
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 10 May 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1294331
 Dina Poplausky1*
Dina Poplausky1* Jade N. Young1
Jade N. Young1 Brandon R. Block1*
Brandon R. Block1* Yeriel Estrada1
Yeriel Estrada1 Giselle K. Singer1
Giselle K. Singer1 Vicky Wong1
Vicky Wong1 Patricia Cabral1
Patricia Cabral1 Yamato Suemitsu2
Yamato Suemitsu2 Randie H. Kim1
Randie H. Kim1 Philip Friedlander3
Philip Friedlander3 Nicholas Gulati1
Nicholas Gulati1While typically low-risk, cutaneous squamous cell carcinoma (cSCC) can infrequently progress to metastatic disease with in-transit lesions, localized to the dermis or subcutaneous tissue between the primary tumor and draining regional lymph nodes. These lesions are associated with poor prognostic values, including decreased survival rates and increased risk of recurrence. We present the case of a 75-year-old male with cSCC and in-transit metastases on his scalp treated with the immune checkpoint inhibitor (ICI) pembrolizumab in conjunction with diphencyprone (DPCP), a topical hapten that induces a delayed-type hypersensitivity reaction in the skin. The patient was enrolled in a clinical trial (NCT05481658) that involved the twice-weekly application of DPCP 0.04% ointment to four of the in-transit metastases on his frontal scalp, concurrent with pembrolizumab 300 mg administered every three weeks. Following effective sensitization and a twelve-week treatment course, complete clearance of all lesions, DPCP-treated and non-DPCP treated, was achieved, with no adverse events. The immunologic profiles of the post-treatment biopsies were analyzed by TaqMan Low Density Array quantitative real-time polymerase chain reaction to measure immune marker gene expression. Relative to the non-DPCP-treated lesion, the DPCP-treated lesion demonstrated increased pro-inflammatory genetic markers and T-cell activation. This case represents the first reported instance of in-transit metastases of cSCC successfully treated with DPCP and an ICI. It highlights the potential safety and efficacy of DPCP with systemic immunotherapy for the management of in-transit metastases of cSCC in patients for whom surgery and radiation may be contraindicated.
Cutaneous squamous cell carcinoma (cSCC) accounts for about 20% of all skin cancers and has a rising incidence in the United States (1). Although typically low-risk, 1.2-5% of cSCCs metastasize (2). In-transit metastases are defined as distinct lesions originating in the dermis or subcutaneous tissues occurring before the first station of regional lymph nodes. Prior research on cutaneous metastasis is limited; however, they typically confer poor prognosis and are a manifestation of end-stage disease (3). A multicentric cohort study in March 2023 found that the size and number of in-transit metastases are associated with an increased risk of relapse, and the number of in-transit metastases is associated with decreased survival (4).
Treatment often includes a combination of surgery, radiation, and chemotherapy or immunotherapy. However, despite aggressive treatment options available, one-year survival is 45-69% (3). Immune checkpoint inhibitors, including programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) inhibitors, are increasingly used in various cancers. The Food and Drug Administration (FDA) approved the PD-1 inhibitors pembrolizumab and cemiplimab for the treatment of cSCCs not curable by surgery or radiation therapy. Pembrolizumab demonstrated a complete response (CR) rate of 10.5% and a partial response (PR) rate of 24.8% for patients with recurrent or metastatic cSCC. Cemiplimab demonstrated a CR rate of 6.8% and a PR rate of 40.7% in patients with metastatic cSCC (5).
Here we report the case of a 75-year-old male with in-transit metastases of cSCC treated with diphencyprone (DPCP) ointment and pembrolizumab.
A 75-year-old male with an extensive history of keratinocyte carcinomas presented to an outside dermatologist with a new scalp lesion. The clinical examination was notable for an ulcerated, erythematous indurated plaque on the vertex scalp. A biopsy was performed which revealed invasive cSCC, poorly differentiated with positive deep and peripheral margins, and a thickness of 5.5 mm. The patient then underwent Mohs micrographic surgery, and the tumor was removed in two stages. The frozen section pathology demonstrated aggregates of atypical keratinocytes in the dermis and subcutaneous tissue. The patient opted to heal by secondary intention. Two months after the primary tumor resection, the patient presented with multiple, firm, erythematous nodules on his scalp (Figure 1A). Biopsies of the right and left crown of the scalp revealed poorly differentiated cSCC representing in-transit metastases.
