
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 10 July 2024
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1293598
This article is part of the Research TopicFuture Frontiers in the Management of Metastatic Colorectal CancerView all 8 articles
Background: There is limited evidence of comparative results among different treatments for patients with unresectable colorectal liver metastases (CRLM) who have failed at least one line of previous systemic therapy. We aimed to compare the efficacy of systemic treatments among these patients through this investigation.
Methods: We collected randomized controlled trials (RCTs) reported in English up until July 2023, from databases including PubMed, Embase, Cochrane Library, ClinicalTrials.gov, and prominent conference databases, for this Bayesian network meta-analysis. Phase II or III trials that evaluated at least two therapeutic regimens were included. Primary outcome was overall survival (OS), secondary outcome was progression-free survival (PFS). Hazards ratios (HRs) with 95% confidence intervals (CIs) were used as effect size. Subgroup analysis was performed based on metastatic sites. The current systematic review protocol was registered on PROSPERO (CRD42023420498).
Results: 30 RCTs were included, with a total of 13,511 patients. Compared to chemotherapy, multi-targeted therapy (HR 0.57, 95% CI 0.37–0.87) and targeted therapy plus chemotherapy (HR 0.78, 95% CI 0.67–0.91) show significant advantages. Targeted therapy (HR 0.92, 95% CI 0.54–1.57) and local treatment plus chemotherapy (HR 1.03, 95% CI 0.85–1.23) had comparable performance. For patients with liver metastases, TAS-102 plus bevacizumab, aflibercept plus fluorouracil-based combination chemotherapy (CTFU), and bevacizumab plus capecitabine-based combination chemotherapy (CTCA) showed the best outcomes in terms of OS. Bevacizumab plus intensified CTFU, bevacizumab plus CTCA, and HAI followed by single-agent chemotherapy (SingleCT) performed the best regarding PFS. For patients with liver-limited metastases, aflibercept plus CTFU is the optimal choice in OS. For PFS, the best options were HAI followed by SingleCT, aflibercept plus CTFU, and panitumumab plus CTFU. For patients with multiple-site metastases, the best treatments were TAS-102 plus bevacizumab, bevacizumab plus CTCA, bevacizumab plus CTFU, and aflibercept plus CTFU.
Conclusion: Multi-targeted therapy and targeted therapy plus chemotherapy are the best treatment mechanisms. TAS-102 plus bevacizumab is superior in OS, the combination of anti-VEGF drugs like bevacizumab and aflibercept with standard chemotherapy is the preferred option for CRLM patients.
Colorectal cancer (CRC) is responsible for around 10% of all cancers diagnosed globally every year, making it the second most prevalent cancer in women and the third most common in men (1). More than half of all CRC patients develop metastases, with the liver being the most common site for distant metastasis (2, 3). Factors associated with colorectal liver metastases (CRLM) may include: the connection of the portal vein system between the colorectal and liver, which provides a rich blood supply; as well as the location and histological type of the primary tumor. Liver metastases are developed in approximately 50% of patients diagnosed with CRC, and synchronous liver metastases are present in 10–15% of patients (4, 5).
For patients with CRLM, curative resection is considered a standard approach (6). The application of this method is limited to only 20–30% of cases because of factors like tumor location, size, patients’ comorbidities, or unresectable disease. As a result, the 5-year survival rate decreases significantly to around 30% (7, 8). The efficacy of radiation therapy for CRLM patients is subject to debate, mainly due to the liver’s low tolerance for radiation doses compared to the higher doses needed to eradicate tumor cells. Nevertheless, percutaneous radiation treatments such as proton therapy and CyberKnife can be highly effective for patients with a few small, strategically located liver metastases. These therapies provide highly precise targeting, thereby minimizing damage to the surrounding healthy tissues. Conventional radiotherapy techniques are suitable for treating CRLM patients with normal liver function (9, 10). To enhance control over local lesions, suitable ablation treatments could be chosen based on location, treatment goals, and complications when surgical removal of liver metastases is not feasible (11). Systemic chemotherapy is typically given to patients with CRLM who are ineligible for resection (12–14). In recent decades, research has shown that patients with CRLM who received systematic chemotherapy experienced a significant improvement in their overall survival (OS) (15, 16). Regional therapies, including hepatic arterial infusion (HAI), conventional transarterial chemoembolization (cTACE), and transarterial radioembolization (TARE), serve as valuable alternatives for patients who exhibit limited response to initial chemotherapy. Particularly in cases of liver-only metastasis, it is essential to assess the number, size, and location of the tumors to effectively tailor the treatment approach. Integrating systemic chemotherapy with regional and percutaneous therapies often leads to superior outcomes. This holistic approach maximizes the benefits of each treatment modality, enhancing the precision and efficacy of the therapy, and ultimately improves patient prognosis (17). During the process of metastasis, tumors enhance their energy supply by promoting angiogenesis, which is why anti-angiogenesis therapy is crucial for the treatment of CRLM. In particular, bevacizumab and cetuximab have been created as molecularly targeted medications (18). According to Saltz et al., the efficacy of incorporating bevacizumab into XELOX or FOLFOX-4 was evaluated in 1401 CRLM patients (19). In the bevacizumab group, the median duration of progression-free survival (PFS) was 9.4 months, which was significantly longer than that of the placebo group (P = 0.0023). Furthermore, the addition of cetuximab to FOLFOX-4, in comparison to using FOLFOX-4 alone, resulted in a significant improvement in overall response. For patients with initially unresectable CRLM, the combination of targeted drugs and chemotherapy can also result in a higher rate of remission and enhanced resectability (20). As more advancements are made in understanding immune checkpoint in various cancer types, particularly in DNA mismatch repair defects (dMMR)/high microsatellite instability (MSI-H) CRC, immunotherapy has emerged as an appealing treatment option alongside targeted therapy (5).
Currently, there are multiple options available for previously treated patients with unresectable CRLM. Meanwhile, there is a lack of relative outcomes among these treatments. Therefore, we conducted this network meta-analysis (NMA) of randomized controlled trials (RCTs) to systematically compare the efficacy of all current treatment regimens on patients with unresectable CRLM who have failed at least one previous line of systemic therapy, and to offer healthcare clinicians, patients, and relevant guidelines with references in clinical medication and disease management.
We conducted our study in accordance with the guidelines outlined in the extension statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21). See Supplementary File 1. This systematic review protocol was registered on PROSPERO (CRD42023420498).
The search strategy is provided in Supplementary File 2. On July 31, 2023, we conducted thorough search included PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov to identify relevant RCTs and published studies. We did not impose any limitations on the publication date, and only considered studies published in English. Additionally, we incorporated abstracts from the European Society for Medical Oncology, American Society of Clinical Oncology starting from 2021.
Initially, titles and abstracts of the included articles were screened by two researchers. The eligibility criteria, which were based on the PICOS framework, were as follows:
(1) Population: Adult patients diagnosed histologically or cytologically with confirmed unresectable CRLM. Meanwhile, patients should receive at least one form of systemic treatment previously. No restrictions were placed on individual-level characteristics. Due to limited reporting in some RCTs, we assumed that patients who had metastases in two or more sites including liver metastases. Considering that the proportion of liver metastases reaches over 90% in patients with metastases in two or more sites (22, 23).
(2) Interventions and comparisons: We evaluated various systematic interventions, including pharmaceutical, surgical, radiological, and multi-mechanism therapies.
(3) Outcomes: Trials that reported hazard ratios (HR) of either OS or PFS.
(4) Study design: Phase II or III studies that evaluated various contrasting treatments were taken into account.
We only considered trials that offered the most recent and informative data to prevent repetition. Moreover, trials that investigated treatments unrelated to any comparisons were disregarded. Additionally, trials that explored different dosages but with the same administrations were also eliminated.
Two independent researchers (YJ and MZ) were responsible for extracting the required data. Any discrepancies that arose were resolved through discussions that involved other researchers (YJ, MZ, WT, and XZ). The extracted information was the characteristics of eligible trials (publication year, registration information, etc.), characteristics of populations (age, sample size, countries, etc.), and characteristics of the program (interventions, outcomes of endpoints, etc.). The clinical outcomes extracted included OS and PFS. The individual patient data from studies that only presented Kaplan-Meier curves without HR or 95% confidence intervals (CIs), was obtained using the tool developed by Liu et al. (24).
