- 1Department of Ophthalmology, Centro Hospitalar Universitário Lisboa Central, Lisbon, Portugal
- 2Department of Ophthalmology, Unidade Local de Saúde de São José, Lisbon, Portugal
- 3Department of Ophthalmology, Hospital CUF Descobertas, Lisbon, Portugal
- 4Department of Anatomical Pathology, Hospital de Montilla, Andaluzia, Spain
- 5Department of Ophthalmology, Hospital Lusíadas de Lisboa, Lisbon, Portugal
- 6Deparment of Ophthalmology, Clínica de São João de Deus, Lisbon, Portugal
- 7Department of Haematology and Oncology, CUF Oncologia, Lisbon, Portugal
- 8Department of Medical Oncology, Hospital de Cascais, Cascais, Portugal
- 9NOVA Medical School (NMS), Faculdade de Ciências Médicas (FCM), Universidade NOVA de Lisboa (UNL), Lisbon, Portugal
- 10Department of Medical Oncology, AIM Cancer Center, Lisbon, Portugal
Breast cancer is a significant global health concern, contributing to substantial morbidity and mortality among women. Hormone receptor-positive (HR+)/HER2-negative (HER2-) breast cancer constitutes a considerable proportion of cases, and significant advancements have been made in its management. CDK4/6 inhibitors (CDK4/6is) are a new targeted therapy that has demonstrated efficacy in adjuvant, advanced and metastatic settings. The propensity of lobular breast carcinomas for estrogen-rich sites, such as periocular tissues and orbital fat, may explain their tendency for orbital metastases. Current treatment strategies for these cases are predominantly palliative, and the prognosis remains poor. This article presents a unique case of a 51-year-old female with progressive right periorbital edema, pain, and limited ocular motility. An imaging work-up showed bilateral intra and extraconal orbital infiltration, which was biopsied. The histopathologic analysis disclosed mild chronic inflammatory infiltrate with thickened fibrous tissue and moderately differentiated lobular carcinoma cells, positive for GATA3 and CK7 markers, with 100% of tumor nuclei expressing estrogen receptors (ER+). A systemic evaluation showed a multicentric nodular formation in both breasts. Further diagnostic assessments unveiled an HR+/HER2- bilateral lobular breast carcinoma with synchronous bilateral orbital metastases. Systemic treatment was initiated with abemaciclib 150mg twice daily and letrozole 2.5mg once a day. However, this regimen was interrupted due to toxicity. After two weeks, treatment was resumed with a reduced abemaciclib dose (100mg twice daily) alongside letrozole, with a reasonable tolerance. Nearly two years after the initial diagnosis of inoperable metastatic cancer, the patient remains on the same systemic treatment regimen with no signs of invasive disease. This case report is the first of a patient presenting with bilateral orbital metastases from bilateral lobular breast cancer, showing an impressive and sustained response to a first-line treatment regimen combining abemaciclib and letrozole. A literature review on bilateral orbital metastases from breast cancer is also presented.
1 Introduction
Breast cancer is the most commonly diagnosed cancer globally and is the primary cause of cancer-related mortality in women (1). Categorized by disease stage and histological features, which include morphology and receptor status, breast cancer heterogeneity plays a crucial role in clinical decision-making (2, 3). Hormone receptor-positive (HR+)/HER2-negative (HER2-) breast cancer constitutes the most prevalent subtype, accounting for around 65% of cases (4). Another shared characteristic in luminal HER2- breast cancer is the hyperactivity of the CDK4/6 pathway, which contributes to resistance against endocrine therapy (5).
In recent years, significant strides have been made in the management of HR+/HER2- breast cancer through the introduction of CDK4/6 inhibitors (CDK4/6is), such as palbociclib, ribociclib, and abemaciclib, thereby improving outcomes for adjuvant, advanced and/or metastatic settings (6–15). CDK4/6is can block retinoblastoma protein hyper-phosphorylation, inducing G1 arrest and curtailing proliferation (16, 17). A novel therapeutic approach by abemaciclib (Verzenio; Eli Lilly), an oral selective small molecule targeting the CDK-RB1-E2F pathway pivotal for cell cycle progression, has garnered substantial attention (16). The MONARCH 3 trial, a phase 3, double-blind, randomized study, recently demonstrated that abemaciclib plus nonsteroidal aromatase inhibitor (NSAI - including letrozole) resulted in more prolonged overall survival compared to placebo plus NSAI (absolute improvement of 13.1 months) (hazard ratio, 0.804; 95% CI, 0.637 to 1.015; p= 0.0664; p-value did not reach threshold for statistical significance) and significantly extended progression-free survival (hazard ratio, 0.535; 95% CI, 0.429 to 0.668; p= <0.0001; 29.0 months in the abemaciclib arm, 14.8 months in the placebo arm) (10, 18). Consequently, combining CDK4/6is with endocrine therapy emerged as one of the preferred regimens for patients with advanced and/or metastatic HR+/HER2- breast cancer.
Furthermore, abemaciclib distinguishes itself as the sole CDK4/6 inhibitor examined in a dedicated clinical trial specifically addressing metastatic disease within the central nervous system (CNS) (NCT02308020, phase II trial, encompassing leptomeningeal disease, a criterion indicative of greater severity) (19, 20). In contrast, trials involving palbociclib and ribociclib had limited inclusion or lacked representation of patients with disease at this CNS level (21–23).
To our knowledge, we report the first clinical case of bilateral orbital metastases as the presenting feature of bilateral breast cancer treated with a CDK4/6i and an aromatase inhibitor.
