- 1Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, CA, United States
- 2Obstetrics and Gynecology, Adventist Health White Memorial Medical Center, Los Angeles, CA, United States
- 3Data Reporting and Analytics, MedHealth Statistical Consulting Inc., Solon, OH, United States
- 4Center for Observational and Real-World Evidence (CORE), Merck & Co., Inc., Rahway, NJ, United States
- 5Gynecology Oncology, Kaiser Permanente Southern California, Riverside, CA, United States
Introduction: The Coronavirus Disease 2019 (COVID-19) pandemic posed critical challenges in providing care to ovarian cancer (OC) patients, including delays in OC diagnosis and treatment initiation. To accommodate for delays in OC surgery, the Society of Gynecologic Oncology (SGO) recommended preferential use of neoadjuvant chemotherapy during the pandemic. The purpose of this study was to assess the association of the COVID-19 pandemic with neoadjuvant chemotherapy use in patients diagnosed with OC.
Methods: This retrospective cohort study included patients diagnosed with stage II-IV ovarian cancer of epithelial subtype between 01/01/2017-06/30/2021 at Kaiser Permanente Southern California (KPSC), a large integrated healthcare system in the United States. Ovarian cancer patients diagnosed between 2017-2020 were identified from KPSC’s Surveillance, Epidemiology, and End Results (SEER)-affiliated cancer registry. Patients diagnosed in 2021 were identified from the electronic medical records (EMR) using ICD-10 diagnosis codes, followed by medical chart review to validate diagnosis and extract information on histology and stage at diagnosis. March 4, 2020 was used as the cut-off to define pre-pandemic and pandemic periods. Patients diagnosed with COVID-19 between OC diagnosis and treatment completion were excluded. Data on neoadjuvant chemotherapy use were extracted from the cancer registry and EMR, supplemented by chart review. Modified Poisson regression was used to evaluate the association of the pandemic with neoadjuvant chemotherapy use.
Results: Of 566 OC patients, 160 (28.3%) were diagnosed in the pandemic period. Patients diagnosed in the pandemic period were slightly younger (mean age 62.7 vs 64.9 years, p=0.07) and had a higher burden of Charlson comorbidities (p=0.05) than patients diagnosed in pre-pandemic period. No differences in time to treatment initiation were observed by pandemic periods. Neoadjuvant chemotherapy use was documented in 58.7% patients during the pandemic period compared to 47.3% in pre-pandemic period (p=0.01). After adjusting for covariates, patients diagnosed in the pandemic period were 29% more likely to receive neoadjuvant chemotherapy than patients diagnosed in pre-pandemic period [RR(95%CI): 1.29(1.12-1.49)].
Discussions: Ovarian cancer patients diagnosed in the COVID-19 pandemic were more likely to receive neoadjuvant chemotherapy than patients diagnosed before the pandemic. Future research on patient outcomes and trends in the post-pandemic period are warranted.
Introduction
The COVID-19 pandemic posed unprecedented challenges in the delivery of healthcare worldwide; care in cancer patients was particularly affected. There were delays in cancer diagnosis and treatment initiation; modifications in cancer treatment regimens were also recommended to reduce potential COVID-19 exposure and cancer treatment-related complications, as well as to prioritize overall COVID-19 response (1). Patients with gynecologic cancers were no exception. Discontinuation of cervical cancer screening services, reduction in emergency visits and urgent referral in patients with suspected cancer, delays in treatment initiation, and alterations/postponement of surgical procedures were reported in gynecologic cancer patients (2–4).
While primary cytoreductive surgery is the first line treatment choice for patients with ovarian cancer, neoadjuvant chemotherapy followed by interval cytoreductive surgery is not inferior to primary debulking surgery in advanced ovarian cancer patients (5, 6). During the COVID-19 pandemic, shortage of resources, including personal protective equipment (PPE) and ventilators, and repurposing of operating rooms as critical care units affected the standard of care (7). After weighing the risks and benefits of COVID-19 infection and surgical delay in gynecologic oncology patients, the American College of Surgeons (ACS) categorized gynecologic cancer cases, including ovarian cancer cytoreductive surgery, as ‘semi-urgent’ (8, 9). At the same time, to accommodate for delays in ovarian cancer surgery and to reduce harm, the Society of Gynecologic Oncology (SGO) recommended the use of neoadjuvant chemotherapy during the pandemic (8); SGO’s recommendation to use neoadjuvant chemotherapy preferentially was consistent with recommendations from other international gynecologic oncology societies (10, 11).
