- 1Department of Surgery, Government Medical College and Hospital, Chandigarh, India
- 2Department of Surgery, University of Puerto Rico School of Medicine, San Juan, Puerto Rico
- 3Department of Surgery, Mayo Clinic, Rochester, MN, United States
- 4Department of Surgery, Dow University of Health Sciences, Karachi, Pakistan
- 5Department of Surgery, University of Florida College of Medicine, Gainesville, FL, United States
- 6Department of Surgery, Florida State University, Tallahassee, FL, United States
- 7Department of Surgery, Duke University Medical Center, Durham, NC, United States
- 8Department of Surgery, University of Alabama, Tuscaloosa, AL, United States
- 9Department of Surgery, Aga Khan University, Karachi, Pakistan
- 10Department of General Surgery, Mayo Clinic Florida, Jacksonville, FL, United States
Introduction: Gastric cancer ranks as the 5th most prevalent cancer and the 4th leading cause of cancer-related deaths worldwide. Various treatment modalities, including surgical resection, chemotherapy, and radiotherapy, are available for gastric cancer patients. However, disparities related to age, sex, race, socioeconomic factors, insurance status, and demographic factors often lead to delayed time to treatment.
Methods: In this retrospective study, conducted between 2004 and 2019, we utilized data from the National Cancer Database (NCDB) to investigate the factors contributing to disparities in the time to first treatment, surgery, chemotherapy, and radiotherapy among gastric cancer patients. Our analysis incorporated several variables, and statistical analysis was conducted to provide valuable insights into these disparities.
Results: We observed notable disparities in the timing of treatment for various demographic groups, including age, sex, race, insurance status, geographic location, and facility type. These disparities include longer time to treatment in males (32.67 vs 30.75), Native Americans (35.10 vs 31.09 in Asians), low-income patients (32 vs 31.15), patients getting treatment in an academic setting (36.11 vs 29.61 in community setting), significantly longer time to chemotherapy in 70+ age group (51.13 vs 40.38 in <40 y age group), black race (55.81 vs 47.05 in whites), low income people (49.64 vs 46.74), significantly longer time to radiotherapy in females (101.61 vs 79.75), blacks and Asians (109.68 and 113.96 respectively vs 92.68 in Native Americans) etc. There are various other disparities in time to surgery, chemotherapy, and radiotherapy.
Conclusions: Understanding these disparities is crucial in developing targeted strategies to improve timely access to appropriate treatments and enhance outcomes for gastric cancer patients. Future research with updated data and prospective study designs can provide a more comprehensive understanding of the factors influencing patient outcomes in gastric cancer.
1 Introduction
In the year 2020, gastric cancer ranked as the 5th most prevalent cancer and the 4th leading cause of cancer-related deaths worldwide (1). Year 2020 reported over 1 million newly diagnosed cases of gastric cancer, with Eastern Asia and Eastern Europe reporting the highest incidences (2). In the United States, it is estimated that approximately 26,500 individuals will be diagnosed with gastric cancer in 2023, with the highest incidence observed in Japanese and Korean populations (2, 3). Despite a declining trend in incidence rates over the past few decades, the global burden of gastric cancer is projected to increase by 62% by 2040 (4). In the United States, black males and Hispanic females exhibit the highest incidence and mortality rates (5).
The primary causative agent of gastric cancer is Helicobacter pylori, responsible for nearly 90% of cases, while other risk factors include cigarette smoking, high salt diet, and processed meat consumption (2, 4). Various treatment modalities are available for gastric cancer, including surgical resection, chemotherapy, and radiotherapy. However, disparities related to age, sex, race, socioeconomic factors, insurance status, and demographic factors often lead to delayed time to treatment for patients with gastric cancer. While there are studies showing poor survival rates with a longer time to treatment in certain cancers (6), there is very little data demonstrating a correlation between time to treatment and overall survival in gastric cancer (5). For individuals who chose to undergo surgery as the initial treatment, there was a bimodal relationship concerning the time to treatment. Specifically, when the time to treatment was 8 weeks or less, a lengthier time to treatment correlated with an extended median overall survival. On the other hand, when the time to treatment ranged from 14 to 20 weeks, a prolonged time to treatment was linked to a diminished median overall survival (7).