Quality of the studies was assessed using the risk of bias (ROB) tool from the Cochrane Collaboration (25). The eligible studies were divided into three categories based on their risk level: high, low, or uncertain (26). The Egger regression test was used to assess the presence of publication bias, considering p-values less than 0.10 as indicating biased results (27).
In this meta-analysis, the primary outcome was OS, while PFS served as a secondary outcome. Network plots were used to compare and visually display the various treatment options. Pooled HRs with 95% CIs were calculated for OS, PFS. In order to examine the synthesized HRs, the fixed effects consistency model was chosen because most of the direct evidence came from a single trial (28). Using the R package Gemtc, the Bayesian network meta-analysis (NMA) was carried out. A total of 50,000 samples were used, divided into four sets of Markov chains. Each set included 10,000 burn-in samples. Non-informative uniform and normal prior distributions were utilized (29). In addition, we performed calculations to determine the probability ranking for all available treatments and presented it using the surface under the cumulative ranking (SUCRA). A higher SUCRA value indicated a higher ranking.
The I2 statistic was used to assess the heterogeneity among the studies, with a moderate level of heterogeneity indicated by a value above 50% (29). Both direct and indirect evidence were considered when assessing the inconsistency of models using the edge-splitting method (29). In order to ensure the reliability of this study, numerous pairwise meta-analyses were conducted for comparison. The convergence of Markov chains was verified by utilizing Gelman-Rubin diagnostic statistics and trace plots (30).
In order to assess the robustness and reliability of the findings, and to assess the influence of metastatic sites, we performed subgroup analyses. We categorized the population into two groups: patients with liver-limited metastases and those with multiple-site metastases.
A total of 4979 records were obtained from the previously mentioned databases, out of which 1365 studies were determined suitable for full-text evaluation. Eventually, the analysis comprised of 30 RCTs, which were represented by 33 articles. The flow chart in Figure 1 illustrates this process. The characteristics of the included studies can be found in Table 1. This research study included a total of 13,511 patients diagnosed with mCRC. In order to form a comprehensive comparison, we divided chemotherapy into single-agent chemotherapy (SingleCT), fluorouracil-based combination chemotherapy (CTFU, defined as mFOLFOX6, FOLFOX4, FOLFOX, FLOX, FOLFIRI, fluorouracil/irinotecan, fluorouracil/leucovorin, or fluorouracil/oxaliplatin), capecitabine-based combination chemotherapy (CTCA, defined as CAPEOX, XELOX, OXXEL, XELIRI or capecitabine/mitomycin), and intensified CTFU (ICTFU, defined as FOLFIRINOX or mFOLFOXIRI). We unified best supportive care (BSC) and placebo as the same. There were 23 treatments involved, comprising aflibercept plus CTFU, anlotinib, bevacizumab plus CTCA, bevacizumab plus CTFU, bevacizumab plus ICTFU, BSC, cetuximab, CTCA, CTFU, famitinib, fruquintinib, HAI followed by SingleCT, napabucasin, nintedanib, panitumumab, panitumumab plus CTFU, ramucirumab, regorafenib, simvastatin plus CTFU, TACE plus CTFU, TARE plus CTFU, TAS-102 (trifluridine/tipiracil), and TAS-102 plus bevacizumab. Six treatment mechanisms were covered, including multi-targeted therapy, targeted therapy plus chemotherapy, targeted therapy, local treatment plus chemotherapy, chemotherapy, and BSC. For multi-targeted therapy, targeted therapy, and local treatment, the definitions are as follows: Multi-targeted therapy combines various agents and approaches to target tumor growth from multiple angles, integrating systemic chemotherapy with specific molecular-targeted drugs and regional treatments. Targeted therapy employs drugs that specifically attack cancer cells based on unique molecular markers, such as angiogenesis inhibitors, thereby minimizing damage to normal cells. Local treatment directly targets the liver tumor, using methods such as surgical resection, radiofrequency ablation (RFA), or transarterial approaches like TACE and TARE to deliver treatments directly to the tumor site.
The assessment of ROB is presented in Supplementary File 3. Overall, ROB in all RCTs was generally low. However, multiple RCTs were open-label (15, 18, 32, 34, 35, 38, 44, 46, 49–51, 53–55, 58–61), thereby raising concerns about participant and personnel blinding, outcome assessment. The results of the Egger test indicated no publication bias in our network, the funnel plots are displayed in Supplementary File 4.
For OS, network plot is provided in Figures 2A, B. Among the 21 intervention options for patients with liver metastases, the top three ranked were TAS-102 plus bevacizumab (SUCRA 0.889), aflibercept plus CTFU (SUCRA 0.818), and bevacizumab plus CTCA (SUCRA 0.798). When compared to CTFU, treatments that had significant advantages were ranked from best to worst as follows: TAS-102 plus bevacizumab (HR 0.58, 95% CI 0.36–0.93), aflibercept plus CTFU (HR 0.65, 95% CI 0.49–0.86), and bevacizumab plus CTCA (HR 0.68, 95% CI 0.49–0.94). No significant difference in comparison to CTFU for the other options. Among the six mechanisms, the rankings from highest to lowest were as follows: multi-targeted therapy (SUCRA 0.985), targeted therapy plus chemotherapy (SUCRA 0.746), targeted therapy (SUCRA 0.503), local treatment plus chemotherapy (SUCRA 0.286), chemotherapy (SUCRA 0.339), and BSC (SUCRA 0.141). Compared to chemotherapy, multi-targeted therapy (HR 0.57, 95% CI 0.37–0.87) and targeted therapy plus chemotherapy (HR 0.78, 95% CI 0.67–0.91) had significant advantages. Targeted therapy (HR 0.92, 95% CI 0.54–1.57) and local treatment plus chemotherapy (HR 1.03, 95% CI 0.85–1.23) showed consistent efficacy, when compared to chemotherapy. Targeted therapy plus chemotherapy was superior. Among them, aflibercept plus CTFU showed the best performance. In comparison to aflibercept plus CTFU, the performance ranked from good to poor were bevacizumab plus CTCA (HR 1.04, 95% CI 0.68–1.6), bevacizumab plus CTFU (HR 1.23, 95% CI 0.85–1.77), panitumumab plus CTFU (HR 1.31, 95% CI 0.9–1.91), and simvastatin plus CTFU (HR 1.41, 95% CI 0.88–2.26). There were no significant differences observed among targeted or local treatment plus chemotherapy regimens. Among local treatment, HAI had the best performance, followed by TACE and TARE. Among target therapies, fruquintinib had the best efficacy. Compared to fruquintinib, the treatments with no significant difference, ordered from best to worst, were regorafenib (HR 1.03, 95% CI 0.7–1.53), TAS-102 (HR 1.14, 95% CI 0.77–1.68), cetuximab (HR 1.19, 95% CI 0.48–2.9), and panitumumab (HR 1.41, 95% CI 0.65–3.05). Comparatively, anlotinib (HR 1.58, 95% CI 1.16–2.16), nintedanib (HR 2.29, 95% CI 1.64–3.19), ramucirumab (HR 1.66, 95% CI 1.12–2.44), and napabucasin (HR 2.29, 95% CI 1.64–3.19) showed significant differences in efficacy compared to fruquintinib. Furthermore, CTCA (HR 0.66, 95% CI 0.2–2.18) had better efficacy compared to CTFU. More details, please see Tables 2, 3 and Figure 3.