Studies conducted in Portugal and Netherlands have reported an increased administration of neoadjuvant chemotherapy in patients with advanced ovarian cancer during the COVID-19 pandemic, compared to pre-pandemic periods (2, 12). However, to our knowledge, evidence on the impact of the COVID-19 pandemic on neoadjuvant chemotherapy use in the United States (US) has not been reported. Our aim was to assess the association of the COVID-19 pandemic with neoadjuvant chemotherapy use in patients diagnosed with ovarian cancer at Kaiser Permanente Southern California (KPSC).
Materials and methods
Study design, setting, and subjects
This retrospective cohort study included patients diagnosed with incident epithelial ovarian cancer between January 1st, 2017, and June 30th, 2021 at KPSC, an integrated healthcare delivery system serving more than 4.7 million racially and ethnically and socioeconomically diverse members in Southern California. Patients diagnosed with epithelial type ovarian cancer between 2017-2020 were identified from KPSC’s Surveillance, Endpoints, & End Results (SEER)-affiliated cancer registry. Patients diagnosed in 2021 were initially identified from KPSC’s electronic medical records (EMR) using ICD-10 diagnosis codes and subsequently confirmed by chart review. Stage II-IV patients were included in the study if they were aged 18-89 years and were active members of KPSC health plan at the time of ovarian cancer diagnosis. Patients were excluded if they a) had any prior ovarian cancer diagnosis, b) had another cancer diagnosis within six months prior to their incident ovarian cancer diagnosis, c) had missing information on age at diagnosis, cancer stage, and/or race and ethnicity, d) had less than 12 months KPSC membership prior to ovarian cancer diagnosis (i.e., insufficient data to assess comorbidity burden), e) terminated KPSC membership within 12 months after ovarian cancer diagnosis, f) did not receive ovarian cancer treatment at KPSC, or g) were diagnosed with COVID-19 anytime between ovarian cancer diagnosis and ovarian cancer treatment completion (first course of treatment). The study was approved by KPSC’s institutional review board (IRB). Due to the use of secondary, de-identified data, the requirement for written or verbal consent was waived.
Data collection
Data on ovarian cancer, including date of diagnosis, cancer stage, histology type, and treatment were extracted from KPSC’s SEER-registry for patients diagnosed in 2017-2020. For 2021, ICD-10 diagnosis codes (were used to identify ovarian cancer patients from KPSC’s EMR. Medical chart reviews were conducted to validate ovarian cancer diagnosis and to extract information on cancer histology and stage in patients identified from the EMR. Data on neoadjuvant chemotherapy use were extracted from the cancer registry and EMR, supplemented by chart review. Chart reviews were also conducted to understand the rationale behind neoadjuvant chemotherapy use in a small number of stage II ovarian cancer patients. Data on sociodemographic and other clinical variables were extracted from the EMR.
Outcome, exposure, and covariates of interest
Use of neoadjuvant chemotherapy (yes/no) was the outcome of interest. Exposure of interest was the COVID-19 pandemic period. March 4, 2020 was used as the cut-off to define pre-pandemic and pandemic periods; this cut-off was based on date of implementation of stay-at-home order in California. Covariates of interest included age at cancer diagnosis, race and ethnicity, cancer stage, KPSC membership years prior to ovarian cancer diagnosis, Charlson Comorbidities Index, and Neighborhood deprivation index (NDI). Cancer stage included FIGO stages II-IV. Modified Charlson Comorbidities Index (unweighted) was calculated based on comorbidities recorded in the EMR within 12 months prior to ovarian cancer diagnosis (13); diagnosis of ovarian cancer was excluded from Charlson Comorbidities Index calculations. Neighborhood deprivation index (in quartiles), a measure for neighborhood socioeconomic status (SES), was geocoded based on patients’ home addresses and was based on the American Community Survey – Census Bureau data.
Statistical analysis
Descriptive statistics comparing pre-pandemic and pandemic period were reported using frequency (percentage) or mean (standard deviation); Chi-square and t-test p-values were reported, as appropriate. Modified Poisson regression with robust variance was used to evaluate unadjusted and adjusted association of the pandemic with neoadjuvant chemotherapy use. Covariates with score test p-value <0.10 in the unadjusted models were included as potential confounders in the adjusted model. To check for effect modification by race and ethnicity, we evaluated the interaction between race and ethnicity and pandemic periods in the adjusted model. In sensitivity analysis, patients with stage II cancer, as well as patients with low-grade serous carcinoma were excluded. Level of significance was set at 0.05 and two-sided p-values were reported. All analyses were conducted in SAS version 9.4 (Cary, NC).