This study aimed to investigate the different disparities affecting the time to treatment for individuals diagnosed with gastric cancer in the United States of America from 2004 to 2019. There have been studies done on disparities in gastric cancer treatment by Lemini et al. (8) and Rana et al. (9) but our paper focuses on more wider spectrum of sociodemographic groups including income, geographic location etc. as showed in below tables. By gaining a comprehensive understanding of these disparities, we may identify targeted strategies and interventions to improve timely access to appropriate treatments and enhance outcomes for all gastric cancer patients.
2 Methods
We performed a retrospective analysis utilizing data from the National Cancer Database (NCDB) covering the period from 2004 to 2019. The National Cancer Data Base (NCDB) is a comprehensive oncology outcomes database, capturing 70% of annual new invasive cancer diagnoses in the U.S. It serves as a crucial clinical surveillance and quality improvement tool for cancer programs under the American College of Surgeons Commission on Cancer approvals program. The information is employed to examine trends in cancer care, set benchmarks at regional and national levels, and facilitate quality improvement initiatives (9, 10). To access this data, the request was submitted through the American College of Surgeons to obtain the NCDB Participant User File (PUF), which is accessible to individuals affiliated with hospitals participating in the Commission on Cancer. Our study did not require Institutional Review Board approval. The study focused on patients who had been diagnosed with gastric cancer and adhered to the guidelines outlined by the American Joint Committee on Cancer (AJCC) 6th and 7th editions (11). The analysis encompassed a wide range of variables, including race, age, sex, income, insurance status, geographic location (rural/urban), treatment facility type, cancer stage, cancer grade, and Charlson-Deyo Comorbidity (CDC) score. Staging, grading and CDC scoring of gastric cancer is done similar to earlier studies (8, 11).
To evaluate the timing of treatment, specifically surgery, chemotherapy, and/or radiation, we computed and summarized the respective durations. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Our research findings were presented through summaries of clinical and demographic characteristics, disease outcome measures, and treatment variables. For continuous variables, such as mean, median, standard deviation, and ranges, the Kruskal-Wallis test was utilized for analysis. Categorical variables were presented as frequencies and relative frequencies, and chi-square tests were employed for analysis.