2 Case report
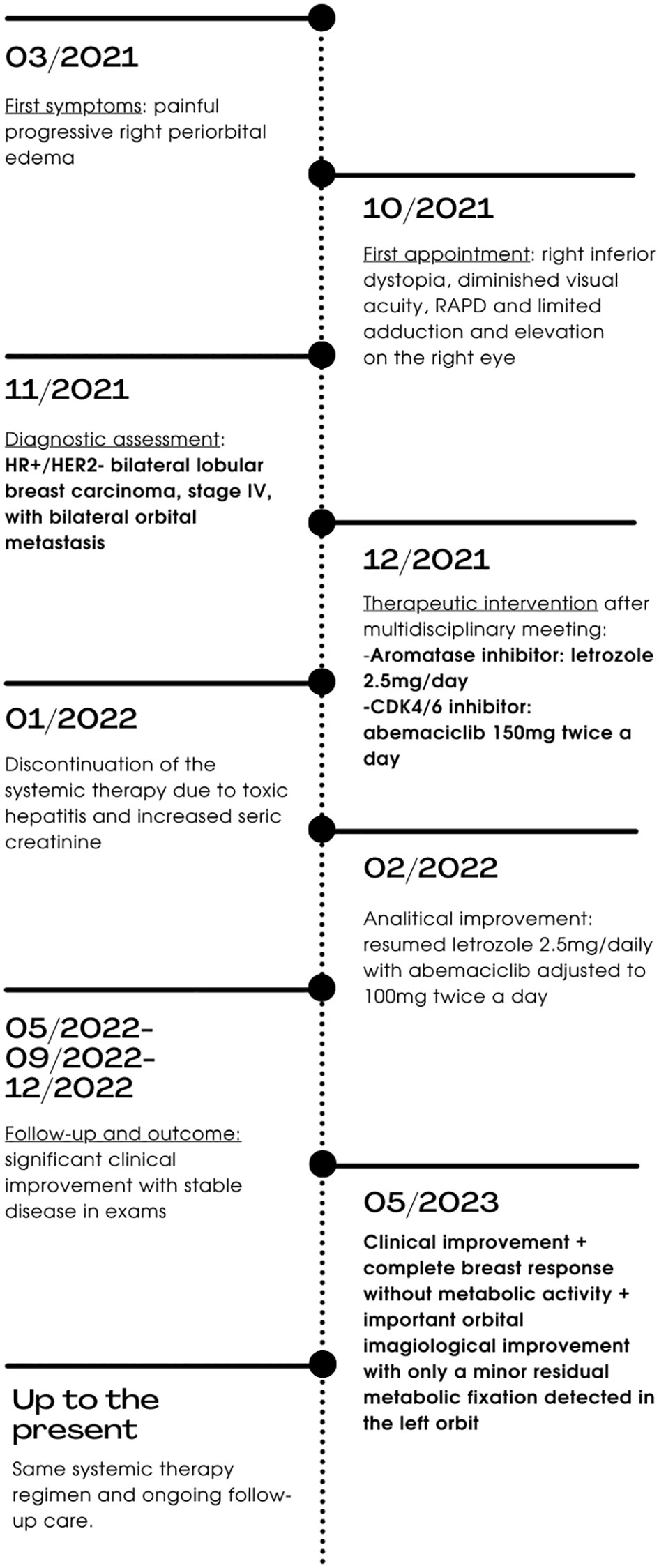
A 51-year-old female presented with a seven-month history of painful progressive periorbital edema and limitation of extraocular movements of the right eye (Figure 1). Her medical history revealed essential hypertension, dyslipidemia, adenomyosis, benign thyroid nodule, gallbladder polyp, major depression, allergic rhinitis, and a smoking history of 32 pack-years. Her pharmacological regimen included candesartan, rosuvastatin, montelukast, paroxetine, lorazepam, mirtazapine, and bupropion. Additionally, she reported an allergy to ibuprofen and had a pertinent family history of prostate cancer in two brothers, diagnosed at 64 and 70 years old. Physical examination revealed inferior dystopia of the right eye with limited horizontal movements on the right eye, without diplopia (Figure 2). The best corrected visual acuity was 20/30 right eye (OD) and 20/20 left eye (OS), Ishihara test 6/10 OD vs. 9/10 OS, and a relative afferent pupillary defect (RAPD) was detected on the right eye. Hertel exophthalmometry showed mild asymmetry of 15mm OD and 16mm OS. In the visual fields, there was an inferonasal paracentral scotoma in the right eye, while the left eye had a normal visual field. Optical coherence tomography (OCT) indicated a mild optic disc edema and a reduction in retinal ganglion cell layer thickness in the right eye, without changes in the nerve fiber layer; no changes were observed in the left eye. Biomicroscopy, intraocular pressure, and ocular fundoscopy findings were unremarkable.
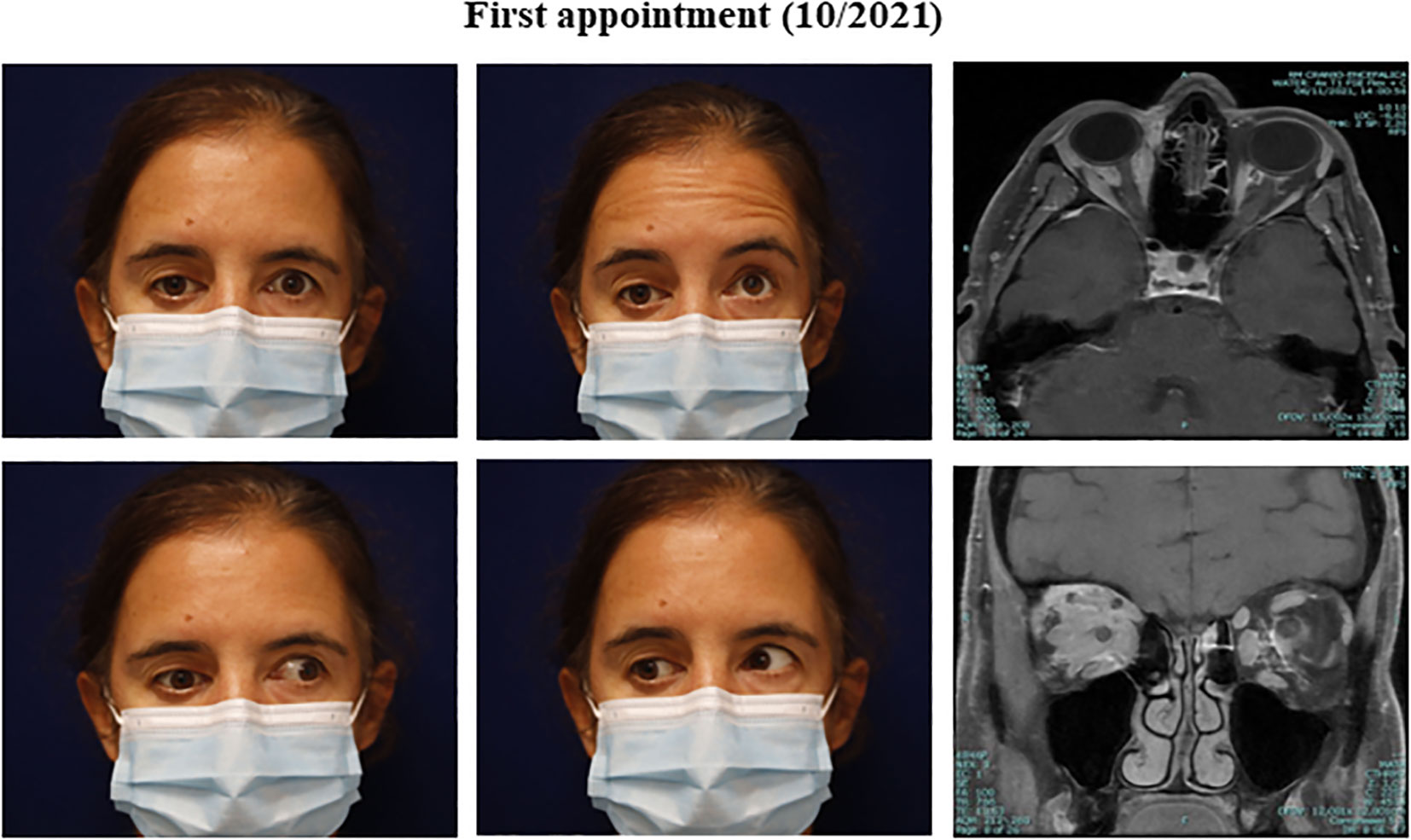
Figure 2 Clinical presentation and orbital findings at the initial appointment. (Clinical pictures) Right inferior dystopia with restriction in extraocular elevation and adduction of the right eye. (Orbit Imaging) Orbit axial and coronal T1 MRI showing post-gadolinium enhancing lesions (intra and extra-conal), with mass effect and inflammatory changes of orbital fat.
2.1 Diagnostic assessment
After the initial presentation, an orbital and cranial magnetic resonance image (MRI) was requested. An extensive intra- and extraconal orbital infiltration involving the optic nerve, extrinsic ocular musculature, and lacrimal gland was found on the right orbit. Similar discrete signal alterations were identified within the left orbit, mainly between the optic nerve and the medial and inferior rectus muscles (Figure 2).
An incisional biopsy of the right orbit was performed, which included several samples collected from the superior and superior-temporal areas through a lid cease approach. Histopathologic examination revealed moderately differentiated lobular carcinoma cells (Figure 3). Immunohistochemical analysis revealed positivity for GATA3 and CK7 markers, with 100% of tumor nuclei expressing estrogen receptors (ER+) (Figure 3). The c-ERB-B2 (HER2/neu) score was 0, and E-cadherin and PD-L1 (combined positive score) expressions were negative.