Results
Sociodemographic and clinical characteristics of the study population are shown in Table 1. Of 566 stage II-IV ovarian cancer patients included, 406 (71.7%) were diagnosed during the pre-pandemic period and the rest [160 (28.3%)] were diagnosed during the pandemic period. Patients diagnosed during the pandemic period were slightly younger than patients diagnosed in the pre-pandemic period [mean age 62.7 vs 64.9 years, respectively, p-value=0.07]. Overall, 47.0% patients were non-Hispanic white, followed by 34.1% Hispanic patients, 10.8% Asian/Pacific Islander/other races, and 8.1% non-Hispanic black patients. No differences in race and ethnicity were observed by the pandemic periods. Overall, 85.5% patients were diagnosed at FIGO stage III-IV. Mean (std dev) time from diagnosis to neoadjuvant treatment initiation was 22.3 (24.5) days, with no differences observed by pandemic periods (p-value=0.17). More than one-third of the patients diagnosed during the pandemic period (38.8%) had more than two Charlson comorbidities compared to 30.5% patients diagnosed during the pre-pandemic period (p-value=0.05). Overall, 50.5% patients received neoadjuvant chemotherapy; 58.7% patients diagnosed in the pandemic period received neoadjuvant chemotherapy compared to 47.3% in the pre-pandemic period (p-value=0.01). Proportion of patients who received neoadjuvant chemotherapy by calendar period (quarters) is shown in Figure 1. The increase in neoadjuvant chemotherapy use corresponded with the first and second waves of the pandemic in California.
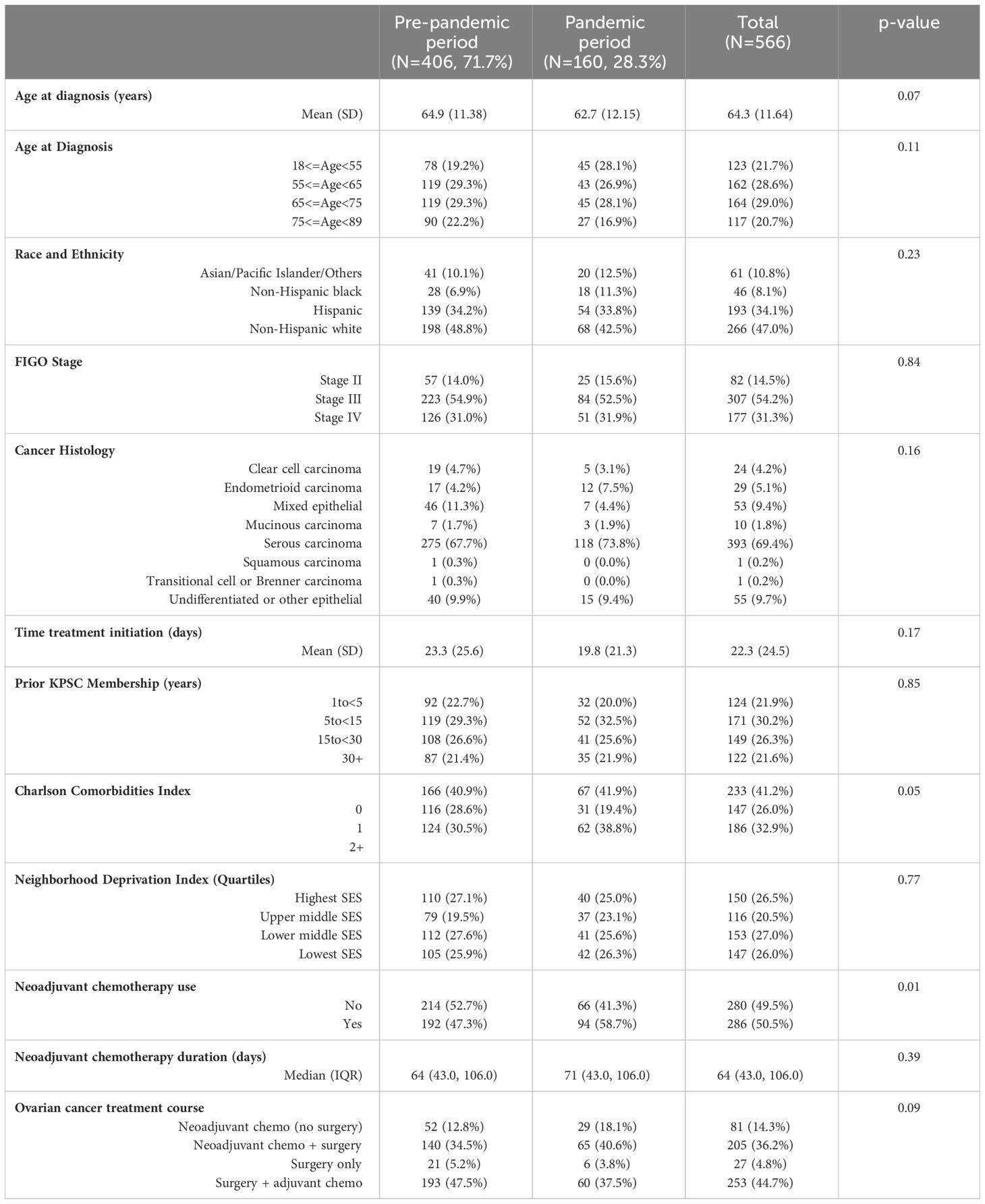
Table 1 Sociodemographic and clinical characteristics of patients diagnosed with stage II-IV ovarian cancer of epithelial origin at Kaiser Permanente Southern California by pandemic periods.
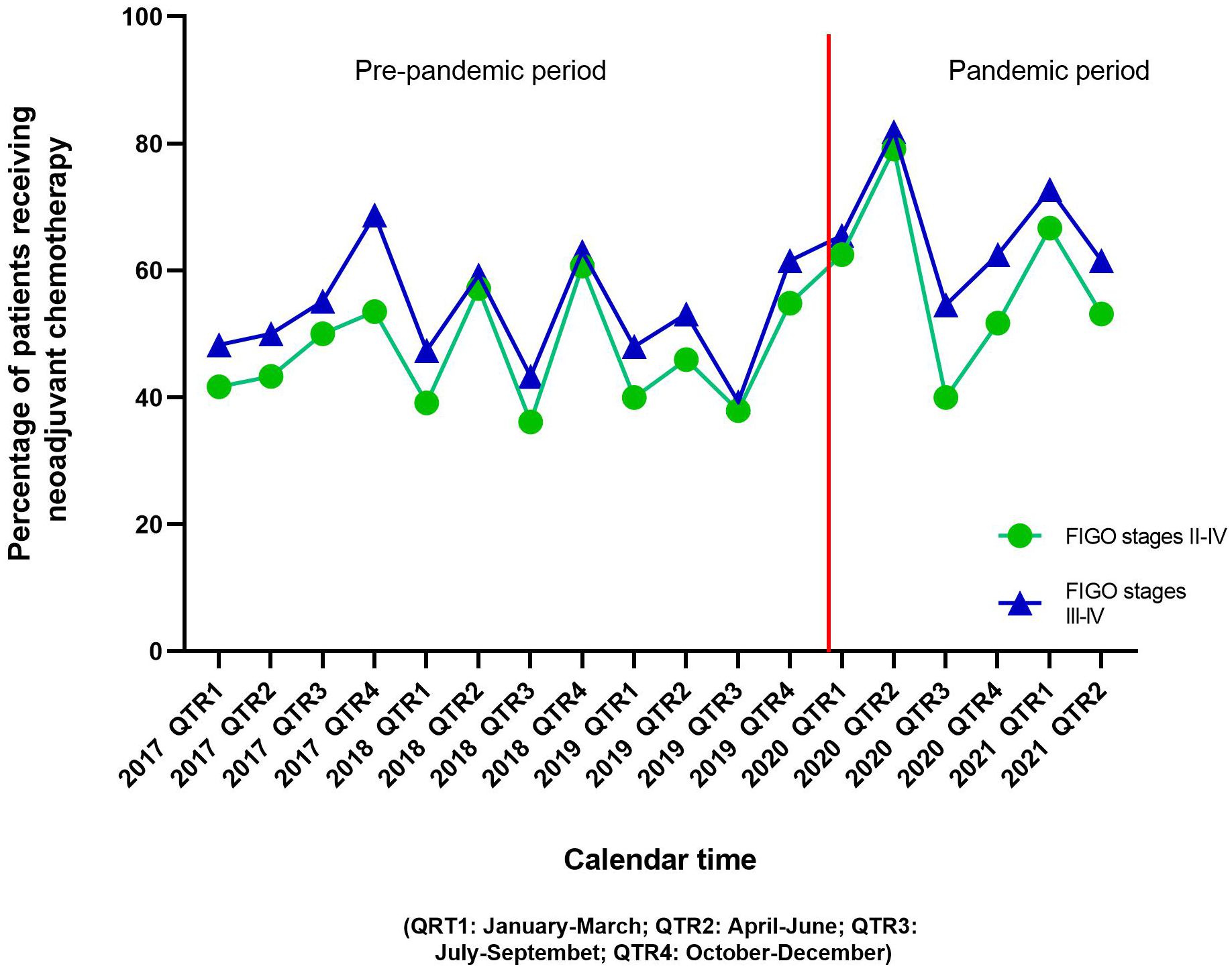
Figure 1 Distribution of ovarian cancer patients receiving neoadjuvant chemotherapy before and after the advent of the COVID-19 pandemic.
