential treatment management strategies tailored to different sizes and/or segments of polyps during their progression toward malignancy.
In summary, this study seeks to reassess the overall CRC risk by considering the entire polyp population, offering a comprehensive perspective and anticipating that our research may contribute to a fresh understanding of colorectal cancer screening and prevention strategies. The results of the study underscore the significance of small and diminutive polyps in the context of CRC development and the potential of considering the total number of small and diminutive polyps as an indicator of HGD incidences. In clinical practice, endoscopists need to weigh the combined risk of HGD from all small and diminutive polyps, ensuring a holistic evaluation to minimize the risk of CRC progression. Future research can focus on region-specific cost–benefit analyses as well as the relationship between the progression of cancer and the size of HGD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision. HS: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing, Data curation. FX: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. SL: Conceptualization, Methodology, Writing – review & editing. GZ: Methodology, Writing – review & editing, Project administration. XX: Methodology, Writing – review & editing. LL: Writing – review & editing. PW: Writing – review & editing, Conceptualization, Data curation, Methodology, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LL is supported by NSFC Grant No.12101397 and No.12090024, Shanghai Science and Technology Commission Grant No.21ZR1431000 and No.21JC1402900, Shanghai Municipal Science and Technology Major Project No.2021SHZDZX0102 and CMA-Shanghai. LL is also affiliated with Shanghai Artificial Intelligence Laboratory and the Smart Justice Lab of Koguan Law School of SJTU.
We thank Dr. Jeremy R. Glissen Brown and Dr. Tyler M. Berzin for providing insightful feedback for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1294745/full#supplementary-material
Supplementary Figure 1 | The relationship between the size, morphology, location, histological subtype of polyps, and the quantity of HGD.
Supplementary Figure 2 | Epidemic risk factors for HGD polyps. Age over 45 and a family history of colorectal cancer are associated with HGD number. *HGD, High-grade dysplasia.
1. Dawwas MF. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med (2014) 370(26):2539–40. doi: 10.1056/NEJMc1405329
2. Vamosi-Nagy I, Koves I. Correlation between colon adenoma and cancer. Eur J Surg Oncol (1993) 19(6):619–24.
3. Calderwood AH, Lasser KE, Roy HK. Colon adenoma features and their impact on risk of future advanced adenomas and colorectal cancer. World J Gastrointest Oncol (2016) 8(12):826–34. doi: 10.4251/wjgo.v8.i12.826
4. Patel A, Williams N, Parsons N, Ali O, Peters F, Ranat R, et al. Risk factors for metachronous adenoma in the residual colon of patients undergoing curative surgery for colorectal cancer. Int J Colorectal Dis (2017) 32(11):1609–16. doi: 10.1007/s00384-017-2881-x
5. Arrospide A, Idigoras I, Mar J, de Koning H, van der Meulen M, Soto-Gordoa M, et al. Cost-effectiveness and budget impact analyses of a colorectal cancer screening programme in a high adenoma prevalence scenario using MISCAN-Colon microsimulation model. BMC Cancer (2018) 18(1):464. doi: 10.1186/s12885-018-4362-1
6. Lam YF, Seto WK, Tong T, Cheung KS, Lo O, Hung IF, et al. Rates of metachronous adenoma after curative resection for left-sided or right-sided colon cancer. Intest Res (2018) 16(4):619–27. doi: 10.5217/ir.2018.00013
7. Rho JH, Ladd JJ, Li CI, Potter JD, Zhang Y, Shelley D, et al. Protein and glycomic plasma markers for early detection of adenoma and colon cancer. Gut (2018) 67(3):473–84. doi: 10.1136/gutjnl-2016-312794
8. Harewood R, Wooldrage K, Robbins EC, Kinross J, von Wagner C, Cross AJ. Adenoma characteristics associated with post-polypectomy proximal colon cancer incidence: a retrospective cohort study. Br J Cancer (2022) 126(12):1744–54. doi: 10.1038/s41416-022-01719-4
9. Yun GY, Moon HS, Kwon IS, Kim JS, Kang SH, Lee ES, et al. Left-sided colectomy: one of the important risk factors of metachronous colorectal adenoma after colectomy for colon cancer. Dig Dis Sci (2018) 63(4):1052–61. doi: 10.1007/s10620-018-4958-y
10. Umar SB, Ramirez FC. The proof is in the pudding: improving adenoma detection rates reduces interval colon cancer development. Transl Gastroenterol Hepatol (2017) 2:99. doi: 10.21037/tgh.2017.11.10