By employing a robust methodology and rigorous statistical analysis, we aimed to provide valuable insights into the disparities related to the timing of treatment among gastric cancer patients and contribute to the advancement of knowledge in this field.
3 Results
3.1 Time to treatment
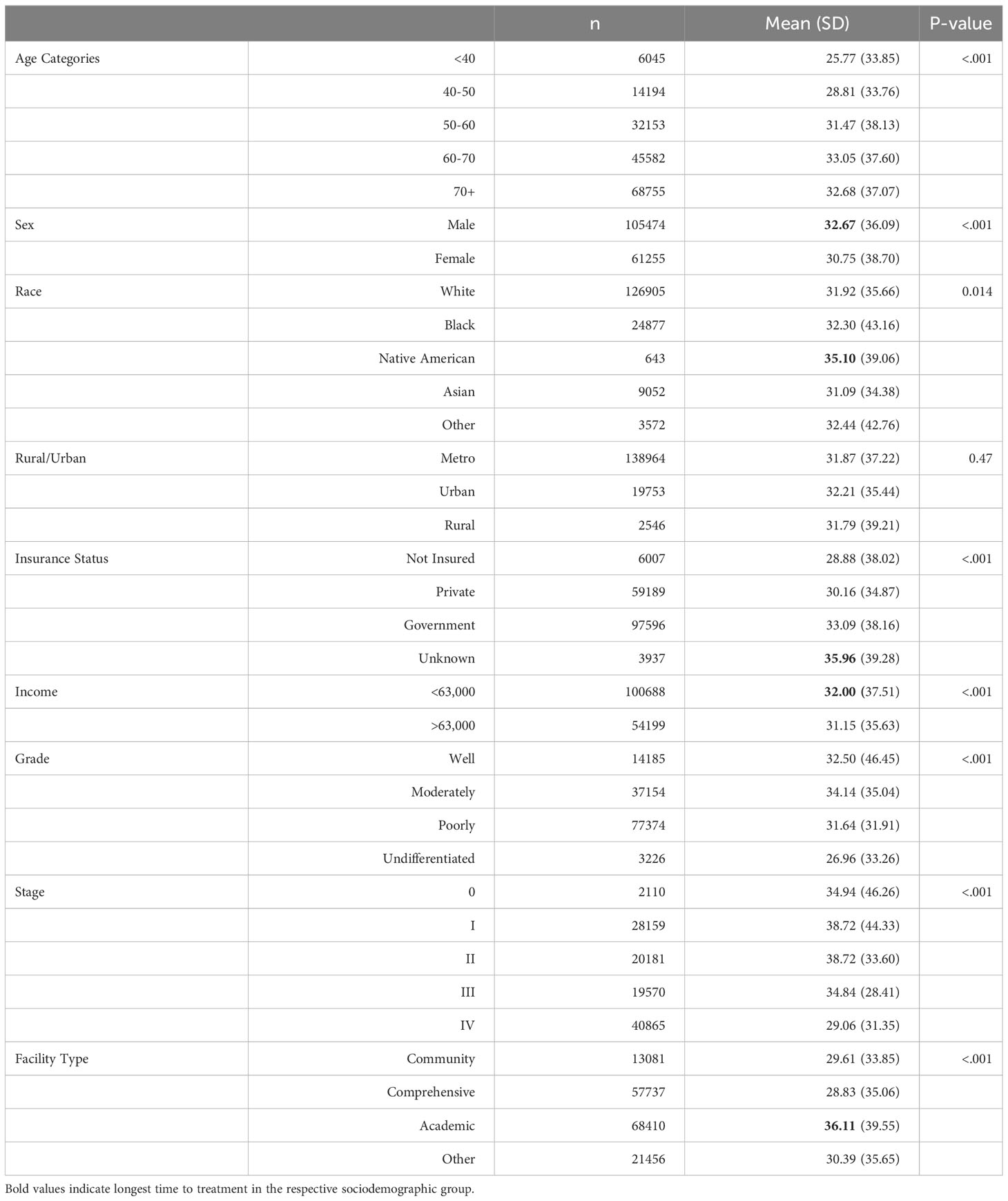
It is a crucial metric in cancer management, yet there is limited data comparing its impact on overall survival in patients with gastric cancer (5). Study by Ramanathan et al. indicates longer time to treatment has no correlation with overall survival (12) whereas according to Fisher et al. patients who had urgent surgery had worse outcomes as compared to elective surgery patients (13). Some studies indicate a bimodal relationship between overall survival and time to surgical treatment, with decreased survival rates observed in patients with treatment initiated either <4 weeks or >14 weeks after diagnosis (7). So, more studies are needed to know about the exact effect of time to treatment on overall survival. Notably, as shown in Table 1, longer time to treatment is associated with specific factors, including male sex, Native American race, Government Insurance, income less than 63000, and treatment received at academic settings.
3.2 Time to surgery
Surgery plays a pivotal role in the treatment of gastric cancer, with surgical intervention serving as the mainstay approach. For patients diagnosed with early-stage gastric cancer, total, subtotal, or distal gastrectomy with D2 lymphadenectomy offers a curative treatment option (14). In recent times, laparoscopic gastrectomy, performed by skilled surgeons, has gained prominence due to its associated benefits, such as reduced blood loss, fewer post-operative complications, and quicker recovery (15). A recent systematic review and meta-analysis of 16 studies comparing robotic gastrectomy (RG) and laparoscopic gastrectomy (LG) for gastric cancer indicated that RG is associated with a decreased risk of postoperative complications, shorter hospital stays, and lower rates of conversion to open surgery. Nevertheless, there were no significant differences observed in terms of overall survival, disease-free survival, or the number of harvested lymph nodes between the two procedures (16). However, disparities in time to surgery have been observed in certain patient groups as shown in Table 2. Males, Native Americans, individuals residing in urban areas, those with insurance coverage, and patients receiving treatment in academic settings experience longer time intervals before undergoing surgery.