Table 2 shows the unadjusted and adjusted association of the pandemic with neoadjuvant chemotherapy use in patients with Stage II-IV ovarian cancer. In the unadjusted model, patients diagnosed in the pandemic period were 24% more likely to receive neoadjuvant chemotherapy compared to patients in the pre-pandemic period [RR (95%CI): 1.24 (1.04-1.47)]. Compared to non-Hispanic white patients, Asian/Pacific Islander/other patients were less likely to receive neoadjuvant chemotherapy [RR (95%CI): 0.66 (0.47-0.94)]. Patients diagnosed at FIGO stage III and IV were more likely to receive neoadjuvant chemotherapy than patients diagnosed at FIGO stage II [RR (95%CI): 5.42 (2.64-11.12) and 9.07 (4.44-18.50), respectively]. Compared to patients with no Charlson comorbidities, patients with ≥2 Charlson comorbidities were 47% more likely to receive neoadjuvant chemotherapy [RR (95%CI): 1.47 (1.22-1.77)]. After adjusting for age, race and ethnicity, FIGO stage, and Charlson comorbidity index, patients in the pandemic period were 29% more likely to receive neoadjuvant chemotherapy compared to patients in the pre-pandemic period [RR (95%CI): 1.29 (1.12-1.49)].
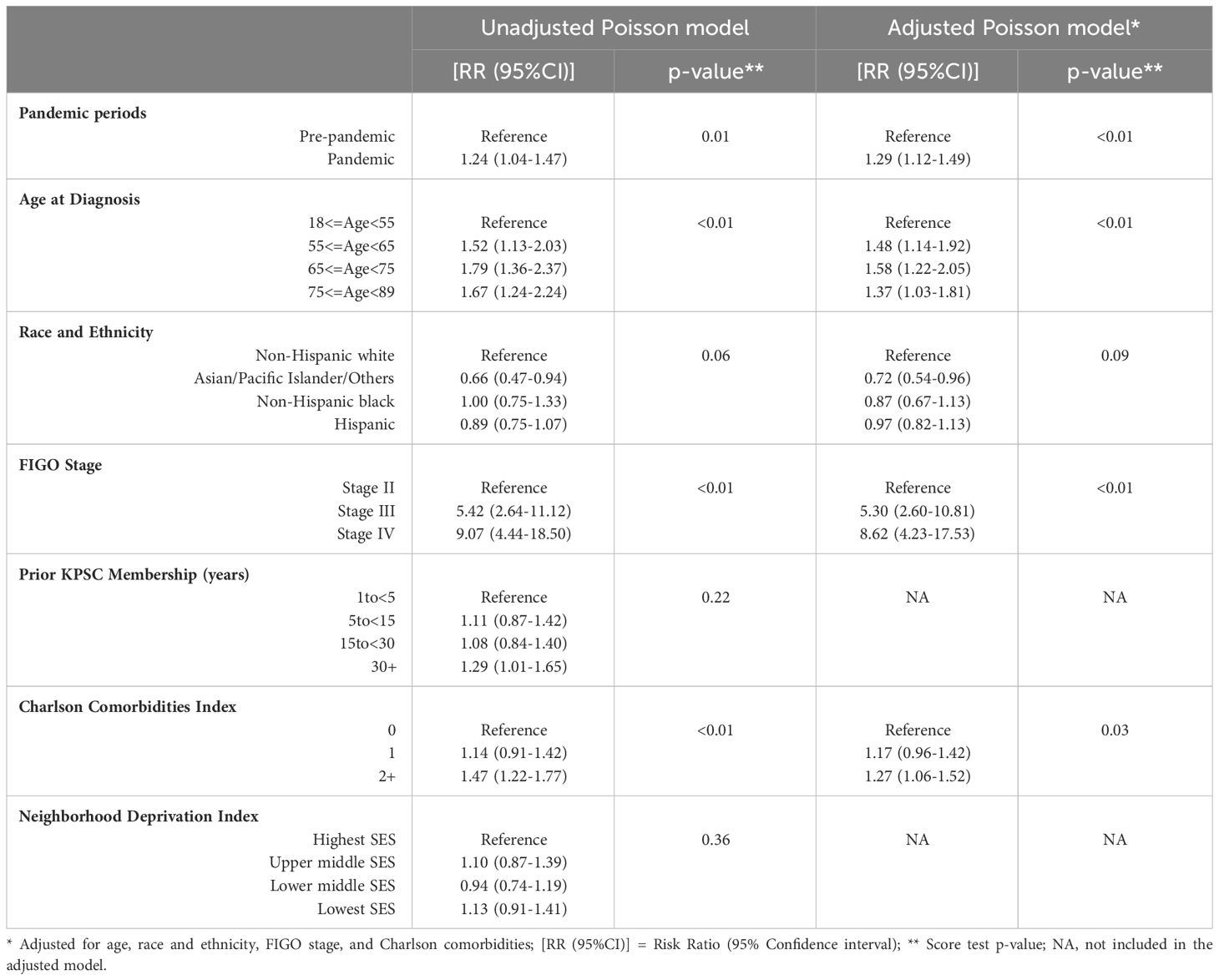
Table 2 Unadjusted and adjusted association of the COVID-19 pandemic with neoadjuvant chemotherapy use in patients with stage II-IV ovarian cancer.
Of the covariates included in the adjusted model, age, cancer stage, and Charlson comorbidities were associated with neoadjuvant chemotherapy use. Older patients were more likely to receive neoadjuvant chemotherapy than patients aged <55 years [RR (95%CI): 1.48 (1.14-1.92) and 1.58 (1.22-2.05) for age groups 55to<65 and 65to<75 years, respectively]. Patients diagnosed at FIGO stages III and IV were more likely to receive neoadjuvant