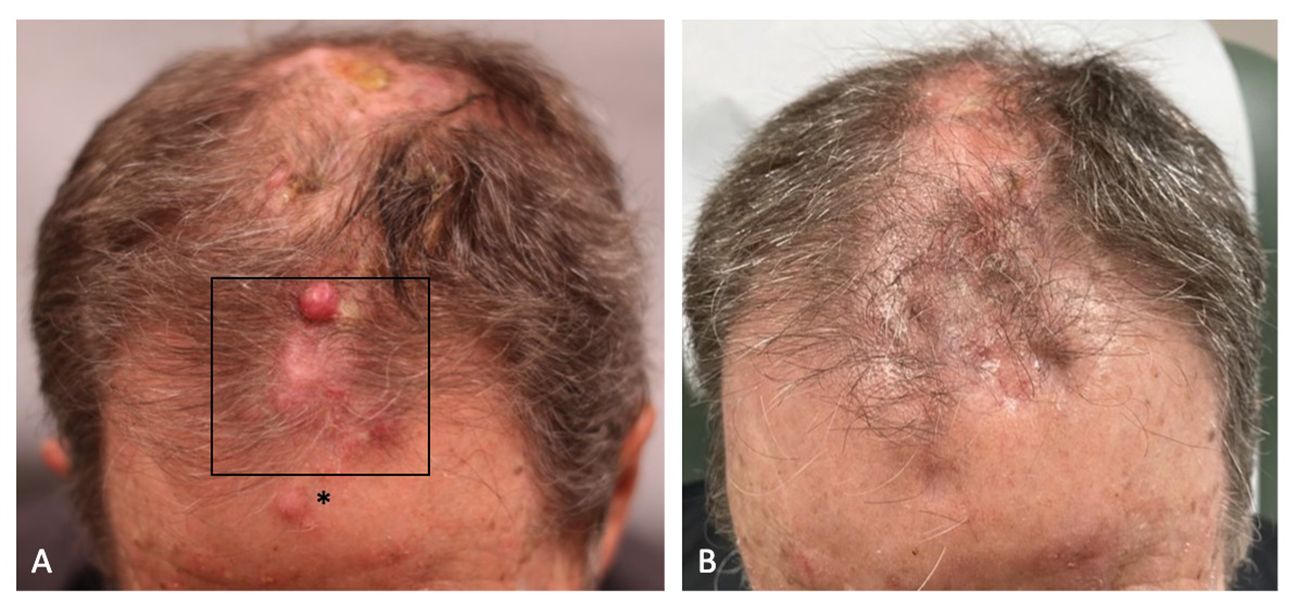
Figure 1 (A) The patient’s frontal scalp demonstrated erythematous nodules, consistent with in-transit metastases of cutaneous squamous cell carcinoma, before combination treatment with pembrolizumab and diphencyprone (DPCP). DPCP was applied to the boxed area. The asterisk denotes the area of sensitization. (B) Regression of the in-transit metastases after a 12-week course of combination treatment with pembrolizumab and DPCP.
Given the extent of his multifocal disease, he was not a candidate for surgical resection or radiotherapy and was therefore referred to medical oncology for systemic treatment. The workup included a positron emission tomography (PET) scan, which demonstrated multiple hypermetabolic soft tissue nodules throughout the scalp, compatible with biopsy-proven cSCC. There was no evidence of internal metastases. The patient began pembrolizumab 300 mg every three weeks as part of standard-of-care treatment. He was simultaneously enrolled in a clinical trial using DPCP ointment in conjunction with PD-1 or PD-L1 immune checkpoint inhibition for the treatment of cancer patients with cutaneous metastases (ClinicalTrials.gov identifier NCT05481658).