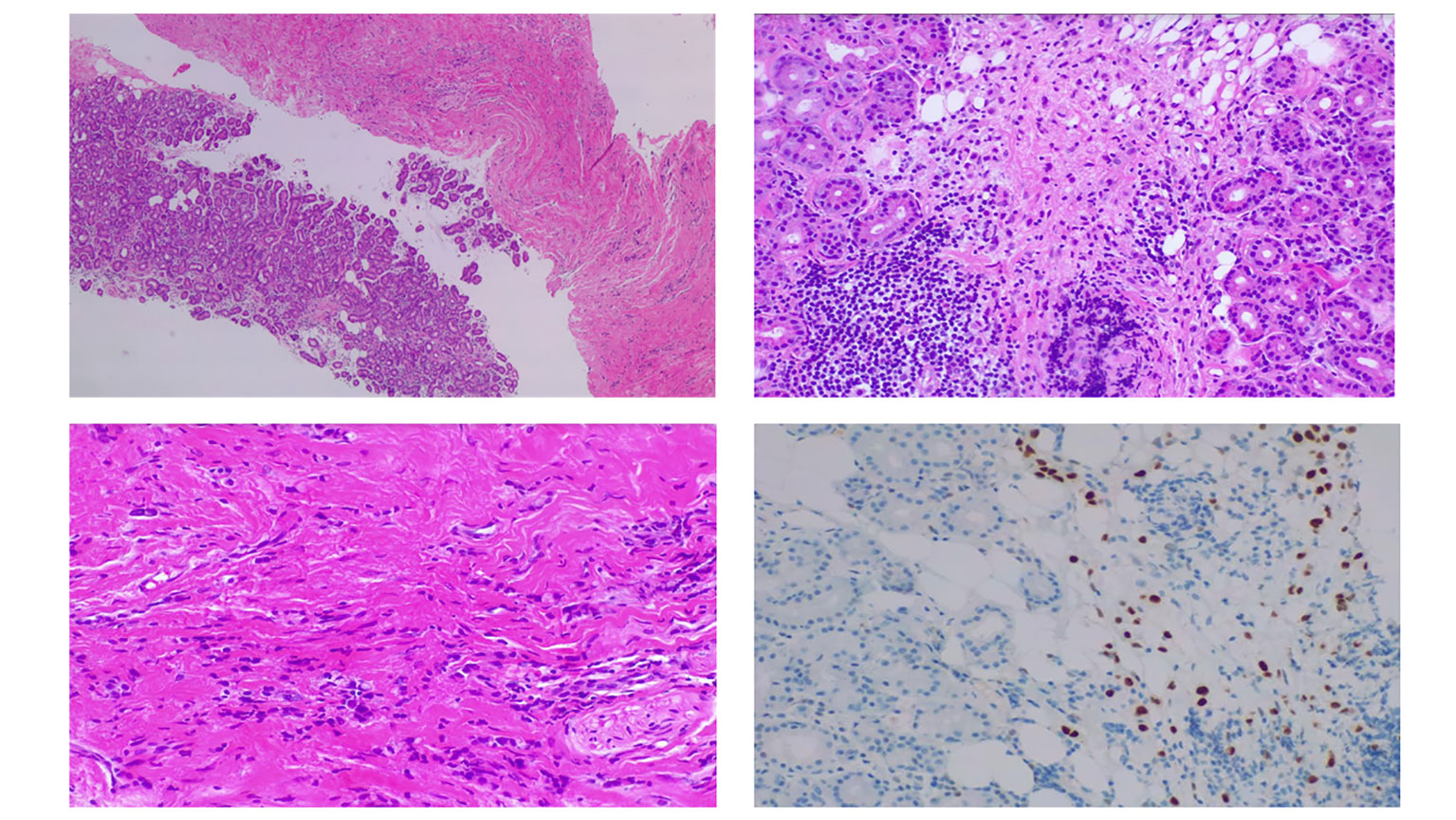
Figure 3 Orbital biopsy. (Supero-left) Orbital biopsy comprised soft tissue and lacrimal gland fragments with infiltration by lobular breast carcinoma. (Supero-right) Lacrimal gland showing discohesive cells with nuclear atypia, many resembling signet-ring cells and containing intracellular mucin. (Infero-left) Thickened fibrous tissue where isolated cells and cell rows of similar histologic characteristics are identified. (Infero-right) Infiltrating cells exhibiting immunoreactivity for estrogen receptors, suggesting breast origin.
Following these findings, a comprehensive work-up was initiated to identify the primary tumor. This encompassed breast ultrasonography, mammography, breast MRI, esophagogastroduodenoscopy, gynecological transvaginal ultrasound, lumbar puncture, and positron emission tomography (PET)/CT scan employing 18-fluorodeoxyglucose (18F-FDG). The PET/CT 18-FDG scan revealed moderate heterogeneous radiopharmaceutical uptake in both orbits, the right axillary lymph node, and mild to moderate metabolic activity in the stomach and uterus. Esophagogastroduodenoscopy uncovered hyperemic gastropathy without neoplastic or dysplastic tissue, and transvaginal ultrasonography identified adenomyosis and leiomyomas. A lumbar puncture revealed suspected neoplastic cells, prompting a neuroaxis MRI that showed no suspected invasive disease. Breast imaging unveiled multicentric nodular formations. These solid, irregularly contoured nodules numbered at least three on the right and two on the left, with a larger, coarser superior-external nodule on the right (10mm) along with notably enhancing right axillary lymph nodes, the largest measuring 19 mm. Given the suspicious nature of the findings in both breasts (BI-RADS Category 4), ultrasound-guided core biopsies were performed on two breast nodules and the right axillary node. Histological analysis revealed invasive carcinoma with a lobular pattern, moderately differentiated (Grade 2). ER was positive in 90% of cells, while progesterone receptor (PR) was 100%. HER2 was negative, as was E-cadherin. The dominant lesion in the right breast exhibited a proliferation index (Ki67) of 10%, and in the left breast, it was 7%. Axillary cytology confirmed these findings.
Hence, the patient was diagnosed with metastatic lobular breast cancer, classified as stage IV disease according to the AJCC 8th edition TNM staging (24). The case was discussed in a multidisciplinary breast cancer tumor board, and, given the metastatic and unresectable nature of the disease, coupled with its unsuitability for local intervention, it was decided to initiate systemic treatment with a CDK4/6i plus an aromatase inhibitor.
2.2 Therapeutic intervention
In December of 2021, based on the results of the MONARCH 3 clinical trial (10), the patient initiated abemaciclib 150mg twice daily, combined with letrozole 2.5mg once a day.
2.3 Follow-up and outcome
In January 2022, just a month after starting systemic therapy, the patient developed analytical toxic hepatitis, marked by elevated ALT and AST levels at grade 3, along with GGT elevation at grade 4, as classified by the Common Terminology Criteria for Adverse Events (CTCAE) (25), which contributed to the temporary withdrawal of treatment. Furthermore, this was accompanied by increased serum creatinine (grade 2). After a two-week interval, during which laboratory parameters were reassessed and showed progressive improvement, the patient resumed letrozole, while the dose of abemaciclib was adjusted to 100mg twice a day. Close monitoring of laboratory values was undertaken. Over the subsequent four months, there was a gradual recovery in hepatic parameters, although the serum creatinine level remained at grade 1.
Concomitantly, the patient encountered grade 1 diarrhea, nausea, and asthenia. While adverse effects progressively resolved, grade 1 diarrhea persisted and was effectively managed through interventions such as loperamide administration, oral hydration, and dietary adjustments.