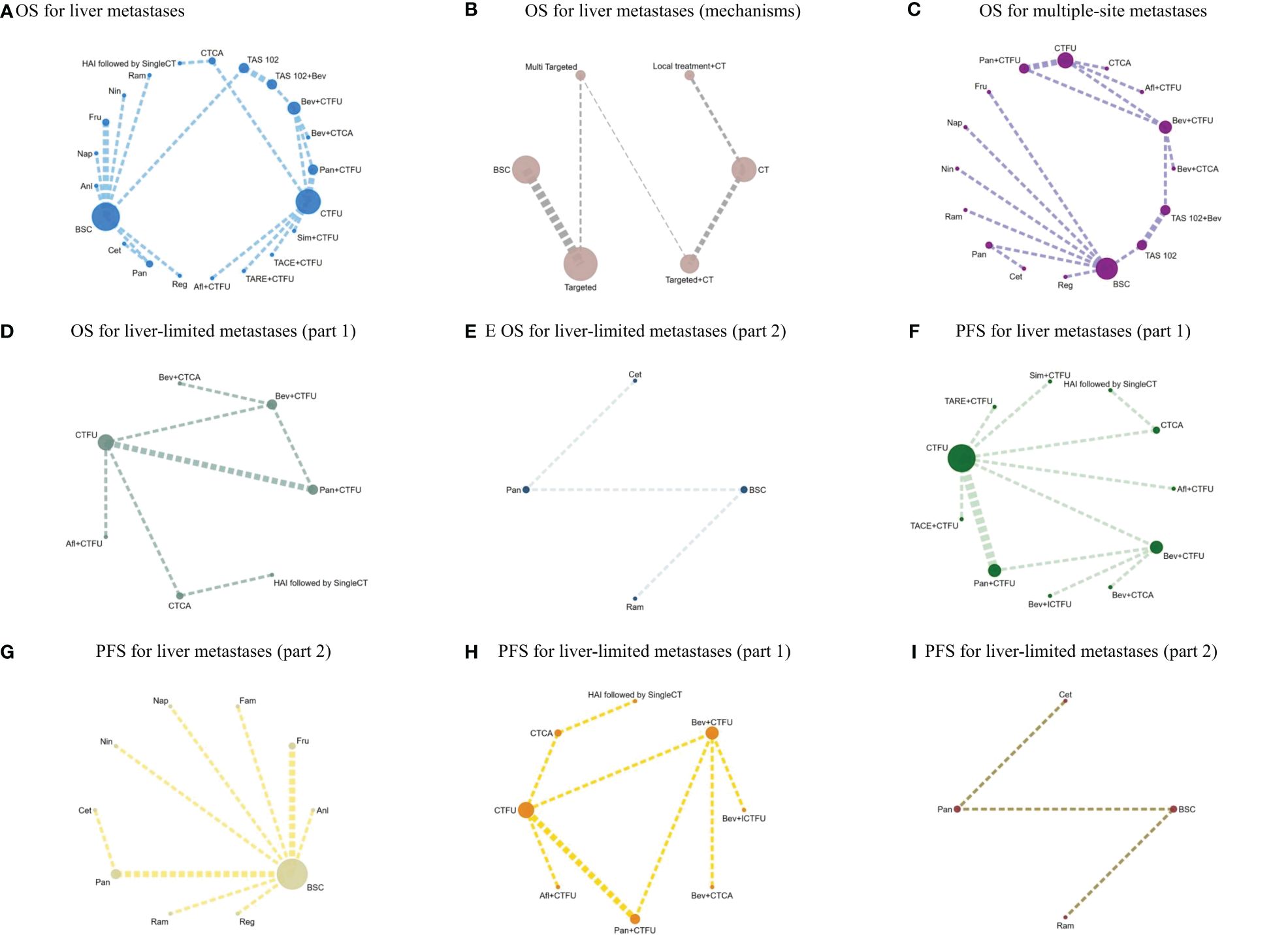
Figure 2 (A) OS for liver metastases; (B) OS for liver metastases (mechanisms); (C) OS for multiple-site metastases; (D) OS for liver-limited metastases (part 1); (E) OS for liver-limited metastases (part 2); (F) PFS for liver metastases (part 1); (G) PFS for liver metastases (part 2); (H) PFS for liver-limited metastases (part 1); (I) PFS for liver-limited metastases (part 2).
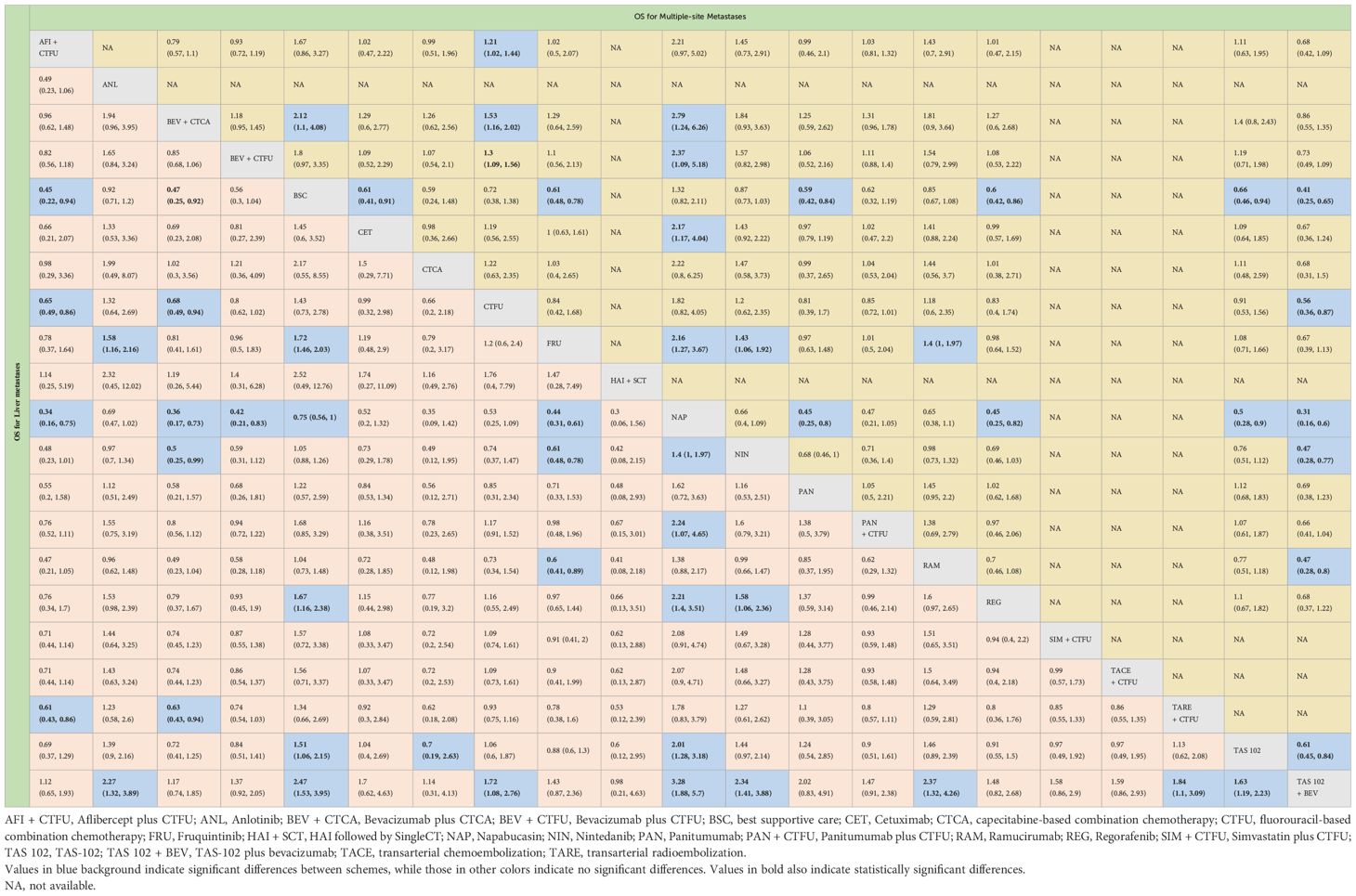
Table 2 Overall survival profiles of treatment regimens in metastatic CRC patients with liver metastases and multiple-site metastases.