Cutaneous punch biopsies of lesional and non-lesional tissue were collected at baseline before systemic and topical oncologic therapy for hematoxylin and eosin (H&E) staining (Figures 2A, B) and were analyzed by TaqMan Low Density Array quantitative real-time polymerase chain reaction (TLDA qRT-PCR) to measure immune marker gene expression. The patient was sensitized to 0.4% DPCP on one of his in-transit metastases and his right upper arm, as well as to 0.04% DPCP on his left lower arm. Effective sensitization was confirmed two weeks later by notable erythema and scaling. The patient was then treated with DPCP 0.04% ointment twice weekly for 12 weeks, applied to four in-transit metastases on the frontal scalp. This topical treatment was started concurrently with pembrolizumab. Five in-transit metastases were not treated with DPCP.
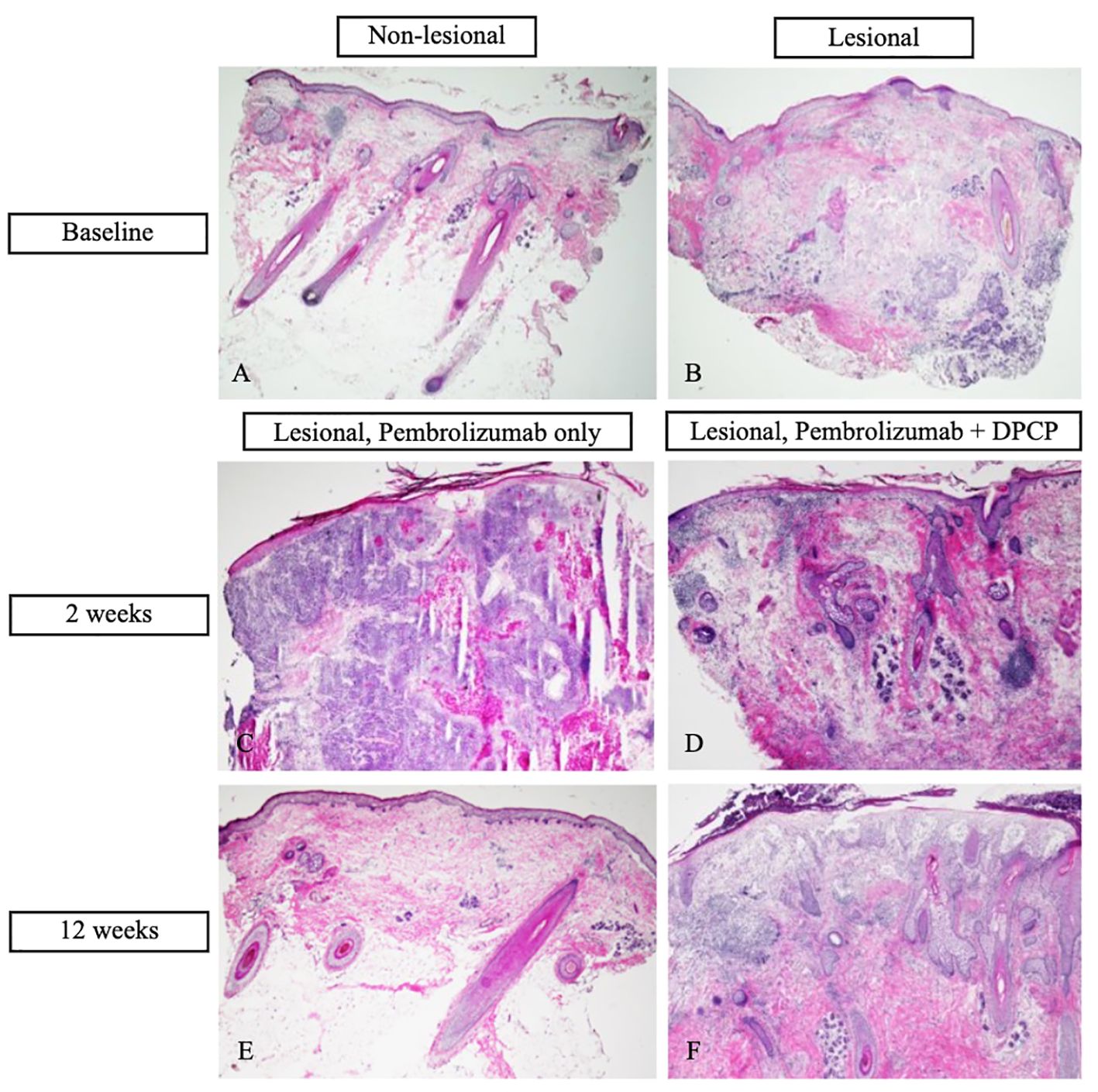
Figure 2 Hematoxylin and eosin (H&E) stains of cutaneous biopsies. (A) Baseline non-lesional skin demonstrates benign skin tissue, negative for tumor (20X magnification). (B) Baseline in-transit metastasis demonstrates moderately to poorly differentiated infiltrative cutaneous squamous cell carcinoma (cSCC) with focal keratinization completely within the dermis. There is mild inflammatory infiltrate associated with tumor proliferation (20X). (C) In-transit metastasis two weeks post-treatment with pembrolizumab only (diphencyprone (DPCP) not applied) demonstrates diffuse dermal infiltration of moderately to poorly differentiated cSCC with focal keratinization (20X). (D) In-transit metastasis two weeks post-treatment with DPCP and pembrolizumab demonstrates lichenoid dermatitis with diffuse dermal inflammatory and fibrotic changes. No cSCC is identified (40X). (E) In-transit metastasis after 12 weeks of treatment with pembrolizumab only demonstrates benign skin tissue with minimal changes including mild dermal fibrosis and perivascular inflammatory infiltrate (20X). (F) In-transit metastasis after 12 weeks of treatment with DPCP and pembrolizumab demonstrates spongiosis, superficial dermal edema, patchy inflammatory infiltrate, and dermal fibrosis. No cSCC is identified (40X).