chemotherapy than stage II patients [RR (95%CI): 5.30 (2.60-10.81) and 8.62 (4.23-17.53), respectively]. Patients with ≥2 Charlson comorbidities were 27% more likely to receive neoadjuvant chemotherapy than patients with no Charlson comorbidities [RR (95%CI): 1.27 (1.06-1.52). Association of race and ethnicity with neoadjuvant chemotherapy was modified by pandemic periods; Hispanic and non-Hispanic white patients were 46% and 27% more likely to receive neoadjuvant chemotherapy during the pandemic period compared to the pre-pandemic period [RR (95%CI): 1.46 (1.13-1.88) and 1.27 (1.04-1.54), respectively] (data not shown).
Sensitivity analysis was limited to patients with advanced ovarian cancer (FIGO stages III-IV, n=484). After adjusting for age, race and ethnicity, FIGO stage, and Charlson Comorbidities Index, patients in the pandemic period were 28% more likely to receive neoadjuvant chemotherapy compared to patients in the pre-pandemic period [RR (95%CI): 1.28 (1.11-1.48)]. Patients with FIGO stage IV cancer were 63% more likely to use neoadjuvant chemotherapy than patients with stage III cancer [RR (95%CI): 1.63 (1.42-1.88)]. Older age and ≥2 Charlson comorbidities were also associated with higher neoadjuvant chemotherapy use (data not shown).
Discussion
The COVID-19 pandemic was associated with increased use of neoadjuvant chemotherapy in patients diagnosed with advanced ovarian cancer in an integrated healthcare delivery system in the US. No statistically significant differences in cancer stage at diagnosis and time to treatment initiation were observed before and during the pandemic. To our knowledge, no previous studies have reported on the impact of the COVID-19 pandemic on neoadjuvant chemotherapy use in ovarian cancer patients in the US.