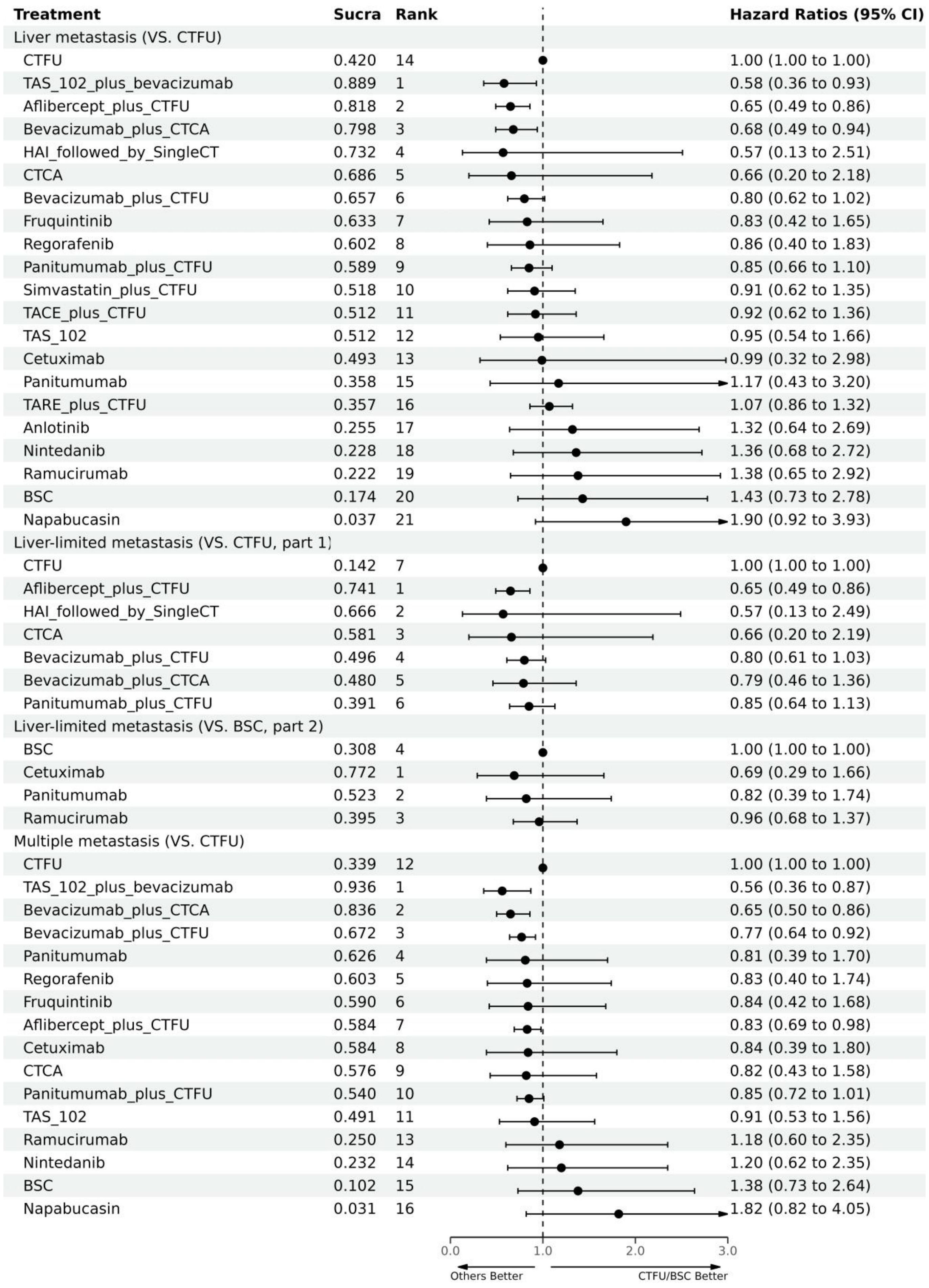
Figure 3 Forest plot for OS. CTFU, fluorouracil-based combination chemotherapy; CTCA, capecitabine-based combination chemotherapy; ICTFU, intensified chemotherapy containing four interventions; SingleCT, single-agent chemotherapy.
For PFS, due to the lack of sufficient data, we can only analyze monotherapy and combination therapies separately. Network plots is shown in Figures 2F, G. For combination therapies, the top three ranked regimes were bevacizumab plus ICTFU (SUCRA 0.887), bevacizumab plus CTCA (SUCRA 0.774), HAI followed by SingleCT (SUCRA 0.688). Targeted combination chemotherapy shows significant advantages over CTFU, specifically, the rankings from high to low were bevacizumab plus ICTFU (HR 0.43, 95% CI 0.29–0.64), bevacizumab plus CTCA (HR 0.51, 95% CI 0.38–0.69), bevacizumab plus CTFU (HR 0.6, 95% CI 0.48–0.76), panitumumab plus CTFU (HR 0.61, 95% CI 0.49–0.76). Overall, targeted therapy plus chemotherapy had the better efficacy than local treatment plus chemotherapy and chemotherapy; the effects of local treatment combination chemotherapy and chemotherapy tended to be consistent. CTFU performed better than CTCA. In monotherapies, the top three ranked were fruquintinib (SUCRA 0.962), anlotinib (SUCRA 0.889), and regorafenib (SUCRA 0.809). Compared to BSC, treatments with significant advantages were fruquintinib (HR 0.24, 95% CI 0.19–0.3), anlotinib (HR 0.27, 95% CI 0.2–0.36), regorafenib (HR 0.32, 95% CI 0.22–0.46), nintedanib (HR 0.53, 95% CI 0.44–0.64). Full information is available in Tables 4, 5 and Figure 4.
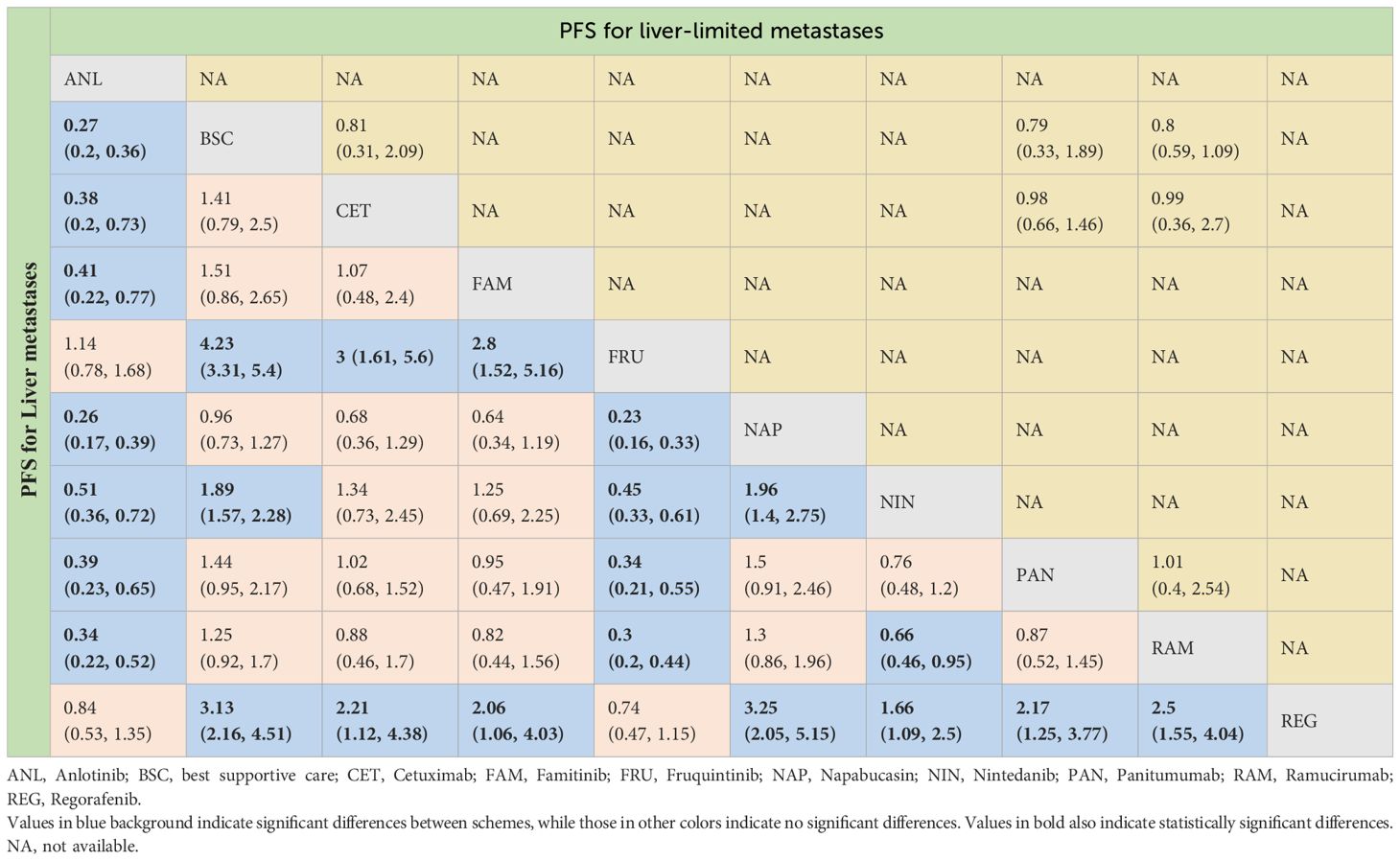
Table 4 Progression-free survival profiles of monotherapy regimens in metastatic CRC patients with liver metastases and liver-limited metastases.