After two weeks of dual treatment with pembrolizumab and DPCP, regression was observed for the DPCP-treated metastases only. Given the positive clinical response, additional cutaneous biopsies were performed of a DPCP-treated metastasis and a non-DPCP-treated metastasis (Figures 2C, D). At the end of the 12-week treatment period, the patient had resolution of all metastases, including the DPCP-treated and non-DPCP-treated lesions (Figure 1B). At this time, follow-up biopsies of a prior DPCP-treated metastasis and a non-DPCP-treated metastasis were taken (Figures 2E, F). The patient has continued pembrolizumab per the treating oncologist. At the time of publication, the patient is on month 18 of pembrolizumab treatment. A repeat PET scan demonstrated a reduction in the metabolic activity of the previously noted soft tissue lesions in the scalp, suggestive of a favorable tumor response. The patient was monitored for adverse events at each twice-weekly visit. The side effects of DPCP included expected erythema, skin irritation, and mild pruritus, all of which were tolerable to the patient and managed with conservative treatment. There were no DPCP drug interruptions due to toxicities. At the patient’s most recent follow-up four months after his last DPCP treatment application, the patient remains clinically clear of cutaneous metastases.
The immunologic profiles of the post-treatment biopsies were characterized by TLDA qRT-PCR. Relative to the non-DPCP-treated lesion, the DPCP-treated lesion demonstrated upregulation of IL-2, ICOS, and PD-1 by 4.96-fold, 4.31-fold, and 10.70-fold, respectively. Moreover, CXCL1 and IL-1B gene expression were upregulated by 4.13-fold and 3.03-fold, respectively.
This is a case of a patient who achieved resolution of in-transit metastases of cSCC with DPCP and an ICI. To date, there is no reported use of topical treatments concurrent with systemic therapy for in-transit metastases of cSCC. Given that survival rates for end-stage cSCC are low, new treatment paradigms must be explored.