During follow-up, the patient exhibited a marked clinical response to treatment, with significant recovery of visual acuity and extraocular motility, which occurred as early as the first cycle of abemaciclib and continued despite the reduced dosage of 100mg twice daily. The patient underwent repeated orbital MRI, breast MRI, and PET/CT with 18F-FDG imaging, confirming a favorable response, with bilateral tumor size reduction on both orbits and breast areas, without new lesions.
In May 2023, after sixteen months of systemic therapy, the patient achieved a complete response in both breasts and a significant improvement on orbital imaging, with practically complete permeabilization of bilateral intraorbital fat with only a minor residual metabolic fixation detected in the left orbit (Figure 4). Visual acuity remained stable at 20/20 OI, with visual field recovery, and extraocular motility improved with only a mild limitation of right eye adduction (Figure 4). OCT revealed an improvement in retinal ganglion cell layer thickness and normalization of the optic disc in the right eye. Hertel exophthalmometry was 14mm OD and 15mm OS, while the rest of the physical examination yielded unremarkable findings.
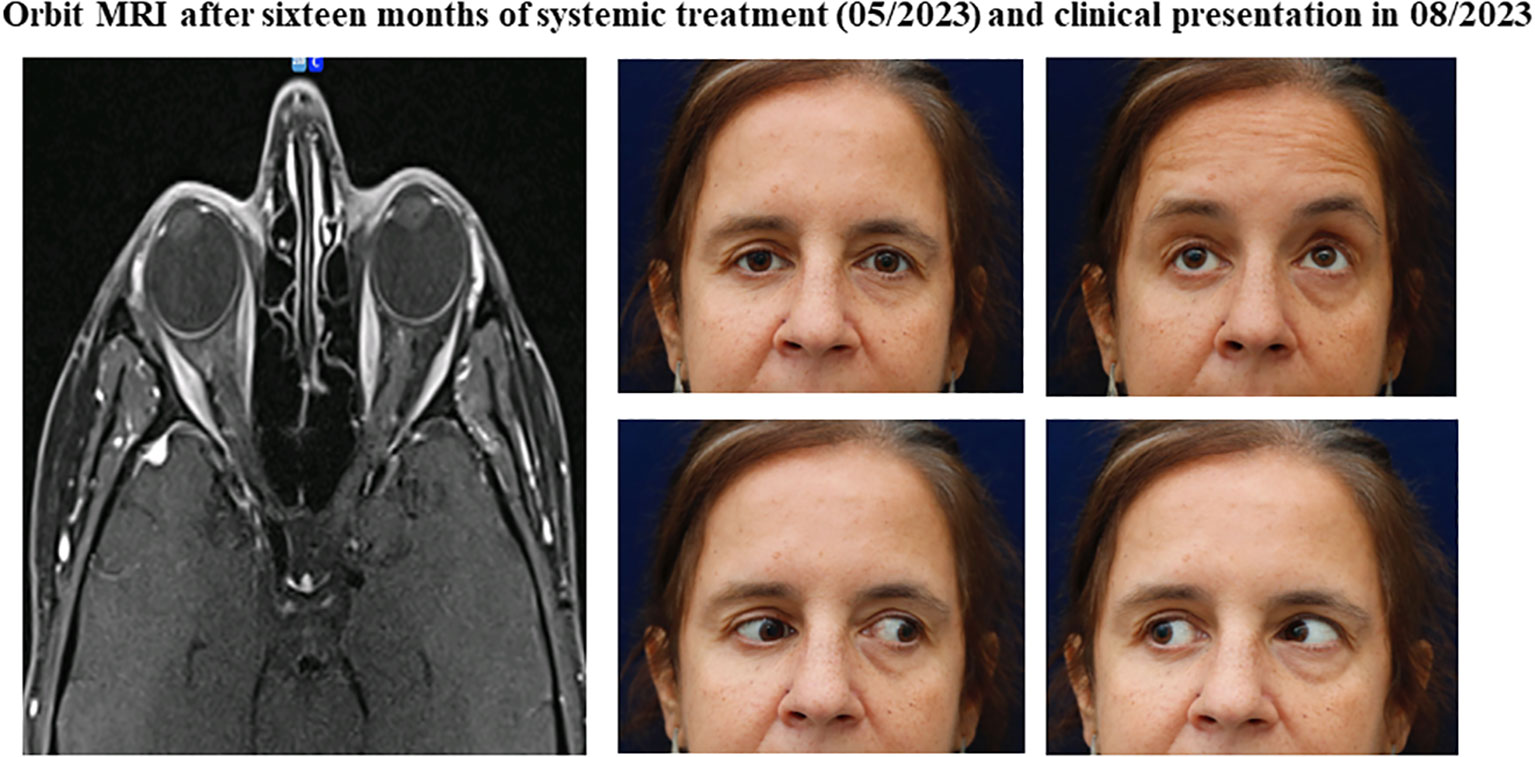
Figure 4 Orbital findings after sixteen months of systemic treatment and clinical presentation in 08/2023. (Orbit Imaging) Orbit axial and coronal T1 MRI showing imaging improvement in the orbital region, marked by permeabilization of bilateral intraorbital fat. (Clinical pictures) Significant clinical improvement in ocular movement restrictions, with only partial limitation remaining on right adduction.
The patient continues to adhere to the same systemic therapy regimen, remains resilient with her progress, and actively participates in follow-up care. In the last follow-up, the patient resumed her professional and social activities, not reporting any limitations in daily tasks.
3 Discussion and conclusion
Orbital metastases represent a complex subset, accounting for 1–13% of all orbital neoplasms and affecting around 2–5% of patients diagnosed with systemic malignancies (26). Notably, breast cancer (36%), melanoma (10%), and prostate cancer (8.5%) emerge as the most common primary sources of orbital metastases (27–29). They are typically unilateral, but clinically evident bilateral metastases are reported in 4–20% of cases (30). They are often identified after the primary tumor diagnosis, with a prevailing interval of 3 to 6 years (31, 32). However, exceptional cases have revealed latency extending over decades post-cancer diagnosis, the longest being 42 years after the primary breast carcinoma identification (33). The median age of orbital metastases from breast cancer is 54 (range 28-77 years) (26, 29).
Various tumors and tumor-like lesions can involve the orbit (34), making imaging a crucial step in the initial differential diagnosis of patients with new symptoms or without a previous diagnosis (35). Thyroid eye disease, granulomatosis with polyangiitis, amyloidosis, sarcoidosis, lymphoproliferative disease, orbital inflammatory pseudotumor, IgG4-related disease, as well as solid tumors, infectious and vascular conditions, are always important to consider when radiologic changes are found in the orbital space (36). A biopsy is warranted when clinicoradiologic findings are inconclusive or a previous histological diagnosis is questioned (36, 37).
Intriguingly, orbital metastases can occasionally serve as the inaugural finding of an undetected primary tumor, appearing in an estimated 10% to 31% of cases (31, 38, 39). Considering histological subtypes, lobular breast carcinoma, comprising 10-15% of all breast cancer cases (40), exhibits an increased expression of ER and PR but has decreased HER2 positivity compared to the no special type (NST)/ductal carcinoma (41). In contrast, E-cadherin expression in ductal breast carcinoma limits cellular dispersion, and therefore, orbital metastases from NST are rare (42). Conversely, it is worth noting the propensity of lobular carcinomas for metastases to sites with a substantial supply of estrogen, such as the gastrointestinal and genitourinary tracts (42–47). This could be attributed to the steroid hormone production in periocular tissues and orbital fat, fostering a conducive milieu to metastases of lobular breast carcinoma (45–47).