Studies conducted in Europe have reported an increase in neoadjuvant chemotherapy use in ovarian cancer patients after the onset of the pandemic (2, 12). Our findings are consistent; we observed a 29% increased use of neoadjuvant chemotherapy in patients diagnosed during the pandemic period compared to pre-pandemic patients. This finding has important clinical implications as it highlights the adoption of SGO’s modified treatment recommendations in ovarian cancer patients during the pandemic, in the US. However, unlike Algera’s and Antunes’ studies (2, 12), we reported the adjusted association of the COVID-19 pandemic with neoadjuvant chemotherapy. In the adjusted model, we observed that patients in the older age groups, patients diagnosed at FIGO stages III and IV, and patients with a higher comorbidity burden were more likely to receive neoadjuvant chemotherapy than their respective counterparts. Although neoadjuvant chemotherapy is mostly recommended in patients with advanced stage (stages III-IV) ovarian cancer, 8.5% of stage II patients in our cohort received neoadjuvant chemotherapy (data not shown); these patients were either too sick and frail to receive primary cytoreductive surgery, had a high comorbidity burden, or did not consent to surgery as the first-line treatment. When we limited our analysis to stage III-IV patients, more advanced cancer stage was still associated with increased neoadjuvant chemotherapy use. These findings are expected based on clinical management guidelines for ovarian cancer (14). Older age, advanced cancer stage, and comorbidities make these patients poor candidates for optimal cytoreductive surgery, and increase their vulnerability to COVID-19 infection and severity (10, 14, 15).
So far, evidence on COVID-19’s impact on ovarian cancer treatment patterns in the US is limited. Frey and colleagues reported treatment delay and change/cancellation of treatment regimens in gynecologic cancer patients receiving care at three New York city hospitals, although they did not assess trends in neoadjuvant chemotherapy use by pandemic periods (3). While Frey and colleagues observed delay in treatment initiation or change in treatment protocols in 38.7% of their study population due to the pandemic, they were unable to determine if treatment delays resulted from hospital policies or from patient-related factors (3). We did not observe any difference in time to treatment initiation before and after the onset of the pandemic. This might be attributed to KPSC’s integrated delivery of care model that ensures timely multidisciplinary care. It is also important to note that all patients included in our study were insured. Since we were interested in assessing the impact of the pandemic on neoadjuvant chemotherapy independent of patient’s COVID infection status, unlike Frey’s study, we only included patients who did not have any COVID-19 infection/diagnosis between cancer diagnosis and completion of first course of treatment.
Our study had some limitations. We did not have information on neoadjuvant chemotherapy dose; we were unable to assess if the COVID-19 pandemic impacted the recommended neoadjuvant chemotherapy dose in ovarian cancer patients. However, when we compared duration of neoadjuvant chemotherapy use before and during the pandemic, no statistically significant differences were observed. We did not have individual level SES data, but we used NDI as a proxy for SES. Our study population consisted of insured patients within an integrated health care system, hence, our findings may not be generalizable to other ovarian cancer cohorts. However, we included a racially and ethnically diverse cohort of ovarian cancer patients, and our extensive EMR database allowed us to capture the complete clinical characteristics of our patient population.
In conclusion, our study provided a snapshot of the impact of the pandemic on ovarian cancer care in the US, without the confounding effects of access to care. We observed increased neoadjuvant chemotherapy use during the pandemic compared to the pre-pandemic period in patients with advanced stage cancer. Future studies are needed to assess the impact of the pandemic on treatment patterns and cancer outcomes, including response to cancer treatments and survival in patients with ovarian cancers.
Data availability statement
Data analyzed during the current study are not publicly available due to the nature of the database (Electronic health records). Requests to access these datasets should be directed to CC, Q2h1bi5SLkNoYW9Aa3Aub3Jn.
Ethics statement
The studies involving humans were approved by Kaiser Permanente Southern California Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because secondary, de-identified data were used.
Author contributions
AM: Investigation, Methodology, Validation, Writing – original draft. NS: Validation, Writing – review & editing. LX: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. KC: Data curation, Investigation, Project administration, Validation, Writing – review & editing. AG: Data curation, Investigation, Validation, Writing – review & editing. ES: Conceptualization, Methodology, Writing – review & editing. MM: Conceptualization, Methodology, Writing – review & editing. MH: Investigation, Methodology, Validation, Writing – review & editing. CC: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and AstraZeneca UK Ltd (PI: CC).
Acknowledgments
The authors thank all patients included in the study.