DPCP is a topical hapten that induces a delayed-type hypersensitivity reaction in the skin. It has historically been employed for the treatment of warts (6), but has more recently shown efficacy in the treatment of cutaneous metastases of melanoma (7). Of note, DPCP has also been used successfully in conjunction with an ICI for cutaneous metastases of melanoma (8).
DPCP has been shown to increase both PD-1 gene expression in tissue and PD-1 protein levels in the serum (9). By increasing the PD-1 targets, DPCP may amplify the inhibition induced by pembrolizumab (9), thus allowing for synergistic anti-tumor immune activation. Clinically and histopathologically, the patient’s DPCP-treated metastases demonstrated earlier regression in comparison to those treated with ICI alone. Therefore, the addition of a topical immunotherapy agent to systemic immunotherapy may be more effective than systemic monotherapy. While all lesions, both DPCP-treated and non-DPCP-treated, ultimately resolved in the setting of pembrolizumab, the earlier resolution of lesions with DPCP demonstrates the synergy with pembrolizumab and may have positive clinical implications for patients. Prior research suggests that DPCP is not systemically absorbed (10); however, the immune response to DPCP may affect areas not topically treated with DPCP (8, 11).
The comparison of non-DPCP-treated lesion and the DPCP-treated lesion isolates and quantifies DPCP synergism with a systemic ICI. IL-2 and ICOS are markers for T-cell activation (12, 13), which may contribute to its anti-tumor effect (14). In line with the current literature, PD-1 was upregulated in the DPCP-treated lesion, thereby augmenting the substrate available for pembrolizumab. Moreover, the upregulation of CXCL1 and IL-1B demonstrates increased activation of pro-inflammatory genetic markers (15, 16).
The patient tolerated the combination therapy well with minimal, expected skin inflammation. This topical modality may be particularly useful in patients for whom surgery or radiation may be contraindicated. Given that in-transit metastases are often a sign of terminal disease, patients may not be able to tolerate more aggressive treatments. Additionally, DPCP is an inexpensive therapy (17), having positive economic impacts on both patients and the healthcare system.
We describe, to our knowledge, the first case of in-transit metastases of cSCC treated successfully with DPCP and a PD-1 inhibitor, potentially due to synergistic immune activation. Our results indicate that, in this patient, DPCP was an effective and safe treatment in conjunction with systemic immunotherapy. Further exploration with long-term data and a larger patient population is needed to evaluate the safety, efficacy, and clinical utility of DPCP in conjunction with an ICI for in-transit metastases of cSCC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Human Research Protection Program at the Icahn School of Medicine at Mount Sinai (ISMMS). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. BB: Investigation, Project administration, Writing – original draft, Writing – review & editing, Data curation, Formal Analysis. YE: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. GS: Resources, Supervision, Writing – review & editing. VW: Resources, Supervision, Writing – review & editing. PC: Project administration, Supervision, Writing – review & editing. YS: Formal analysis, Resources, Writing – review & editing. RK: Formal analysis, Resources, Writing – review & editing. PF: Resources, Supervision, Writing – review & editing. NG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. American Skin Association, The Black Family Stem Cell Institute/The Skin Biology and Diseases Resource-based Center, and P30 Cancer Center Support Grant.