Despite advances, therapeutic strategies for managing orbital metastases remain a challenge due to the scarcity of data. Current treatment approaches generally lean toward a palliative plan, especially as orbital metastases from breast cancer often arise in the context of advanced end-stage disease (48). Even with treatment, the prognosis for patients diagnosed with orbital metastases yields a mean survival of 31 months (1-116 months) (31, 49).
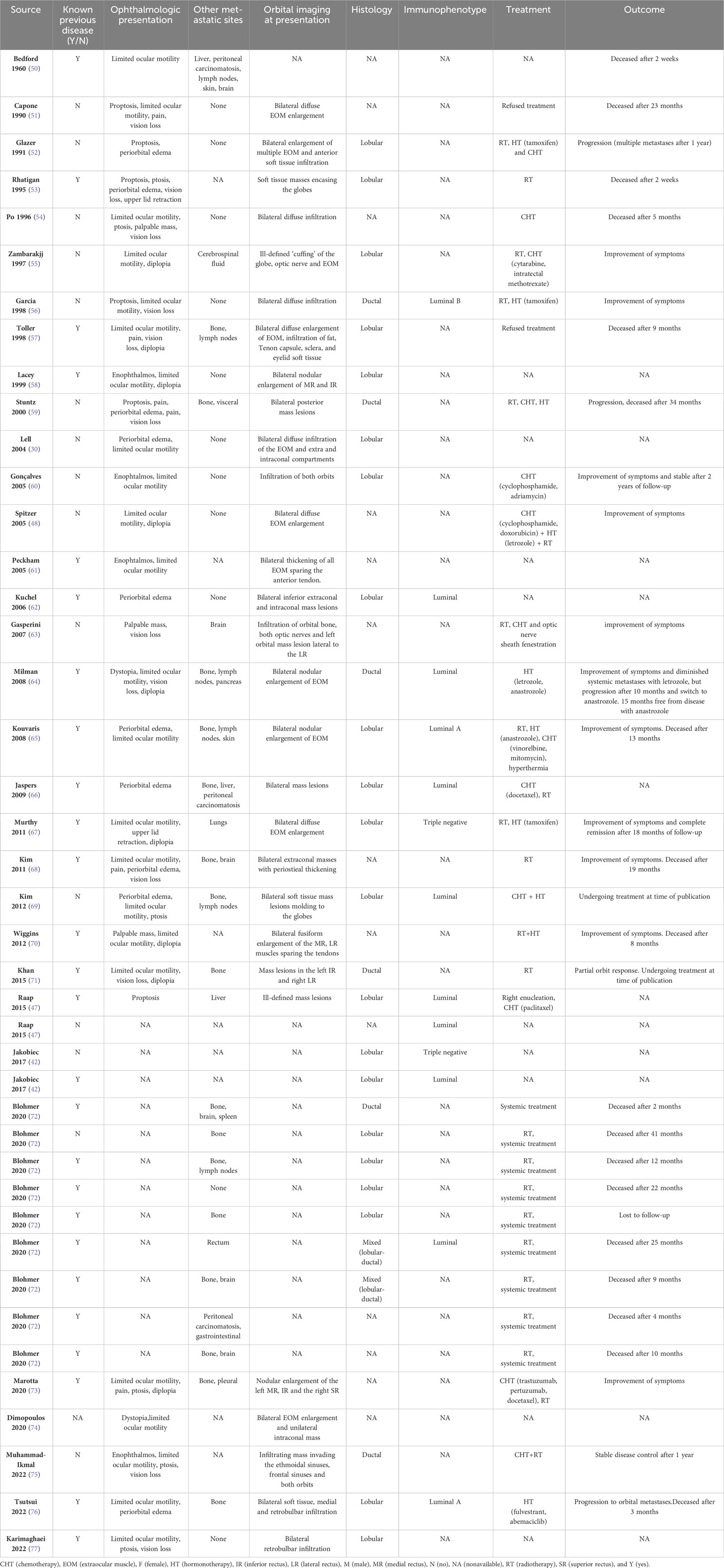
A review of cases involving bilateral orbital metastases from breast cancer, as reported in English-language literature, was conducted through PubMed, Medline, and Google Scholar databases using the appropriate controlled [MESH] keywords “breast cancer”, “bilateral”, “metastases”, “ocular” and “orbit” and acknowledging references list. The selected articles included case reports and case series that provided detailed clinical, histological, and treatment descriptions (30, 42, 47, 48, 50–77). The summarized findings are presented in Table 1. Forty-two patients, mostly females (95%), were found. The mean age was 59 years (ranging from 36 to 83 years). The majority (64%) had known breast cancer (42, 47, 50, 53, 57, 58, 61, 62, 64–68, 70–73, 76, 77), and orbital metastases were usually identified around 4.8 years after the first diagnosis. Due to the anatomical constraints of the compact orbit space, these metastases usually present as space-occupying lesions, leading to significant clinical symptoms (31). Affected patients commonly exhibit limited ocular motility (55%) (30, 48, 50, 51, 54–58, 60, 61, 64, 65, 67–71, 73–77), vision loss (29%) (51, 53, 54, 56, 57, 59, 63, 64, 68, 71, 75, 77), periorbital edema (24%) (30, 52, 53, 59, 62, 65, 66, 68, 69, 76), diplopia (21%) (48, 55, 57, 58, 64, 67, 70, 71, 73), proptosis (14%) (47, 51–53, 56, 59), ptosis (14%) (53, 54, 69, 73, 75, 77), palpable mass (7%) (54, 63, 70), as well as dystopia (64, 74), and upper lid retraction (53, 67) (both 5%). A notable and intriguing occurrence is enophthalmos, observed in 2 cases (5%) (61, 75). This is likely due to the infiltration of neoplastic cells into the extraocular muscles and retro-bulbar stromal tissues, leading to desmoplasia, fibrosis, and the retraction of the eye globe (78). The majority of orbital metastases exhibit lobular histology (50%) (30, 42, 47, 52, 53, 55, 57, 58, 60, 62, 65–67, 69, 72, 76, 77) vs. ductal (14%) (56, 59, 64, 71, 72, 75) (48, 61, 74) vs. mixed (5%) (72), a trend that is consistent throughout existing literature (42). The immunophenotype of these clinical cases is predominantly hormone receptor-positive in breast cancer, specifically belonging to the luminal subtype (42, 47, 56, 62, 64–66, 69, 72, 76). These metastases often demonstrate a diffuse infiltration pattern within the orbit, affecting bones and extraocular muscles. Invasion of intracranial structures is rare, with brain metastases identified in only 6 cases (14%) (50, 63, 68, 72). Despite various forms of palliative treatment, bilateral orbital metastasis from breast cancer remains a poor prognostic factor, with a mean survival of 16 months following diagnosis (range 0.5 to 41 months) (31).
The emergence of CDK4/6is, such as palbociclib, ribociclib, and abemaciclib, has brought a remarkable shift in the paradigm of the treatment of advanced and/or metastatic HR+/HER2- breast cancer (6–13). Notably, none of the included treatment guidelines name specific CDK4/6is treatments but recommend the class broadly, as there have been no head-to-head clinical trials to date comparing the three approved CDK4/6is, and the efficacy of each appears to be similar (22, 79, 80). Nevertheless, the latest comprehensive survival data imply possible distinctions between the different CDK4/6is, indicating a trend in preferred choices, as palbociclib did not increase overall survival (23).