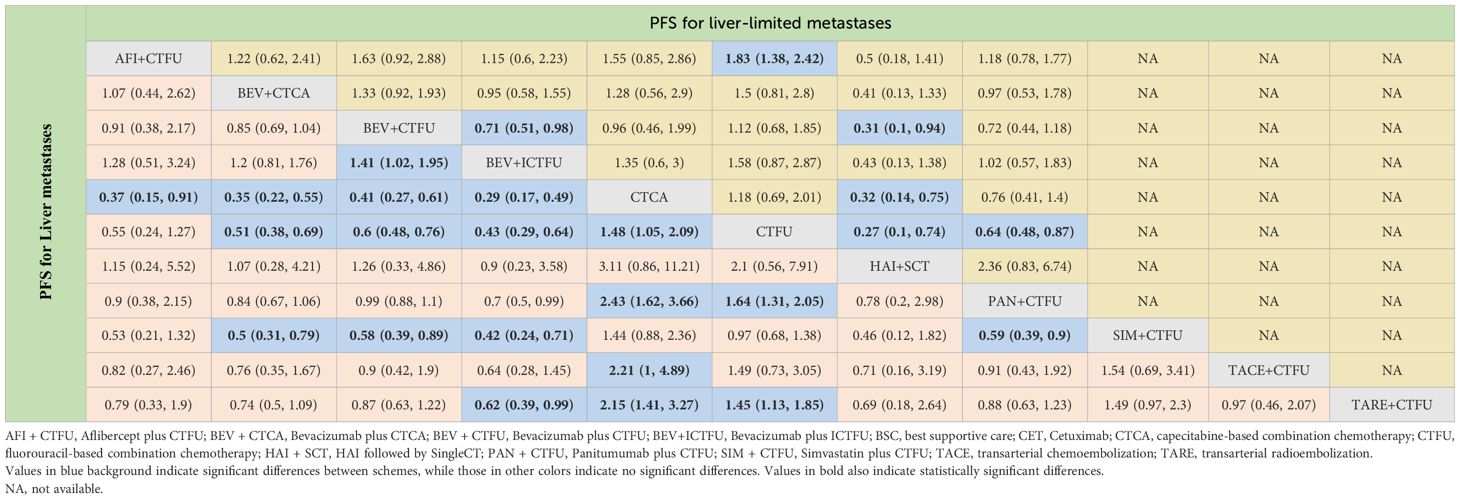
Table 5 Progression-free survival profiles of combined therapies in metastatic CRC patients with liver metastases and liver-limited metastases.
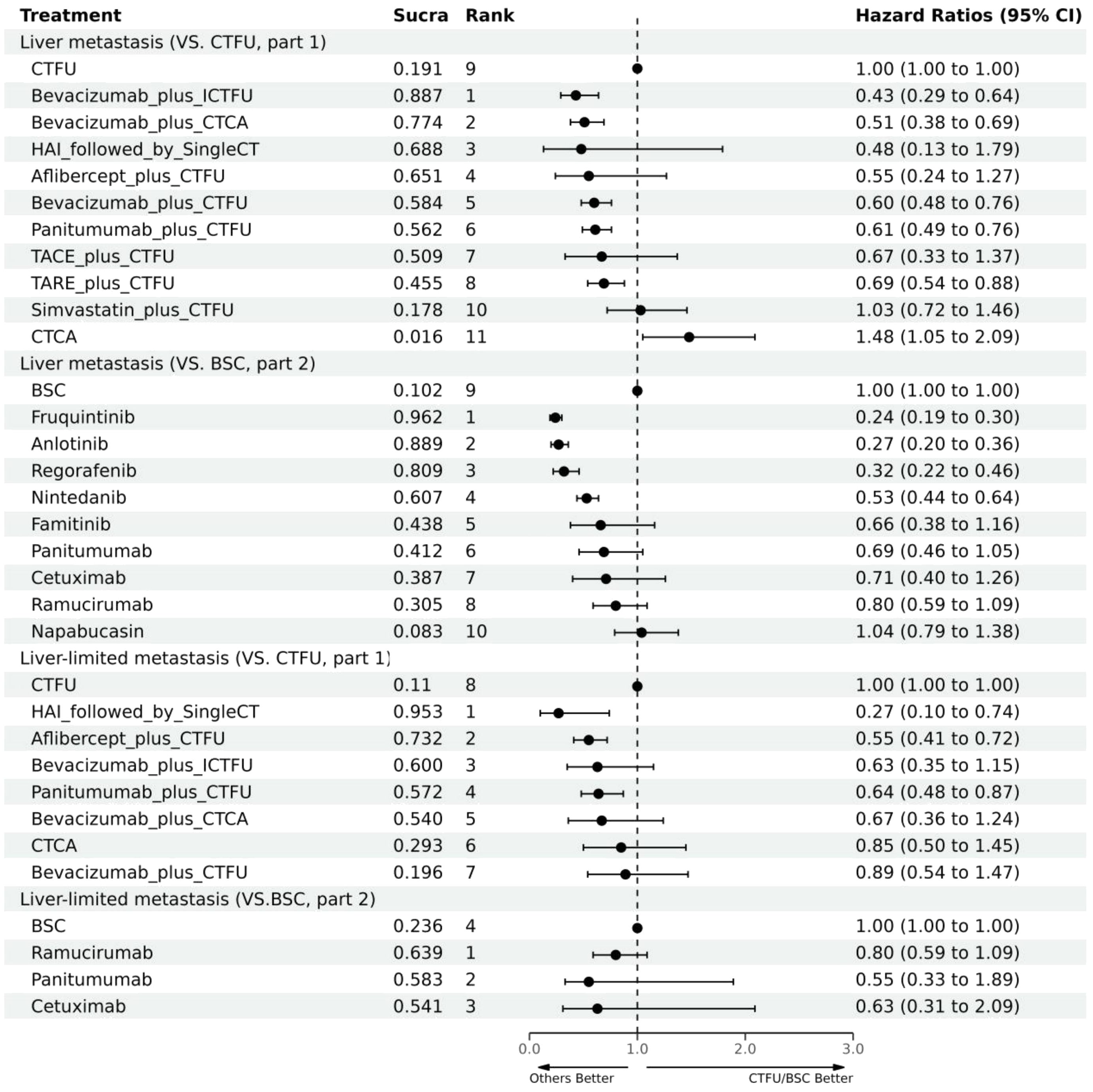
Figure 4 Forest plot for PFS. CTFU, fluorouracil-based combination chemotherapy; CTCA, capecitabine-based combination chemotherapy; ICTFU, intensified chemotherapy containing four interventions; SingleCT, single-agent chemotherapy.
In terms of OS, similarly, due to data insufficiency, we divided this section into two parts for analysis: In the combination therapy, compared to CTFU, only aflibercept plus CTFU (HR 0.65, 95% CI 0.49–0.86) showed the significant advantage. Meanwhile, there was no significant difference in efficacy between the combination therapies. For monotherapy, cetuximab (HR 0.69, 95% CI 0.29–1.66), panitumumab (HR 0.82, 95% CI 0.39–1.74), ramucirumab (HR 0.96, 95% CI 0.68–1.37) were all superior to BSC, but the advantages were not statistically significant. Network plots is shown in Figure 2D, E, forest plots and league table are provided in Figure 3 and Table 6.