We would like to thank Dr. Digpal Gour, PhD for his contributions to this project, specifically his efforts in the laboratory and the generation of our qRT-PCR data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Jong E, Lammerts MUPA, Genders RE, Bouwes Bavinck JN. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. (2022) 36:6–10. doi: 10.1111/jdv.17728
2. Caudill J, Thomas JE, Burkhart CG. The risk of metastases from squamous cell carcinoma of the skin. Int J Dermatol. (2023) 62:483–6. doi: 10.1111/ijd.16164
3. Klein JC, McKesey J, Srivastava D, Nijhawan RI. In transit metastases of cutaneous squamous cell carcinoma: A single institution case series. J Am Acad Dermatol. (2023) 88:943–5. doi: 10.1016/j.jaad.2022.11.020
4. Marti-Marti I, Podlipnik S, Cañueto J, Ferrándiz-Pulido C, Deza G, Sanmartín O. Prognostic factors for satellitosis or in-transit metastasis in cutaneous squamous cell carcinoma: A multicentric cohort study. J Am Acad Dermatol. (2023) doi: 10.1016/j.jaad.2023.02.048
5. Hughes BGM, Munoz-Couselo E, Mortier L, Bratland Å, Gutzmer R, Roshdy O. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. (2021) 32:1276–85. doi: 10.1016/j.annonc.2021.07.008
6. Upitis JA, Krol A. The use of diphenylcyclopropenone in the treatment of recalcitrant warts. JCMS. (2002) 6:214–7. doi: 10.1007/s10227-001-0050-9>
7. Lôbo M de M, Calsavara VF, Ricci BV, Lopes Pinto CA, Bertolli E, Duprat Neto JP. Response rates of cutaneous melanoma metastases to diphencyprone: A meta-analysis. J Am Acad Dermatol. (2020) 83:1812–3. doi: 10.1016/j.jaad.2020.04.023
8. Gulati N, Carvajal RD, Postow MA, Wolchok JD, Krueger JG. Definite regression of cutaneous melanoma metastases upon addition of topical contact sensitizer diphencyprone to immune checkpoint inhibitor treatment. Exp Dermatol. (2016) 25:553–4. doi: 10.1111/exd.13030
9. Han J, Agarwal A, Young JN, Owji S, Luu Y, Poplausky D, et al. Proteomic profiling of a patient with cutaneous melanoma metastasis regression following topical contact sensitizer diphencyprone and immune checkpoint inhibitor treatment. Sci Rep. (2022) 12:22364. doi: 10.1038/s41598-022-27020-1
10. Berth-Jones J, Mc Burney A, Hutchinson PE. Diphencyprone is not detectable in serum or urine following topical application. Acta Derm Venereol. (1994) 74:312–3. doi: 10.2340/0001555574312313
11. Buckley, Keane, Munn, Fuller, Higgins, Vivier D. Recalcitrant viral warts treated by diphencyprone immunotherapy. Br J Dermatol. (1999) 141:292–6. doi: 10.1046/j.1365-2133.1999.02978.x
12. Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol. (2018) 36:411–33. doi: 10.1146/annurev-immunol-042617-053352
13. Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. (2001) 409:97–101. doi: 10.1038/35051100
14. Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. (1998) 10:588–94. doi: 10.1016/S0952-7915(98)80228-8
15. Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, et al. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. (2016) 6:33123. doi: 10.1038/srep33123
16. Cai Y, Xue F, Quan C, Qu M, Liu N, Zhang Y. A critical role of the IL-1β-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J Invest Dermatol. (2019) 139:146–56. doi: 10.1016/j.jid.2018.07.025
Keywords: cutaneous metastases, metastatic squamous cell carcinoma, diphencyprone (DCP, DPCP), immune check inhibitor (ICI), open-label clinical study, phase 1 cancer trials
Citation: Poplausky D, Young JN, Block BR, Estrada Y, Singer GK, Wong V, Cabral P, Suemitsu Y, Kim RH, Friedlander P and Gulati N (2024) Case report: Regression of in-transit metastases of cutaneous squamous cell carcinoma with combination pembrolizumab and topical diphencyprone. Front. Oncol. 14:1294331. doi: 10.3389/fonc.2024.1294331
Received: 14 September 2023; Accepted: 15 April 2024;
Published: 10 May 2024.
Edited by:
Simona Kranjc Brezar, Institute of Oncology Ljubljana, SloveniaReviewed by:
Rolando Perez-Lorenzo, Columbia University, United StatesCopyright © 2024 Poplausky, Young, Block, Estrada, Singer, Wong, Cabral, Suemitsu, Kim, Friedlander and Gulati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dina Poplausky, ZGluYS5wb3BsYXVza3lAdHVmdHMuZWR1; Brandon R. Block, YnJhbmRvbi5ibG9ja0BpY2Fobi5tc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.