3.3 Time to chemotherapy
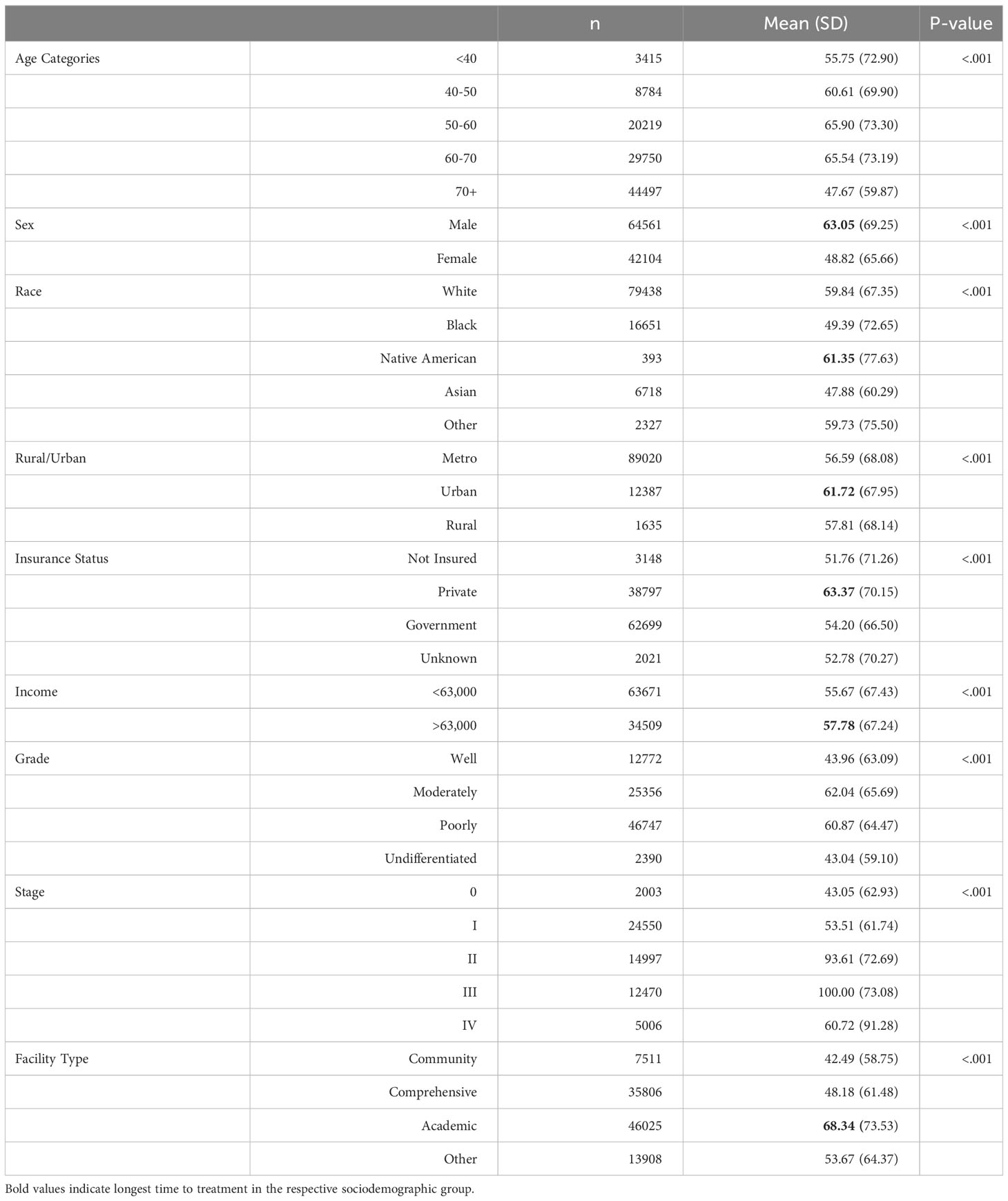
It plays a vital role in the management of advanced gastric cancers, serving as a mainstay approach to improve patient outcomes. Studies have demonstrated that chemotherapy can extend survival by approximately 7 months compared to best supportive treatment (17). Additionally, neoadjuvant chemotherapy has been shown to reduce mortality in patients with advanced gastric cancer without increasing complications or post-operative mortality rates (18). However, certain patient groups experience longer time intervals before initiating chemotherapy as shown in Table 3. The 70+ age group, females, individuals of Asian or black race, those with low income, government insurance, and patients receiving treatment at academic settings tend to encounter delays in receiving chemotherapy.