Notably, abemaciclib has exhibited efficacy in managing intraocular metastases originating from breast cancer, as elucidated in two case reports (81, 82). A woman 57 years old with iris metastases, which regressed within four months and remained undetectable through an eight-month follow-up using a combination of abemaciclib and letrozole (82). In a second case, a woman in her 50s with bilateral choroid metastases stemming from breast cancer positively responded to abemaciclib and fulvestrant within four months after the beginning of treatment (81). The significant response observed to abemaciclib in treating intraocular metastases aligns with preclinical and clinical evidence showing its ability to penetrate the central nervous system (19, 20). This suggests that abemaciclib holds promise as a viable therapeutic option in this specific clinical scenario. No cases of orbital metastases treated with these targeted therapies were found.
To the best of our knowledge, we present the first case of a patient whose initial presentation had bilateral orbital metastases originating from bilateral lobular breast cancer with a substantial and dramatic response to a first-line treatment regimen that combined abemaciclib and letrozole.
Interestingly, our case report emphasizes that even with a reduced dose of 100mg, abemaciclib demonstrated efficacy without compromising the outcome. Similar to the MONALEESA trials, overall survival outcomes for patients with HR+/HER2- advanced breast cancer exhibited comparable results between those who underwent dose reductions of ribociclib and those who received the standard dose (83). This observation prompts the intriguing idea of tailoring treatment by personalizing doses for individual patients, considering their unique responses and tolerances.
Further comprehensive investigations are warranted to fully comprehend the potential of CDK4/6is in managing orbital metastases. It is essential to conduct rigorous studies that evaluate the safety and efficacy of different CDK4/6is through head-to-head comparisons and explore the impact of varying doses. These studies will provide valuable insights into optimizing treatment strategies and potentially improving outcomes for HR+/HER2- breast cancer patients with orbital metastases.
Therefore, the selection of CDK4/6i depends mainly on the toxicity profile and comorbidities of the patient. For instance, it is conceivable to avoid abemaciclib in patients with inflammatory bowel disease, while ribociclib should be avoided in patients with prolonged QT interval alterations on electrocardiogram (23). Conversely, palbociclib should be cautiously approached in patients with compromised bone marrow reserve (23).
Notably, the most frequent adverse effect observed during abemaciclib treatment is diarrhea, primarily of grade 1 severity (10), which aligns with our clinical case. Additionally, our patient exhibited analytical findings of hepatic toxicity and a mild increase in serum creatinine one month after initiating systemic treatment. These events, known and expected in the MONARCH trials (10), resolved upon withdrawal and subsequent reduction of abemaciclib dosage to 100mg twice a day. Adverse events and toxicities have been recognized in certain instances to correlate with positive treatment outcomes in cancer therapy (84, 85). Nevertheless, the current understanding of predictive factors for response to available breast cancer treatments remains insufficient. This uncertainty prompts the exploration of unconventional factors, such as the microbiota’s role in offering insights into individual risk and prognosis, pharmacokinetics, pharmacodynamics, and clinical efficacy (86, 87). Recent research has demonstrated the capacity of the gut microbiota to influence the effectiveness and adverse effects of cancer treatments, as both cancer and anticancer therapies have bidirectional interactions with gut microbiota (86, 88, 89).
While the correlation is intriguing, it is essential to acknowledge that it may not be straightforward. The connection between adverse effects and treatment response can be intricate, influenced by various patient-specific elements, tumor characteristics, and the complex interplay of the drug with the body’s physiological systems. Thus, while a correlation between diarrhea, changes in hepatic parameters, and treatment response in breast cancer with abemaciclib is captivating, further investigations are imperative to establish a causal relationship and unveil the underlying mechanisms linking these observations.
As we navigate these investigations, we must recognize the limitations inherent in single-case reports and exercise caution in extrapolating results to similar presentations and the long-term effects that may extend beyond sixteen months.
In conclusion, this clinical case underscores the potential of combining CDK4/6is, especially abemaciclib, with endocrine therapy in treating HR+/HER2- orbital metastatic breast cancer. While this case report highlights promising therapeutic avenues, it underscores the need for comprehensive studies, acknowledging the complexities of individual responses and the influence of factors like microbiota. As we advance toward more personalized oncology approaches, these findings encourage us to delve deeper into the interplay between treatments, adverse effects, and patient outcomes to optimize therapeutic strategies in metastatic breast cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NRA: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Supervision. AD: Conceptualization, Data curation, Formal analysis, Resources, Supervision, Validation, Visualization, Writing – review & editing. DR: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft. RS: Data curation, Investigation, Resources, Visualization, Writing – original draft. BC: Data curation, Writing – review & editing. DAC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The funding was for open access publication fee. Payed by CUF Oncology.
Acknowledgments
The authors would like to express their gratitude to Nuno Caçador, MD, neuroradiologist, for his invaluable work and diagnostic assessment.
Conflict of interest
DC has received honoraria from the Portuguese Navy, CUF Oncologia, RiberaSalud, and NTT DATA, and has served as a speaker, advisory board member, or has received research or education funding from AstraZeneca, CUF Oncologia, Daiichi Sankyo, Gilead, Hoffmann-La Roche, Merck KGaA, Merck Sharp & Dohme, Nestlé, Novartis, Pfizer, Nanobiotix, Puma Bio-technology Inc., Sanofi, Seagen Inc., and Uriage. DC is supported by two research grants: AstraZeneca Produtos Farmacêuticos LDA and Grupo José de Mello.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Seung SJ, Traore AN, Pourmirza B, Fathers KE, Coombes M, Jerzak KJ. A population-based analysis of breast cancer incidence and survival by subtype in ontario women. Curr Oncol (2020) 27(2):191–8. doi: 10.3747/co.27.5769
3. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PloS Med (2010) 7(5):e1000279. doi: 10.1371/journal.pmed.1000279
4. NIH SEER Program. Cancer stat facts: female breast cancer subtypes. 2022. Cancer stat facts: female breast cancer subtypes. Available at: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (Accessed 1st August 2023).
5. Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Related Cancer. (2011) 18(3):333–45. doi: 10.1530/ERC-10-0262
6. Li J, Fu F, Yu L, Huang M, Lin Y, Mei Q, et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res Treat (2020) 180(1):21–32. doi: 10.1007/s10549-020-05528-2
7. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. New Engl J Med (2016) 375(18):1738–48. doi: 10.1056/NEJMoa1609709
8. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med (2016) 375(20):1925–36. doi: 10.1056/NEJMoa1607303
9. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol (2016) 17(4):425–39. doi: 10.1016/S1470-2045(15)00613-0
10. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35(32):3638–46. doi: 10.1200/JCO.2017.75.6155
11. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol (2018) 19(7):904–15. doi: 10.1016/S1470-2045(18)30292-4
12. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. New Engl J Med (2019) 381(4):307–16. doi: 10.1056/NEJMoa1903765
13. Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2. JAMA Oncol (2020) 6(1):116. doi: 10.1001/jamaoncol.2019.4782
14. Slamon DJ, Fasching PA, Hurvitz S, Chia S, Crown J, Martín M, et al. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2– early breast cancer. Ther Adv Med Oncol (2023) 15:175883592311781. doi: 10.1177/17588359231178125
15. Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol (2023) 24(1):77–90. doi: 10.1016/S1470-2045(22)00694-5
16. Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs (2021) 81(3):317–31. doi: 10.1007/s40265-020-01461-2
17. Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev (2016) 45:129–38. doi: 10.1016/j.ctrv.2016.03.002
18. Goetz M, Toi M, Huober J, Sohn J. MONARCH 3: Final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. Texas (2023).
19. Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor–positive breast cancer. Clin Cancer Res (2020) 26(20):5310–9. doi: 10.1158/1078-0432.CCR-20-1764
20. Schlam I, Tolaney SM. Is there a role for CDK 4/6 inhibitors in breast cancer brain metastases? Oncotarget (2021) 12(9):873–5. doi: 10.18632/oncotarget.27904
21. De Laurentiis M, Borstnar S, Campone M, Warner E, Bofill JS, Jacot W, et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population. Breast Cancer Res Treat (2021) 189(3):689–99. doi: 10.1007/s10549-021-06334-0
22. Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol (2021) 39(35):3959–77. doi: 10.1200/JCO.21.01392
23. Grinshpun A, Tolaney SM, Burstein HJ, Jeselsohn R, Mayer EL. The dilemma of selecting a first line CDK4/6 inhibitor for hormone receptor-positive/HER2-negative metastatic breast cancer. NPJ Breast Cancer. (2023) 9(1):15. doi: 10.1038/s41523-023-00520-7
24. Teichgraeber DC, Guirguis MS, Whitman GJ. Breast cancer staging: updates in the AJCC cancer staging manual , 8th edition, and current challenges for radiologists, from the AJR special series on cancer staging. Am J Roentgenol (2021) 217(2):278–90. doi: 10.2214/AJR.20.25223
25. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. CTCAE versión 5.0. Evaluación de la gravedad de los eventos adversos dermatológicos de las terapias antineoplásicas. Actas Dermo-Sifiliográficas. (2021) 112(1):90–2. doi: 10.1016/j.ad.2019.05.009
26. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions. Ophthalmology (2004) 111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002
27. Ahmad SM, Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol (2007) 18(5):405–13. doi: 10.1097/ICU.0b013e3282c5077c
28. Palmisciano P, Ferini G, Ogasawara C, Wahood W, Bin Alamer O, Gupta AD, et al. Orbital metastases: A systematic review of clinical characteristics, management strategies, and treatment outcomes. Cancers (2021) 14(1):94. doi: 10.3390/cancers14010094
29. Valenzuela AA, Archibald CW, Fleming B, Ong L, O’Donnell B, Crompton JJ, et al. Orbital metastasis: clinical features, management and outcome. Orbit (2009) 28(2–3):153–9. doi: 10.1080/01676830902897470
30. Lell M, Schulz-Wendtland R, Hafner A, Magener A, Bautz WA, Tomandl BF. Bilateral orbital tumour as the presentation of mammographically occult breast cancer. Neuroradiology (2004) 46(8):682–5. doi: 10.1007/s00234-003-1106-x
31. Shields JA, Shields CL, Brotman HK, Carvalho C, Perez N, Eagle RC. Cancer metastatic to the orbit. Ophthal Plast Reconstruct Surg (2001) 17(5):346–54. doi: 10.1097/00002341-200109000-00009
32. Goldberg RA, Rootman J, Cline RA. Tumors metastatic to the orbit: A changing picture. Surv Ophthalmol (1990) 35(1):1–24. doi: 10.1016/0039-6257(90)90045-W
33. Spitofsky NR, Barke MR, Shields CL. Orbital and eyelid metastases 42 years after primary breast carcinoma. Ophthal Plast Reconstruct Surg (2023) 39(4):e135. doi: 10.1097/IOP.0000000000002304
34. Weber AL, Romo LV, Sabates NR. PSEUDOTUMOR OF THE ORBIT. Radiol Clinics North America (1999) 37(1):151–68. doi: 10.1016/S0033-8389(05)70084-1
35. Ben Simon GJ, Annunziata CC, Fink J, Villablanca P, McCann JD, Goldberg RA. Rethinking orbital imaging. Ophthalmology (2005) 112(12):2196–207. doi: 10.1016/j.ophtha.2005.09.013
36. Rana K, Juniat V, Patel S, Selva D. Extraocular muscle enlargement. Graefe’s Arch Clin Exp Ophthalmol (2022) 260(11):3419–35. doi: 10.1007/s00417-022-05727-1
37. Mombaerts I, Rose GE, Verity DH. Diagnosis of enlarged extraocular muscles. Curr Opin Ophthalmol (2017) 28(5):514–21. doi: 10.1097/ICU.0000000000000395
38. Tomizawa Y, Ocque R, Ohori NP. Orbital metastasis as the initial presentation of invasive lobular carcinoma of breast. Internal Med (2012) 51(12):1635–8. doi: 10.2169/internalmedicine.51.7641
39. Framarino-dei-Malatesta M, Chiarito A, Bianciardi F, Fiorelli M, Ligato A, Naso G, et al. Metastases to extraocular muscles from breast cancer: case report and up-to-date review of the literature. BMC Cancer. (2019) 19(1):36. doi: 10.1186/s12885-018-5253-1
40. Portschy PR, Marmor S, Nzara R, Virnig BA, Tuttle TM. Trends in incidence and management of lobular carcinoma in situ: A population-based analysis. Ann Surg Oncol (2013) 20(10):3240–6. doi: 10.1245/s10434-013-3121-4
41. Christgen M, Steinemann D, Kühnle E, Länger F, Gluz O, Harbeck N, et al. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol - Res Practice (2016) 212(7):583–97. doi: 10.1016/j.prp.2016.05.002
42. Jakobiec FA, Stagner AM, Homer N, Yoon MK. Periocular breast carcinoma metastases: predominant origin from the lobular variant. Ophthal Plast Reconstruct Surg (2017) 33(5):361–6. doi: 10.1097/IOP.0000000000000793
43. Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery (1993) 114(4):637–41.