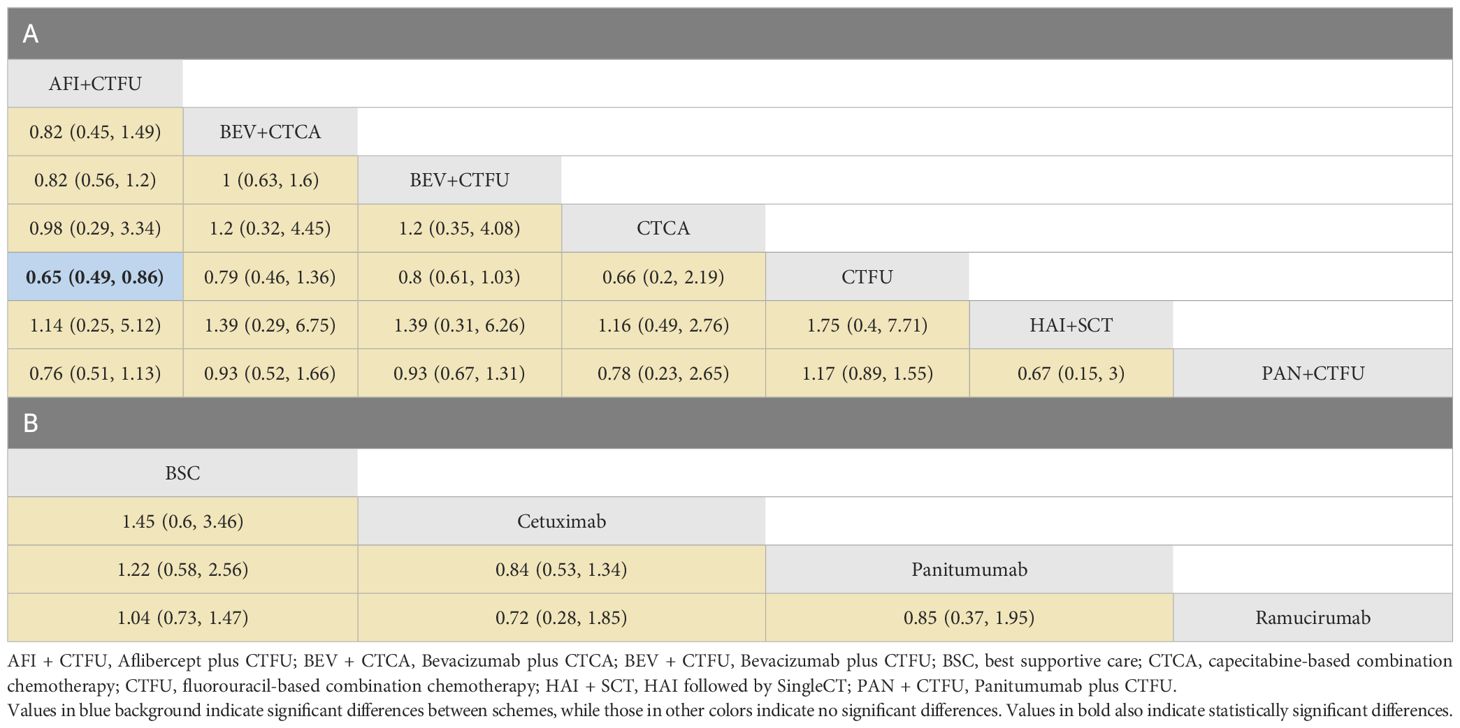
Table 6 Overall survival profiles of combined therapies (A) and monotherapy regimens (B) in metastatic CRC patients with liver-limited metastases.
In terms of PFS, the top three rankings in combination therapy were as follows: HAI followed by SingleCT (SUCRA 0.953), aflibercept plus CTFU (SUCRA 0.732), and bevacizumab plus ICTFU (SUCRA 0.6). Compared to CTFU, the ones with significant advantages were HAI followed by SingleCT (HR 0.27, 95% CI 0.1–0.74), aflibercept plus CTFU (HR 0.55, 95% CI 0.41–0.72), and panitumumab plus CTFU (HR 0.64, 95% CI 0.48–0.87). Among the monotherapy options, ramucirumab performed the best. However, compared to BSC, ramucirumab (HR 0.8, 95% CI 0.59–1.09), panitumumab (HR 0.55, 95% CI 0.33–1.89), and cetuximab (HR 0.63, 95% CI 0.31–2.09) did not demonstrate significant advantages. Other information is presented in Figures 2H, I, 4 and Tables 4, 5.
Due to insufficient data, this section only considered OS. Network plot is provided as Figure 2C. The top three rankings were as follows: TAS-102 plus bevacizumab (SUCRA 0.936), bevacizumab plus CTCA (SUCRA 0.836), and bevacizumab plus CTFU (SUCRA 0.672). Compared to CTFU, the strategies with significant advantages were TAS-102 plus bevacizumab (HR 0.56, 95% CI 0.36–0.87), bevacizumab plus CTCA (HR 0.65, 95% CI 0.5–0.86), bevacizumab plus CTFU (HR 0.77, 95% CI 0.64–0.92), and aflibercept plus CTFU (HR 0.83, 95% CI 0.69–0.98). The efficacy of multi-targeted therapy and targeted therapy plus chemotherapy regimens were superior to targeted therapy and chemotherapy. Among targeted therapy plus chemotherapy regimens, bevacizumab plus CTCA or CTFU performed the best, followed by aflibercept plus CTFU and panitumumab plus CTFU, although there was no significant difference in efficacy between these regimens. For monotherapies, panitumumab (HR 0.81, 95% CI 0.39–1.7), regorafenib (HR 0.83, 95% CI 0.4–1.74), fruquintinib (HR 0.84, 95% CI 0.42–1.68), cetuximab (HR 0.84, 95% CI 0.39–1.8), and TAS-102 (HR 0.91, 95% CI 0.53–1.56) had advantages over CTFU, although without statistical significance. Ramucirumab (HR 1.18, 95% CI 0.6–2.35), nintedanib (HR 1.2, 95% CI 0.62–2.35), and napabucasin (HR 1.82, 95% CI 0.82–4.05) performed worse compared to CTFU. More details are provided in Figure 3 and Table 2.
The results of the heterogeneity test are summarized in Supplementary File 5. It was observed that there was low heterogeneity in almost all of the comparisons. However, high heterogeneity was only detected in targeted therapy VS. BSC (77.2%) in the mechanism comparison.
This NMA systematically compared the efficacy of treatment options for patients with unresectable CRLM who have not responded to at least one prior line of previous systemic therapy. The main findings of this study are summarized as follows:
1. In comparison to chemotherapy, multi-targeted therapy (HR 0.57, 95% CI 0.37–0.87) and targeted therapy plus chemotherapy (HR 0.78, 95% CI 0.67–0.91) show significant advantages; Targeted therapy (HR 0.92, 95% CI 0.54–1.57) and local treatment plus chemotherapy (HR 1.03, 95% CI 0.85–1.23) had comparable performance.
2. For patients with liver metastases, TAS-102 plus bevacizumab, aflibercept plus CTFU, and bevacizumab plus CTCA showed the best outcomes in terms of OS. Bevacizumab plus ICTFU, bevacizumab plus CTCA, and HAI followed by SingleCT performed the best in terms of PFS.
3. For patients with liver-limited metastases, aflibercept plus CTFU was the optimal choice for OS. In terms of PFS, the best options were HAI followed by SingleCT, aflibercept plus CTFU, and panitumumab plus CTFU.
4. For patients with multiple-site metastases, the best treatments were TAS-102 plus bevacizumab, bevacizumab plus CTCA, bevacizumab plus CTFU, and aflibercept plus CTFU.
The use of multi-targeted therapies, such as TAS-102 plus bevacizumab, showed significant effectiveness in prolonging the OS. TAS-102 is an orally active and well tolerated drug composed of trifluridine and tipiracil. Trifluridine, the active antitumor component, is phosphorylated by thymidine kinase within cancer cells to produce trifluridine triphosphate. This trifluridine triphosphate is then substituted for thymidine in DNA. Although the precise mechanism of action for bevacizumab is not fully understood, it likely normalizes tumor blood vessel structure, thereby boosting the blood supply to the tumor. By combining bevacizumab with TAS-102, there is a possibility of elevating trifluridine concentrations specifically within tumor DNA without causing increased overall systemic exposure or toxicity of trifluridine. At the same time, previously treated patients with metastatic CRC exhibit acceptable toxicity when treated with TAS-102 and bevacizumab together (34). Cetuximab, an EGFR inhibitor, showed a strong correlation with increased tumor response. Moreover, it expedited symptom relief in patients with responsive tumors, providing additional benefits (62). Panitumumab, another EGFR inhibitor, is the standard treatment for patients with wild-type metastatic CRC. Studies have shown that anti-EGFR therapy can induce tumor-specific adaptive immune responses and immunogenic cell death. Aflibercept is also a VEGF inhibitor, similar to bevacizumab. It is a recombinant fusion protein that includes parts of human VEGF receptors 1 and 2, fused to the Fc portion of human immunoglobulin G1. This fusion protein blocks the activity of VEGFA, VEGFB, and placental growth factor by acting as a high-affinity ligand trap, preventing these ligands from binding to their natural receptors. Therefore, combined anti-angiogenesis therapies with chemotherapy can further improve the patients’ survival (63).