3.4 Time to radiotherapy
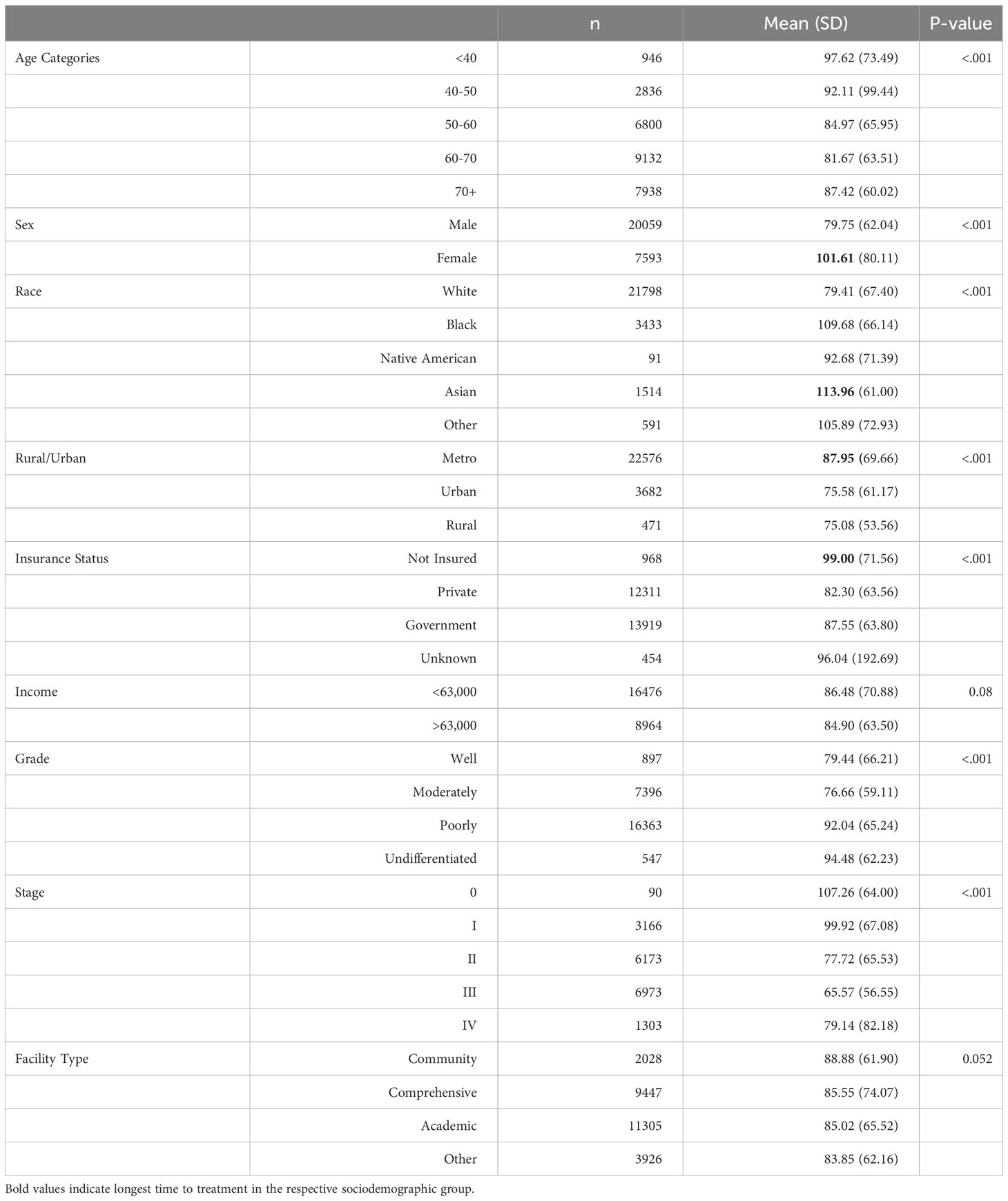
It is a critical aspect in the management of locally advanced gastric cancer, particularly for alleviating local symptoms such as bleeding, pain, and obstruction. Palliative radiotherapy has shown promising response rates for these symptoms, with studies, like Tey et al. reporting rates as high as 74% for bleeding, 67% for pain, and 68% for obstruction (19). Low-dose radiotherapy is often preferred to minimize adverse effects, as it yields similar response rates with fewer side effects (19). However, certain patient groups experience delays in receiving radiotherapy as shown in Table 4. Females, individuals of black and Asian ethnicity, uninsured patients, and those residing in metropolitan cities tend to encounter longer intervals before commencing radiotherapy.
4 Discussion
In our study, we have investigated the factors contributing to disparities in the time to first treatment, surgery, chemotherapy, and radiotherapy among gastric cancer patients. Age, sex, race, insurance, income, facility type, and geographic setting emerged as influential factors influencing these treatment timelines.