11. Akimoto Y, Kudo SE, Ichimasa K, Kouyama Y, Misawa M, Hisayuki T, et al. Small invasive colon cancer with adenoma observed by endocytoscopy: A case report. World J Gastrointest Endosc (2020) 12(9):304–9. doi: 10.4253/wjge.v12.i9.304
12. Uraoka T, Horii J, Goto O, Shimoda M, Yahagi N. Metachronous adenoma on ileorectal anastomosis suture line and submucosal deep invasive cancer suspected of rapid growth in rectal remnant following long-term interval after curative surgery for advanced colon cancer. Dig Endosc (2013) 25 Suppl 2:46–51. doi: 10.1111/den.12094
13. Kushima R. The updated WHO classification of digestive system tumours-gastric adenocarcinoma and dysplasia. Pathologe (2022) 43(1):8–15. doi: 10.1007/s00292-021-01023-7
14. Washington MK, Goldberg RM, Chang GJ, Limburg P, Lam AK, Salto-Tellez M, et al. Diagnosis of digestive system tumours. Int J Cancer (2021) 148(5):1040–50. doi: 10.1002/ijc.33210
15. Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol (2009) 193(1):40–6. doi: 10.2214/AJR.08.1709
16. Gschwantler M, Kriwanek S, Langner E, Göritzer B, Schrutka-Kölbl C, Brownstone E, et al. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol (2002) 14(2):183–8. doi: 10.1097/00042737-200202000-00013
17. Ponz DLM, Di Gregorio C. Pathology of colorectal cancer. Dig Liver Dis (2001) 33(4):372–88. doi: 10.1016/S1590-8658(01)80095-5
18. Schoefl R, Ziachehabi A, Wewalka F. Small colorectal polyps. Dig Dis (2015) 33(1):38–41. doi: 10.1159/000366034
19. Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol (2006) 4(3):343–8. doi: 10.1016/j.cgh.2005.12.021
20. Ponugoti PL, Cummings OW, Rex DK. Risk of cancer in small and diminutive colorectal polyps. Dig Liver Dis (2017) 49(1):34–7. doi: 10.1016/j.dld.2016.06.025
21. Jeong YH, Kim KO, Park CS, Kim SB, Lee SH, Jang BI. Risk factors of advanced adenoma in small and diminutive colorectal polyp. J Korean Med Sci (2016) 31(9):1426–30. doi: 10.3346/jkms.2016.31.9.1426
22. Halfter K, Bauerfeind L, Schlesinger-Raab A, Schmidt M, Schubert-Fritschle G, Hölzel D, et al. Colonoscopy and polypectomy: beside age, size of polyps main factor for long-term risk of colorectal cancer in a screening population. J Cancer Res Clin Oncol (2021) 147(9):2645–58. doi: 10.1007/s00432-021-03532-7
23. Ito T, Takeuchi Y, Hanaoka N, Matsuura N, Hamada K, Uedo N, et al. Ten-millimeter advanced transverse colon cancer accompanied by a sessile serrated adenoma and/or polyp. Gastrointest Endosc (2015) 82(2):419–20; discussion 420. doi: 10.1016/j.gie.2015.02.010
24. Bretagne JF, Manfredi S, Piette C, Hamonic S, Durand G, Riou F. Yield of high-grade dysplasia based on polyp size detected at colonoscopy: a series of 2295 examinations following a positive fecal occult blood test in a population-based study. Dis Colon Rectum (2010) 53(3):339–45. doi: 10.1007/DCR.0b013e3181c37f9c
25. Vleugels JLA, Hazewinkel Y, Fockens P, Dekker E. Natural history of diminutive and small colorectal polyps: a systematic literature review. Gastrointest Endosc (2017) 85(6):1169–1176.e1. doi: 10.1016/j.gie.2016.12.014
26. Wang P, Xiao X, Glissen Brown JR, Berzin TM, Tu M, Xiong F, et al. Development and validation of a deep-learning algorithm for the detection of polyps during colonoscopy. Nat BioMed Eng (2018) 2(10):741–8. doi: 10.1038/s41551-018-0301-3
27. Wang P, Berzin TM, Glissen BJ, Bharadwaj S, Becq A, Xiao X, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut (2019) 68(10):1813–9. doi: 10.1136/gutjnl-2018-317500
28. Barkun AN, von Renteln D, Sadri H. Cost-effectiveness of artificial intelligence-aided colonoscopy for adenoma detection in colon cancer screening. J Can Assoc Gastroenterol (2023) 6(3):97–105. doi: 10.1093/jcag/gwad014
29. Martinez ME, Baron JA, Lieberman DA, Schatzkiner SJ, Greenberg ER. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology (2009) 136(3):832–41. doi: 10.1053/j.gastro.2008.12.007
30. Liu P, Wang P, Glissen BJ, Berzin TM, Zhou Liu X. The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study. Therap Adv Gastroenterol (2020) 13:1756284820979165. doi: 10.1177/1756284820979165
31. Wang P, Liu X, Berzin TM, Glissen Brown JR, Liu P, Zhou C, et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol (2020) 5(4):343–51. doi: 10.1016/S2468-1253(19)30411-X
32. Wang P, Liu P, Glissen Brown JR, Berzin TM, Zhou G, Lei S, et al. Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Gastroenterology (2020) 159(4):1252–1261.e5. doi: 10.1053/j.gastro.2020.06.023
33. Utsumi T, Yamada Y, Diaz-Meco MT, Moscat J, Nakanishi Y. Sessile serrated lesions with dysplasia: is it possible to nip them in the bud? J Gastroenterol (2023) 58(8):705–17. doi: 10.1007/s00535-023-02003-9
34. Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut (2020) 69(2):201–23. doi: 10.1136/gutjnl-2019-319858
35. Turner KO, Genta RM, Sonnenberg A. Lesions of all types exist in colon polyps of all sizes. Am J Gastroenterol (2018) 113(2):303–6. doi: 10.1038/ajg.2017.439
36. Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc (2012) 75(6):1218–25. doi: 10.1016/j.gie.2012.02.010
37. Chen TA. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med (2010) 363(14):1372–3. doi: 10.1056/NEJMc1006842
38. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology (2011) 140(1):65–72. doi: 10.1053/j.gastro.2010.09.006
39. Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med (2008) 359(12):1218–24. doi: 10.1056/NEJMoa0803597
40. Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M Interval cancers after negative colonoscopy: population-based case-control study. Gut (2012) 61(11):1576–82. doi: 10.1136/gutjnl-2011-301531
41. Park JH, Kang SH, Joo JS, Rou WS, Kim JS, Eun HS, et al. Effect of diminutive adenoma with high-grade dysplasia on surveillance colonoscopy interval. Dig Dis (2022) 40(5):545–52. doi: 10.1159/000520829
42. Vleugels JLA, Hassan C, Senore C, Cassoni P, Baron JA, Rex DK, et al. Diminutive polyps with advanced histologic features do not increase risk for metachronous advanced colon neoplasia. Gastroenterology (2019) 156(3):623–634.e3. doi: 10.1053/j.gastro.2018.10.050
43. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol (2006) 101(2):343–50. doi: 10.1111/j.1572-0241.2006.00390.x
44. Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol (2012) 13(1):55–64. doi: 10.1016/S1470-2045(11)70283-2
45. Koyama Y, Fukuzawa M, Kono S, Madarame A, Morise T, Uchida K, et al. Diagnostic efficacy of the Japan NBI Expert Team classification with dual-focus magnification for colorectal tumors. Surg Endosc (2022) 36(7):5032–40. doi: 10.1007/s00464-021-08863-7
46. Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, et al. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol (2012) 10(9):1016–20; quiz e79. doi: 10.1016/j.cgh.2012.05.004
47. Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology (2013) 144(1):81–91. doi: 10.1053/j.gastro.2012.09.054
48. Paggi S, Rondonotti E, Amato A, Terruzzi V, Imperiali G, Mandelli G, et al. Resect and discard strategy in clinical practice: a prospective cohort study. Endoscopy (2012) 44(10):899–904. doi: 10.1055/s-0032-1309891
49. Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc (2013) 78(4):625–32. doi: 10.1016/j.gie.2013.04.185
50. Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol (2009) 10(12):1171–8. doi: 10.1016/S1470-2045(09)70329-8
51. Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology (2008) 134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002
52. Zachariah R, Samarasena J, Luba D, Duh E, Dao T, Requa J, et al. Prediction of polyp pathology using convolutional neural networks achieves “Resect and discard” Thresholds. Am J Gastroenterol (2020) 115(1):138–44. doi: 10.14309/ajg.0000000000000429
53. Wang Y, Li WK, Wang YD, Liu KL, Wu J. Diagnostic performance of narrow-band imaging international colorectal endoscopic and Japanese narrow-band imaging expert team classification systems for colorectal cancer and precancerous lesions. World J Gastrointest Oncol (2021) 13(1):58–68. doi: 10.4251/wjgo.v13.i1.58
54. Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc (2016) 28(5):526–33. doi: 10.1111/den.12644
55. Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc (2017) 86(1):18–33. doi: 10.1016/j.gie.2017.04.003
56. ASGE Technology Committee, Abu DB, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc (2015) 81(3):502.e1–502.e16. doi: 10.1016/j.gie.2014.12.022
Keywords: colorectal cancer, CRC screening, small polyp, high-grade dysplasia (HGD), composition ratio
Citation: Zhang J, Sun H, Xiong F, Lei S, Zhou G, Xiao X, Liu L and Wang P (2024) The absolute number of small and diminutive adenomas with high-grade dysplasia is substantially higher compared with large adenomas: a retrospective pooled study. Front. Oncol. 14:1294745. doi: 10.3389/fonc.2024.1294745
Received: 15 September 2023; Accepted: 22 January 2024;
Published: 12 February 2024.
Edited by:
Nina Zidar, University of Ljubljana, SloveniaReviewed by:
Kateřina Kamarádová, University Hospital Hradec Kralove, CzechiaCopyright © 2024 Zhang, Sun, Xiong, Lei, Zhou, Xiao, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pu Wang, d2FuZ3B1aHVheGlAcXEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.