Currently, there have been no studies that have conducted a systematic evaluation of treatment options for previously treated liver metastases in patients with CRC. There are only a few articles that have conducted a meta-analysis on the partial treatments for unresectable CRLM, but the quality and quantity of the included trials were insufficient. Sanne et al. compared ablation, irreversible electroporation, and stereotactic ablative body radiotherapy (SABR) for unresectable CRLM, while no RCTs were included (64). The control rates varied between 22% and 90% for all techniques; thermal ablation had a range of 22% to 89%, irreversible electroporation had 44%, and SABR had a range of 67% to 90% depending on the radiation dose. Focal ablative therapy was a safe option that can lead to long-term disease control. Simone et al. conducted a comparison between HAI and systemic chemotherapy for unresectable CRLM (65). They discovered that the tumor response rate was 42.9% for HAI and 18.4% for systemic chemotherapy. However, no significant difference in the meta-risk of death was observed between the two treatments. Jordan et al. studied on intra-arterial treatments for unresectable and chemorefractory CRLM. They discovered that the combined response rates for cTACE, drug-eluting embolic TACE (DEE-TACE), and TARE were 23%, 36%, and 23% respectively (66). The median survival times and ranges for cTACE, DEB-TACE, and TARE were 16 months (9–23), 16 months (7–25), and 12 months (7–15) respectively. Daniel et al. examined how HAI treatment could potentially be used as a neoadjuvant therapy performing hepatic resection. They discovered that 50% of patients responded positively to HAI, and 18% of patients were able to undergo surgery afterwards (67). Joseph et al. assessed the effectiveness of HAI, cTACE, DEE-TACE, and TARE, in combinations with systemic chemotherapy, for treating unresectable CRLM (68). They discovered that the combination of DEE-TACE and systemic chemotherapy yielded the most favorable oncological results and had the greatest potential for conversion to resection. Cardiovascular complications, including arrhythmias, hypertension, and more severe outcomes such as heart failure and myocardial infarction, are particularly pertinent due to the high vascular nature of the liver and the intense metabolic demands of metastatic cancer. The impact of these cardiovascular side events on overall survival is significant, as they can limit treatment efficacy and adversely affect patient quality of life. In the patents discussed, polydatin, a nutraceutical derived from Polygonum cuspidatum, is highlighted for its potential to enhance the effectiveness of anticancer drugs such as 5-fluorouracil, cisplatin, and tyrosine kinase inhibitors. Polydatin has shown cardioprotective effects and the ability to boost the efficacy of these treatments in clinical studies. Its dual role in reducing cardiovascular side effects and improving treatment outcomes offers a promising avenue for integrated cancer therapies (69).
This study conducted the first systematic evaluation and NMA of the effectiveness of different strategies in previously treated CRLM patients. This has significant implications for the development of clinical medications and related guidelines. In contrast to previous studies, this research meticulously grouped interventions based on the type and mechanism of chemotherapy to minimize heterogeneity to the greatest extent. Moreover, the heterogeneity of this study’s results was low, indicating that the conclusions of this study are relatively reliable. Additionally, we performed multiple subgroup analyses, which hold extraordinary significance for the precise treatment of CRC. We differentiated the analysis between patients with liver-limited metastases and those with multiple-site metastases, providing more evidence support for clinical diagnosis and treatment decisions. After a systematic comparison, the viewpoints we propose are innovative and deserve further discussion, as they will also provide new directions for future clinical research.
Due to data availability, this study has some limitations. The majority of included RCTs did not report safety data for CRLM patients, therefore, we could not systematically evaluate the safety. For the same reason, we were unable to analyze patients with biomarker mutations; Furthermore, due to lack of individual data and most studies only reporting HR, we had to use the Cox proportional hazards model for indirect comparison, instead of other risk-varying models. To conduct a more comprehensive comparison, we considered patients with multiple-site metastases to have liver metastases, though liver metastases in these patients accounting for over 90%, it introduced some uncertainty. However, when comparing results from patients with liver-limited metastases and patients with multiple-site metastases, the conclusions were consistent. Therefore, we believe the impact of this assumption is limited. Lastly, the relative efficacy between many treatment regimens was obtained through indirect comparison, and more evidence from head-to-head RCTs is needed to validate our findings. Although the combination of anti-VEGF drugs with systemic therapy has been studied for its improvement of survival rates, our research further validates this finding. The value of this study lies in the integrated analysis of existing data, providing valuable insights for future research and clinical decision-making.
For CRLM patients who have failed at least one line of previous systemic therapy, multi-targeted therapy and targeted therapy plus chemotherapy are the best mechanisms. TAS-102 plus bevacizumab is superior in OS, and the combination of anti-VEGF drugs like bevacizumab and aflibercept with standard chemotherapy is the preferred option.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MZ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. WT: Funding acquisition, Resources, Software, Supervision, Validation, Writing – review & editing. XZ: Project administration, Resources, Software, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Program of National Natural Science Foundation of China (grant no. 72174207).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1293598/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Lee HY, Woo IS. Perioperative systemic chemotherapy for colorectal liver metastasis: recent updates. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13184590
3. Tauriello DV, Calon A, Lonardo E, Batlle E. Determinants of metastatic competency in colorectal cancer. Mol Oncol. (2017) 11:97–119. doi: 10.1002/1878-0261.12018
4. Pan Z, Peng J, Lin J, Chen G, Wu X, Lu Z, et al. Is there a survival benefit from adjuvant chemotherapy for patients with liver oligometastases from colorectal cancer after curative resection? Cancer Commun (Lond). (2018) 38:29. doi: 10.1186/s40880-018-0298-8
5. Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. (2022) 7:70. doi: 10.1038/s41392-022-00922-2
6. Acciuffi S, Meyer F, Bauschke A, Croner R, Settmacher U, Altendorf-Hofmann A. Solitary colorectal liver metastasis: overview of treatment strategies and role of prognostic factors. J Cancer Res Clin Oncol. (2022) 148:657–65. doi: 10.1007/s00432-021-03880-4
7. Al BM, Kim NK. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Review). Oncol Rep. (2017) 37:2553–64. doi: 10.3892/or.2017.5531
8. Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. (2020) 9:49–58. doi: 10.21037/hbsn
9. Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver Malignancies. Int J Radiat Oncol Biol Phys. (2010) 78:486–93. doi: 10.1016/j.ijrobp.2009.08.020
10. Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. (2005) 62:1371–8. doi: 10.1016/j.ijrobp.2005.01.002
11. Abdalla EK. Commentary: Radiofrequency ablation for colorectal liver metastases: do not blame the biology when it is the technology. Am J Surg. (2009) 197:737–9. doi: 10.1016/j.amjsurg.2008.06.029
12. Angelsen JH, Horn A, Sorbye H, Eide GE, Løes IM, Viste A. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg. (2017) 104:580–9. doi: 10.1002/bjs.10457
13. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
14. Mohamed F, Kallioinen M, Braun M, Fenwick S, Shackcloth M, Davies RJ, et al. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: summary of NICE guidance. Br J Surg. (2020) 107:943–5. doi: 10.1002/bjs.11609
15. Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. (2020) 21:497–507. doi: 10.1016/S1470-2045(19)30862-9
16. Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. (2007) 25:1670–6. doi: 10.1200/JCO.2006.09.0928
17. Liu Y, Chang W, Zhou B, Wei Y, Tang W, Liang F, et al. Conventional transarterial chemoembolization combined with systemic therapy versus systemic therapy alone as second-line treatment for unresectable colorectal liver metastases: randomized clinical trial. Br J Surg. (2021) 108:373–9. doi: 10.1093/bjs/znaa155
18. Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. (2014) 15:569–79. doi: 10.1016/S1470-2045(14)70118-4
19. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. (2008) 26:2013–9. doi: 10.1200/JCO.2007.14.9930
20. Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. (2010) 11:38–47. doi: 10.1016/S1470-2045(09)70330-4
21. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
22. Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. (2016) 6:29765. doi: 10.1038/srep29765
23. Reboux N, Jooste V, Goungounga J, Robaszkiewicz M, Nousbaum JB, Bouvier AM. Incidence and survival in synchronous and metachronous liver metastases from colorectal cancer. JAMA Netw Open. (2022) 5:e2236666. doi: 10.1001/jamanetworkopen.2022.36666
24. Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
27. Shao T, Zhao M, Liang L, Tang W. A systematic review and network meta-analysis of first-line immune checkpoint inhibitor combination therapies in patients with advanced non-squamous non-small cell lung cancer. Front Immunol. (2022) 13:948597. doi: 10.3389/fimmu.2022.948597
28. Zhao Y, Liu J, Cai X, Pan Z, Liu J, Yin W, et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: systematic review and network meta-analysis. BMJ. (2019) 367:l5460. doi: 10.1136/bmj.l5460
29. Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. (2008) 26:753–67. doi: 10.2165/00019053-200826090-00006
30. Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graphi Stat. (1998) 7:434–55. doi: 10.1080/10618600.1998.10474787
31. Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. (2023) 402:41–53. doi: 10.1016/S0140-6736(23)00772-9
32. Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, et al. Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br J Cancer. (2023) 128:1897–905. doi: 10.1038/s41416-023-02212-2
33. Chi Y, Shu Y, Ba Y, Bai Y, Qin B, Wang X, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist. (2021) 26:e1693–703. doi: 10.1002/onco.13857
34. Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. (2020) 21:412–20. doi: 10.1016/S1470-2045(19)30827-7
35. Pietrantonio F, Lobefaro R, Antista M, Lonardi S, Raimondi A, Morano F, et al. Capecitabine and temozolomide versus FOLFIRI in RAS-mutated, MGMT-methylated metastatic colorectal cancer. Clin Cancer Res. (2020) 26:1017–24. doi: 10.1158/1078-0432.CCR-19-3024
36. Van Cutsem E, Yoshino T, Lenz HJ, Lonardi S, Falcone A, Limón ML, et al. Nintedanib for the treatment of patients with refractory metastatic colorectal cancer (LUME-Colon 1): a phase III, international, randomized, placebo-controlled study. Ann Oncol. (2018) 29:1955–63. doi: 10.1093/annonc/mdy241
37. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
38. Xu RH, Muro K, Morita S, Iwasa S, Han SW, Wang W, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. (2018) 19:660–71. doi: 10.1016/S1470-2045(18)30140-2
39. Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol Hepatol. (2018) 3:263–70. doi: 10.1016/S2468-1253(18)30009-8
40. Xu RH, Shen L, Wang KM, Wu G, Shi CM, Ding KF, et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer. (2017) 36:97. doi: 10.1186/s40880-017-0263-y
41. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. (2015) 16:499–508. doi: 10.1016/S1470-2045(15)70127-0
42. Obermannová R, Van Cutsem E, Yoshino T, Bodoky G, Prausová J, Garcia-Carbonero R, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol. (2016) 27:2082–90. doi: 10.1093/annonc/mdw402
43. Lim SH, Kim TW, Hong YS, Han SW, Lee KH, Kang HJ, et al. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br J Cancer. (2015) 113:1421–6. doi: 10.1038/bjc.2015.371
44. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. (2010) 28:4706–13. doi: 10.1200/JCO.2009.27.6055
45. Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res. (2015) 21:5469–79. doi: 10.1158/1078-0432.CCR-15-0526
46. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI {+/-} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. (2014) 25:107–16. doi: 10.1093/annonc/mdt523
47. Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. (2023) 388:1657–67. doi: 10.1056/NEJMoa2214963
48. Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. (2018) 36:350–8. doi: 10.1200/JCO.2017.74.3245
49. Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. (2008) 19:1720–6. doi: 10.1093/annonc/mdn370
50. Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. (2016) 115:1206–14. doi: 10.1038/bjc.2016.309
51. Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. (2015) 26:724–30. doi: 10.1093/annonc/mdv012
52. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2015) 16:619–29. doi: 10.1016/S1470-2045(15)70156-7
53. Mulcahy MF, Mahvash A, Pracht M, Montazeri AH, Bandula S, Martin RCG 2nd, et al. Radioembolization with chemotherapy for colorectal liver metastases: A randomized, open-label, international, multicenter, phase III trial. J Clin Oncol. (2021) 39:3897–907. doi: 10.1200/JCO.21.01839
54. Ghiringhelli F, Vincent J, Bengrine L, Borg C, Jouve JL, Loffroy R, et al. Hepatic arterial chemotherapy with raltitrexed and oxaliplatin versus standard chemotherapy in unresectable liver metastases from colorectal cancer after conventional chemotherapy failure (HEARTO): a randomized phase-II study. J Cancer Res Clin Oncol. (2019) 145:2357–63. doi: 10.1007/s00432-019-02970-8
55. Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, et al. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. (2015) 14:72–80. doi: 10.1016/j.clcc.2014.12.009
56. Price T, Kim TW, Li J, Cascinu S, Ruff P, Suresh AS, et al. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur J Cancer. (2016) 68:51–9. doi: 10.1016/j.ejca.2016.08.010
57. Tabernero J, Van Cutsem E, Lakomý R, Prausová J, Ruff P, van Hazel GA, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. (2014) 50:320–31. doi: 10.1016/j.ejca.2013.09.013
58. Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. (2013) 14:29–37. doi: 10.1016/S1470-2045(12)70477-1
59. Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. (2007) 25:1658–64. doi: 10.1200/JCO.2006.08.1620
60. Modest DP, Karthaus M, Kasper S, Moosmann N, Keitel V, Kiani A, et al. FOLFOX plus panitumumab or FOLFOX alone as additive therapy following R0/1 resection of RAS wild-type colorectal cancer liver metastases - The PARLIM trial (AIO KRK 0314). Eur J Cancer. (2022) 173:297–306. doi: 10.1016/j.ejca.2022.07.012
61. Kim TW, Elme A, Park JO, Udrea AA, Kim SY, Ahn JB, et al. Final analysis of outcomes and RAS/BRAF status in a randomized phase 3 study of panitumumab and best supportive care in chemorefractory wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. (2018) 17:206–14. doi: 10.1016/j.clcc.2018.03.008
62. Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. (2015) 33:692–700. doi: 10.1200/JCO.2014.59.4812
63. Lee MS, Loehrer PJ, Imanirad I, Cohen S, Ciombor KK, Moore DT, et al. Phase II study of ipilimumab, nivolumab, and panitumumab in patients with KRAS/NRAS/BRAF wild-type (WT) microsatellite stable (MSS) metastatic colorectal cancer (mCRC), in: ASCO GI, Abs 7, J Clin Oncol. (2021) 39(3_suppl). doi: 10.1200/JCO.2021.39.3_suppl.7
64. Nieuwenhuizen S, Dijkstra M, Puijk RS, Geboers B, Ruarus AH, Schouten EA, et al. Microwave ablation, radiofrequency ablation, irreversible electroporation, and stereotactic ablative body radiotherapy for intermediate size (3–5 cm) unresectable colorectal liver metastases: a systematic review and meta-analysis. Curr Oncol Rep. (2022) 24:793–808. doi: 10.1007/s11912-022-01248-6
65. Mocellin S, Pasquali S, Nitti D. Fluoropyrimidine-HAI (hepatic arterial infusion) versus systemic chemotherapy (SCT) for unresectable liver metastases from colorectal cancer. Cochrane Database Syst Rev. (2009) CD007823. doi: 10.1002/14651858.CD007823.pub2
66. Levy J, Zuckerman J, Garfinkle R, Acuna SA, Touchette J, Vanounou T, et al. Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB (Oxford). (2018) 20:905–15. doi: 10.1016/j.hpb.2018.04.001
67. Chan DL, Alzahrani NA, Morris DL, Chua TC. Systematic review and meta-analysis of hepatic arterial infusion chemotherapy as bridging therapy for colorectal liver metastases. Surg Oncol. (2015) 24:162–71. doi: 10.1016/j.suronc.2015.06.014
68. Zhao JJ, et al. Intra-arterial therapy for unresectable colorectal liver metastases: A meta-analysis. J Vasc Interv Radiol. (2021) 32:1536–1545.e38. doi: 10.1016/j.jvir.2021.05.032
Keywords: metastatic colorectal cancer, unresectable liver metastases, network meta-analysis, treatment selection, overall survival
Citation: Jiang Y, Zhao M, Tang W and Zheng X (2024) Comparison of systemic treatments for previously treated patients with unresectable colorectal liver metastases: a systematic review and network meta-analysis. Front. Oncol. 14:1293598. doi: 10.3389/fonc.2024.1293598
Received: 13 September 2023; Accepted: 24 June 2024;
Published: 10 July 2024.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Vincenzo Quagliariello, Istituto Nazionale Tumori-IRCCS-Fondazione G. Pascale, ItalyCopyright © 2024 Jiang, Zhao, Tang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zheng, emhlbmd4cEBuanVjbS5lZHUuY24=; Wenxi Tang, dG9rYW1teUBjcHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.