Intriguingly, our data reveals that the time to surgery is notably shorter for individuals aged <40 years and >70 years compared to other age groups. The primary reason for this disparity likely lies in the early-stage diagnosis and lower prevalence of comorbidities among individuals under 40 years, making them highly suitable candidates for early surgical intervention (20). Conversely, patients over 70 years of age are typically diagnosed at an advanced stage of the disease, necessitating immediate surgical action due to the severity of their condition. This finding aligns with the observations made by Brenkman et al., who demonstrated that individuals with advanced tumor stages undergo surgery more promptly than those with early-stage tumors (21).
In our study, a notable disparity was observed between females and males regarding the time to first treatment and surgery, as well as the time to chemotherapy and radiotherapy. The findings suggest that females tended to receive earlier treatment, particularly surgical intervention, potentially hindering the progression to advanced stages of gastric cancer. In contrast, males experienced delays in surgical treatment compared to females, leading to a higher incidence of advanced-stage disease and subsequent initiation of chemotherapy and radiotherapy at an earlier stage.
The underlying reasons for this sex-based variation may stem from inherent sex-related characteristics. Males, in general, have been reported to display reluctance in utilizing healthcare services, whereas females tend to be more frequent users of such services (22). This discrepancy in healthcare-seeking behavior may contribute to the observed differences in treatment timelines. Furthermore, existing literature on other cancer types, such as lung cancer, has shown that women are more likely than men to opt for surgical treatments. This factor may account for the prevention of advanced disease progression in females, leaving males with limited options and necessitating earlier reliance on chemotherapy and radiotherapy (22).
In the studied population, we observed significant differences in the time to first treatment and time to surgery between the Asian and Native American groups. Asian individuals exhibited notably shorter intervals to treatment initiation and surgical intervention, while Native Americans experienced prolonged time to treatment and surgery, yet shorter time to chemotherapy and radiotherapy. This disparity can be attributed to higher awareness levels among Asian individuals regarding gastric cancer, likely influenced by the higher incidence of gastric cancer in this population. Consequently, this heightened awareness leads to earlier diagnosis, rendering Asian individuals better candidates for prompt surgical treatment (5, 8, 23, 24).
It is a known fact that gastric cancer patients without insurance have higher mortality rates compared to those with insurance (9, 25). Surprisingly, patients without insurance demonstrated significantly shorter time to treatment and time to surgery when compared to their insured counterparts. This observation may seem counterintuitive at first, but the most plausible explanation for this difference lies in the worse presentation of the disease at the time of diagnosis among patients without insurance, which necessitates more urgent surgical intervention.
Patients without insurance often face barriers to accessing healthcare services, resulting in delayed diagnosis and limited access to regular medical care. Consequently, gastric cancer may be detected at more advanced stages, leading to a more critical condition that requires immediate surgical intervention. On the other hand, patients with insurance, who likely have better access to healthcare services and early diagnosis, may have the luxury of time for a more comprehensive evaluation and preparatory measures before surgery.
Our study unveiled a concerning trend among gastric cancer patients, indicating that individuals with lower income experience significantly longer intervals to treatment, chemotherapy, and radiotherapy. Notably, this disparity may be compounded by lower education levels, which further diminish the likelihood of receiving timely medical intervention, possibly due to limited health literacy and a lack of awareness regarding the potential consequences of forgoing treatment (23, 26, 27). Tragically, this phenomenon contributes to higher mortality rates among gastric cancer patients from lower-income backgrounds and with lower educational attainment (28).
In our study, we observed notable differences in the time to treatment, surgery, and chemotherapy between patients receiving care in academic settings and those in other healthcare settings. This observation is consistent with findings from previous research for gastric cancer patients and patients with other malignancies (6, 29). The longer treatment duration at academic centers could be attributed to several factors, including a higher patient load, reduced scheduling flexibility, and a substantial number of referral cases that necessitate thorough reanalysis before offering treatment. Academic medical centers often serve as referral centers, receiving complex cases from various regions. Consequently, the need for comprehensive evaluations and consultations can lead to longer timeframes before treatment initiation.
Similarly, time to surgery and radiotherapy was more prolonged in patients living in urban and metropolitan cities respectively than in rural areas. Clinics in rural areas often experience a lower patient load than their urban and metropolitan counterparts. This lower patient volume can be advantageous in offering early appointments and enabling timely treatment for patients in rural communities.
It is essential to acknowledge that while the study identified statistically significant disparities in various factors, these differences may not have significant clinical implications. The large number of patients included in the database enabled the attainment of statistical significance, but the actual differences in the time to treatment among various treatment variables might not result in substantial variations in patient outcomes. While our study extensively covers various aspects in time to treatment disparities of gastric cancer, the analysis of survival rates is beyond the scope of our study. Future research could explore survival rates in the context of gastric cancer.