Conflict of interest
Author LX was employed by MedHealth Statistical Consulting Inc. Authors ES and MM are employees of Merck & Co, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and AstraZeneca UK Ltd. The funder was involved in the study design and interpretation of data.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. (2020) 1:565–7. doi: 10.1038/s43018-020-0074-y
2. Algera MD, van Driel WJ, Slangen BFM, Kruitwagen R, Wouters M, participants of the Dutch Gynecological Oncology Collaborator g. Impact of the COVID-19-pandemic on patients with gynecological Malignancies undergoing surgery: A Dutch population-based study using data from the 'Dutch Gynecological Oncology Audit'. Gynecol Oncol. (2022) 165:330–8. doi: 10.1016/j.ygyno.2022.02.013
3. Frey MK, Fowlkes RK, Badiner NM, Fishman D, Kanis M, Thomas C, et al. Gynecologic oncology care during the COVID-19 pandemic at three affiliated New York City hospitals. Gynecol Oncol. (2020) 159:470–5. doi: 10.1016/j.ygyno.2020.09.005
4. Jacome LS, Deshmukh SK, Thulasiraman P, Holliday NP, Singh S. Impact of COVID-19 pandemic on ovarian cancer management: adjusting to the new normal. Cancer Manag Res. (2021) 13:359–66. doi: 10.2147/CMAR.S287152
5. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. (2010) 363:943–53. doi: 10.1056/NEJMoa0908806
6. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. (2015) 386(9990):249–57. doi: 10.1016/S0140-6736(14)62223-6
7. Al-Jabir A, Kerwan A, Nicola M, Alsafi Z, Khan M, Sohrabi C, et al. Impact of the Coronavirus (COVID-19) pandemic on surgical practice - Part 1. Int J Surg. (2020) 79:168–79. doi: 10.1016/j.ijsu.2020.05.022
8. Fader AN, Huh WK, Kesterson J, Pothuri B, Wethington S, Wright JD, et al. When to operate, hesitate and reintegrate: society of gynecologic oncology surgical considerations during the COVID-19 pandemic. Gynecol Oncol. (2020) 158:236–43. doi: 10.1016/j.ygyno.2020.06.001
9. COVID-19 Guidelines for Triage of Gynecology Patients. Chicago, IL: American College of Surgeons (2020). Available at: https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/gynecology/.
10. Colombo I, Zaccarelli E, Del Grande M, Tomao F, Multinu F, Betella I, et al. ESMO management and treatment adapted recommendations in the COVID-19 era: gynaecological Malignancies. ESMO Open. (2020) 5. doi: 10.1136/esmoopen-2020-000827
11. Akladios C, Azais H, Ballester M, Bendifallah S, Bolze PA, Bourdel N, et al. Recommendations for the surgical management of gynecological cancers during the COVID-19 pandemic - FRANCOGYN group for the CNGOF. J Gynecol Obstet Hum Reprod. (2020) 49:101729. doi: 10.1016/j.jogoh.2020.101729
12. Antunes D, Mendonca L, Melo A, Goncalves S, Nogueira Martins F, Nogueira Martins N. Impact of the COVID-19 pandemic on diagnosis and management of gynecological cancer: A single-center analysis. Medicina (Kaunas). (2022) 58. doi: 10.3390/medicina58121862
13. Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother Psychosom. (2022) 91:8–35. doi: 10.1159/000521288
14. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:191–226. doi: 10.6004/jnccn.2021.0007
Keywords: COVID-19 pandemic, ovarian cancer, neoadjuvant chemotherapy, surgery, race/ethnicity
Citation: Mukherjee A, Shammas N, Xu L, Cannavale KL, Gilfillan AD, Szamreta EA, Monberg M, Hodeib M and Chao CR (2024) Impact of the Coronavirus Disease 2019 pandemic on neoadjuvant chemotherapy use in patients diagnosed with epithelial type ovarian cancer. Front. Oncol. 14:1290719. doi: 10.3389/fonc.2024.1290719
Received: 07 September 2023; Accepted: 13 March 2024;
Published: 27 March 2024.
Edited by:
Analisa DiFeo, University of Michigan, United StatesReviewed by:
Mohammadjavad Ashrafi Mahabadi, Iran University of Medical Sciences, IranTommaso Bianchi, University of Milano Bicocca, Italy
Copyright © 2024 Mukherjee, Shammas, Xu, Cannavale, Gilfillan, Szamreta, Monberg, Hodeib and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun R. Chao, Q2h1bi5SLkNoYW9Aa3Aub3Jn
†ORCID: Chun R. Chao, orcid.org/0000-0002-6072-4954
 Amrita Mukherjee
Amrita Mukherjee Natalie Shammas2
Natalie Shammas2 Chun R. Chao
Chun R. Chao