Although utilizing a large database enhances the generalizability of the study, it is crucial to recognize it as a noteworthy limitation. The sheer volume of patient information may lead to missing data and inaccurately documented information, potentially affecting the study’s reliability. Additionally, the study’s retrospective nature poses limitations, as the data might not fully represent current practices and disparities in the field. Changes in healthcare practices and advancements in treatments over time could influence the relevance of the study’s findings to current medical practices. Moreover, the inherent variability in hospital charges for cancer treatment poses a limiting factor. Different hospitals, particularly private institutions versus safety net hospitals, may employ distinct cost structures for healthcare services which could result in different time to treatment for patients. NCDB, which serves as the primary data source for our study does not provide any information on this. Nonetheless, our study appears to be the first that analyses disparities among gastric cancer patients across all stages and socioeconomic factors.
5 Conclusions
In conclusion, our retrospective study identified significant disparities in the time to treatment, surgery, chemotherapy, and radiotherapy among gastric cancer patients. Age, sex, race, insurance, income, facility type, and geographic setting were key factors influencing treatment timelines. Understanding these disparities is crucial for targeted interventions to improve timely access to care and enhance patient outcomes. However, it is important to interpret the findings cautiously due to the potential limitations of the study. Future research with updated data and prospective designs will further enhance our understanding of these disparities and help develop effective strategies to address them.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SS: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. EG: Supervision, Writing – review & editing. HM: Writing – review & editing. PJ: Writing – review & editing. SR: Writing – review & editing. SA: Writing – review & editing. RP: Writing – review & editing. KP: Writing – review & editing. KS: Writing – review & editing. GK: Writing – review & editing. FM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that author EG was an associate editor and they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. E Clin Med (2022) 47:101404. doi: 10.1016/j.eclinm.2022.101404
3. Cancer.Net. Stomach Cancer - Statistics (2012). Available at: https://www.cancer.net/cancer-types/stomach-cancer/statistics.
4. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol (2023) 20(5):338–49. doi: 10.1038/s41571-023-00747-0
5. Cordova-Marks FM, Carson WO, Monetathchi A, Little A, Erdrich J. Native and indigenous populations and gastric cancer: A worldwide review. Int J Environ Res Public Health (2022) 19(9):5437. doi: 10.3390/ijerph19095437
6. Sukniam K, Kasbi AA, Ashary MA, Popp K, Attwood K, George A, et al. Disparities in time to treatment for breast cancer. Anticancer Res (2022) 42(12):5813–8. doi: 10.21873/anticanres.16088
7. Kaslow SR, He Y, Sacks GD, Berman RS, Lee AY, Correa-Gallego C. Time to curative-intent surgery in gastric cancer shows a bimodal relationship with overall survival. J Gastrointest Surg (2023) 27(5):855–65. doi: 10.1007/s11605-023-05585-0
8. Lemini R, Jorgensen MS, Attwood K, Almerey T, Elli EF, Colibaseanu DT, et al. Racial disparities in outcomes among Asians with gastric cancer in the USA. Anticancer Res (2020) 40(2):881–9. doi: 10.21873/anticanres.14021
9. Rana N, Gosain R, Lemini R, Wang C, Gabriel E, Mohammed T, et al. Socio-demographic disparities in gastric adenocarcinoma: A population-based study. Cancers (Basel) (2020) 12(1):157. doi: 10.3390/cancers12010157
10. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The national cancer data base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol (2008) 15(3):683–90. doi: 10.1245/s10434-007-9747-3
11. Hallinan JTPD, Venkatesh SK. Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. Cancer Imag (2013) 13(2):212–27. doi: 10.1102/1470-7330.2013.0023
12. Ramanathan S, Shen N, Johnson T, Cheng C, Tuma F, Serpa E, et al. Longer wait times do not adversely impact 90-day mortality in patients with stages I-III gastric cancer. Cureus (2023) 15(10):e46494. doi: 10.7759/cureus.46494
13. Fisher BW, Fluck M, Young K, Shabahang M, Blansfield J, Arora TK. Urgent surgery for gastric adenocarcinoma: A study of the national cancer database. J Surg Res (2020) 245:619–28. doi: 10.1016/j.jss.2019.07.073
14. Panda SK, Sahoo PK, Agarwala SK, Houghton TT, Chandrapattan PP, Sankar KV, et al. Evolution of treatment in gastric cancer- a systematic review. J Egypt Natl Canc Inst (2022) 34(1):12. doi: 10.1186/s43046-022-00114-7
15. Lou S, Yin X, Wang Y, Zhang Y, Xue Y. Laparoscopic versus open gastrectomy for gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Int J Surg (2022) 102:106678. doi: 10.1016/j.ijsu.2022.106678
16. Mocan L. Surgical management of gastric cancer: A systematic review. J Clin Med (2021) 10(12):2557. doi: 10.3390/jcm10122557
17. Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (2017) 8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4
18. Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg (2018) 51:120–7. doi: 10.1016/j.ijsu.2018.01.008
19. Tey J, Soon YY, Koh WY, Leong CN, Choo BA, Ho F, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget (2017) 8(15):25797–805. doi: 10.18632/oncotarget.15554
20. Trumbull D, Lemini R, Elli EF, Bagaria SP, Attwood K, Gabriel E. Age-based trends of gastric adenocarcinoma in the United States. Am Surg (2020) 86(12):1721–7. doi: 10.1177/0003134820947395
21. Brenkman HJF, Visser E, Van Rossum PSN, Siesling S, Van Hillegersberg R, Ruurda JP. Association between waiting time from diagnosis to treatment and survival in patients with curable gastric cancer: A population-based study in the Netherlands. Ann Surg Oncol (2017) 24(7):1761–9. doi: 10.1245/s10434-017-5820-8
22. Rana RH, Alam F, Alam K, Gow J. Gender-specific differences in care-seeking behaviour among lung cancer patients: a systematic review. J Cancer Res Clin Oncol (2020) 146(5):1169–96. doi: 10.1007/s00432-020-03197-8
23. Liu N, Molena D, Stem M, Blackford AL, Sewell DB, Lidor AO. Underutilization of treatment for regional gastric cancer among the elderly in the USA. J Gastrointest Surg (2018) 22(6):955–63. doi: 10.1007/s11605-018-3691-3
24. Trumbull D, Lemini R, Attwood K, Kukar M, Gabriel E. Gastric cancer disparities among Asian American subpopulations. Anticancer Res (2020) 40(11):6381–5. doi: 10.21873/anticanres.14659
25. Arias-Ortiz NE, de Vries E. Health inequities and cancer survival in Manizales, Colombia: a population-based study. Colomb Med (Cali) (2018) 49(1):63–72. doi: 10.25100/cm.v49i1.3629
26. Stessin AM, Sherr DL. Demographic disparities in patterns of care and survival outcomes for patients with resected gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev (2011) 20(2):223–33. doi: 10.1158/1055-9965.EPI-10-0158
27. Sha J. An analysis of the correlation between the educational levels and economic status of the Chinese urban elderly population. Chin J Popul Sci (1990) 2(1):1–8.
28. Lamm R, Hewitt DB, Li M, Powell AC, Berger AC. Socioeconomic status and gastric cancer surgical outcomes: A national cancer database study. J Surg Res (2022) 275:318–26. doi: 10.1016/j.jss.2022.02.004
Keywords: time to treatment, gastric cancer, disparities, disparities in treatment, cancer, sociodemographic factors
Citation: Sharan S, Bansal S, Manaise HK, Jimenez PB, Raikot SR, Ahmed SH, Popp R, Popp K, Sukniam K, Kowkabany G, Mubarak F and Gabriel E (2024) Time to treatment disparities in gastric cancer patients in the United States of America: a comprehensive retrospective analysis. Front. Oncol. 14:1292793. doi: 10.3389/fonc.2024.1292793
Received: 24 October 2023; Accepted: 19 January 2024;
Published: 09 February 2024.
Edited by:
Syed Ahsan Raza, University of Pittsburgh, United StatesReviewed by:
Seyed Aria Nejadghaderi, Shahid Beheshti University of Medical Sciences, IranHsiang-Lin Lee, Chung Shan Medical University Hospital, Taiwan
Copyright © 2024 Sharan, Bansal, Manaise, Jimenez, Raikot, Ahmed, Popp, Popp, Sukniam, Kowkabany, Mubarak and Gabriel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seema Sharan, seemasharan2198@gmail.com
†These authors share first authorship
 Seema Sharan
Seema Sharan