44. Ferlicot S, Vincent-Salomon A, Médioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. (2004) 40(3):336–41. doi: 10.1016/j.ejca.2003.08.007
45. Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Davis S, et al. Local estrogen biosynthesis in males and females. Endocrine-related cancer (1999) 6:131–7. doi: 10.1677/erc.0.0060131
46. Spelsberg H, Klueppel M, Reinhard T, Glaeser M, Niederacher D, Beckmann MW, et al. Detection of Oestrogen receptors (ER) α and β in conjunctiva, lacrimal gland, and tarsal plates. Eye (2004) 18(7):729–33. doi: 10.1038/sj.eye.6701314
47. Raap M, Antonopoulos W, Dämmrich M, Christgen H, Steinmann D, Länger F, et al. High frequency of lobular breast cancer in distant metastases to the orbit. Cancer Med (2015) 4(1):104–11. doi: 10.1002/cam4.331
48. Spitzer SG, Bersani TA, Mejico LJ. Multiple bilateral extraocular muscle metastases as the initial manifestation of breast cancer. J Neuro-Ophthalmol (2005) 25(1):37–9. doi: 10.1097/00041327-200503000-00010
49. Dieing A, Schulz CO, Schmid P, Roever AC, Lehenbauer-Dehm S, Jehn C, et al. Orbital metastases in breast cancer: report of two cases and review of the literature. J Cancer Res Clin Oncol (2004) 130(12):745–8. doi: 10.1007/s00432-004-0606-3
50. Bedford PD, Daniel PM. Discrete carcinomatous metastases in the extrinsic ocular muscles* *From the department of ophthalmology, mount Sinai hospital and clinic. Am J Ophthalmol (1960) 49(4):723–6. doi: 10.1016/0002-9394(60)92047-X
51. Capone A. Discrete metastasis of solid tumors to extraocular muscles. Arch Ophthalmol (1990) 108(2):237. doi: 10.1001/archopht.1990.01070040089037
52. Glazer LC, Harris GJ, Simons KB. Orbital metastasis as the presenting sign of adenocarcinoma of the breast. Ophthal Plast Reconstruct Surg (1991) 7(4):252–5. doi: 10.1097/00002341-199112000-00003
53. Rhatigan MC, Ashworth JL, Shah S, Bonshek RE, Leatherbarrow B. Bilateral orbital metastases from breast carcinoma masquerading as thyroid eye disease. Eye (1995) 9(5):653–5. doi: 10.1038/eye.1995.161
54. Po SM. Bilateral lagophthalmos. Arch Ophthalmol (1996) 114(9):1139. doi: 10.1001/archopht.1996.01100140341019
55. Zambarakji HJ, Simcock PR, Kinnear PE. Bilateral orbital metastases in a woman with breast carcinoma. J R Soc Med (1997) 90(12):684–4. doi: 10.1177/014107689709001214
56. Garcia GH, Weinberg DA, Glasgow BJ, Hunt KE, Venegas R, Goldberg RA. Carcinoma of the male breast metastatic to both orbits. Ophthal Plast Reconstruct Surg (1998) 14(2):130–3. doi: 10.1097/00002341-199803000-00010
57. Toller KK, Gigantelli JW, Spalding MJ. Bilateral orbital metastases from breast carcinoma. Ophthalmology (1998) 105(10):1897–901. doi: 10.1016/S0161-6420(98)91037-5
58. Lacey B, Chang W, Rootman J. Nonthyroid causes of extraocular muscle disease. Surv Ophthalmol (1999) 44(3):187–213. doi: 10.1016/S0039-6257(99)00101-0
59. Stuntz M, Yamini D, Moss J, Klein S, Khalkhali I. Bilateral orbital metastases as the presenting finding in a male patient with breast cancer: A case report and review of the literature. Breast J (2000) 6(3):204–8. doi: 10.1046/j.1524-4741.2000.97090.x
60. Gonçalves AC, Moura FC, Monteiro MLR. Bilateral progressive enophthalmos as the presenting sign of metastatic breast carcinoma. Ophthal Plast Reconstruct Surg (2005) 21(4):311–3. doi: 10.1097/01.iop.0000167786.00697.0b
61. Peckham EL, Giblen G, Kim AK, Sirdofsky MD. Bilateral extraocular muscle metastasis from primary breast cancer. Neurology (2005) 65(1):74–4. doi: 10.1212/01.WNL.0000156359.93206.99
62. Kuchel JM, Bowling JC. Bilateral lower eyelid masses—Quiz case. Arch Dermatol (2006) 142(10). doi: 10.1001/archderm.142.10.1351-c
63. Gasperini J, Black E, Van Stavern G. Perineural metastasis of breast cancer treated with optic nerve sheath fenestration. Ophthal Plast Reconstruct Surg (2007) 23(4):331–3. doi: 10.1097/IOP.0b013e318073cc6d
64. Milman T, Pliner L, Langer PD. Breast carcinoma metastatic to the orbit: an unusually late presentation. Ophthal Plast Reconstruct Surg (2008) 24(6):480–2. doi: 10.1097/IOP.0b013e31818b6adc
65. Kouvaris JR, Gkongkou PV, Papadimitriou CA, Papacharalampous XN, Antypas CE, Balafouta MJ, et al. Bilateral metastases to extraocular muscles from lobular breast carcinoma. Onkologie (2008) 31(7):387–9. doi: 10.1159/000129689
66. Jaspers H, Blaisse R, Maessen-Visch B, Mattijssen V. Bilateral swollen eyelids occurring during adjuvant treatment with tamoxifen for early breast cancer. Netherlands J Med (2009) 67(6):245–6.
67. Murthy R, Gupta A, Hegde S, Honavar S. Bilateral multiple extraocular muscle metastasis from breast carcinoma. Indian J Ophthalmol (2011) 59(5):381. doi: 10.4103/0301-4738.83616
68. Kim JH, Choi SY, Cho CK, Yang KM, Noh WC, Kim MS. Bilateral orbital metastases from breast cancer: A case report of successful palliation using stereotactic radiotherapy. Breast J (2011) 17(6):669–71. doi: 10.1111/j.1524-4741.2011.01165.x
69. Kim HJ, Wojno TH, Grossniklaus H. Atypical bilateral orbital metastases of lobular breast carcinoma. Ophthal Plast Reconstruct Surg (2012) 28(6):e142–3. doi: 10.1097/IOP.0b013e318249d5c0
70. Wiggins RE, Byrne SF. Metastatic tumor to the extraocular muscles: Report of 5 cases. J Am Assoc Pediatr Ophthalmol Strabismus (2012) 16(5):489–91. doi: 10.1016/j.jaapos.2012.06.009
71. Khan NA, Morlese J, Khan A. Radiological foresight: a rare case of breast cancer metastases to the extraocular muscles. BMJ Case Rep (2015), bcr2015211264. doi: 10.1136/bcr-2015-211264
72. Blohmer M, Zhu L, Atkinson JM, Beriwal S, Rodríguez-López JL, Rosenzweig M, et al. Patient treatment and outcome after breast cancer orbital and periorbital metastases: a comprehensive case series including analysis of lobular versus ductal tumor histology. Breast Cancer Res (2020) 22(1):70. doi: 10.1186/s13058-020-01309-3
73. Marotta DA, Jabaay MJ, Zadourian A, Kesserwani H. Bilateral orbital metastases masquerading as ocular myasthenia gravis: A case report and review of the literature. Cureus (2020) 12(7):1–5. doi: 10.7759/cureus.9105
74. Dimopoulos A, Zeng J, Shinder R. Breast carcinoma metastatic to the bilateral orbits. Orbit (2020) 39(2):153–3. doi: 10.1080/01676830.2019.1603314
75. Muhammad-Ikmal MK, Masnon NA, Hayati F, Wan HItam WH. Sino-orbital metastasis as the initial presentation of advanced breast cancer. BMJ Case Rep (2022) 15(11):e250108. doi: 10.1136/bcr-2022-250108
76. Tsutsui S, Kawata K, Ubagai T, Okimoto S, Fujihara M, Maeda T, et al. Orbital metastasis of invasive lobular carcinoma of the breast. J Surg Case Rep (2022) 2022(1):1–3. doi: 10.1093/jscr/rjab619
77. Karimaghaei S, Raviskanthan S, Mortensen PW, Malik AI, Lee AG. Metastatic breast cancer presenting as progressive ophthalmoplegia 30 years after initial cancer diagnosis. J Neuro-Ophthalmol (2022) 42(1):e446–7. doi: 10.1097/WNO.0000000000001385
78. El-Khazen Dupuis J, Marchand M, Javidi S, Nguyen TQT. Enophthalmos as the initial systemic finding of undiagnosed metastatic breast carcinoma. Int Med Case Rep J (2021) 14:25–31. doi: 10.2147/IMCRJ.S282113
79. Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol (2021) 32(12):1475–95. doi: 10.1016/j.annonc.2021.09.019
80. Thill M, Lüftner D, Kolberg-Liedtke C, Albert US, Banys-Paluchowski M, Bauerfeind I, et al. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2022. Breast Care (2022) 17(4):421–9. doi: 10.1159/000524789
81. Barke MR, Agrawal KU, Shields CL. Abemaciclib and fulvestrant for bilateral choroidal metastasis from breast carcinoma. JAMA Ophthalmol (2022) 140(10):1026. doi: 10.1001/jamaophthalmol.2022.3453
82. Goduni L, Ashkenazy N, Hansen E, Soyano-Muller A, Correa ZM, Harbour JW. Iris metastasis from breast cancer successfully treated with abemaciclib and letrozole. Retinal cases Brief Rep (2023) 17(2):123–5. doi: 10.1097/ICB.0000000000001176
83. Burris HA, Chan A, Bardia A, Thaddeus Beck J, Sohn J, Neven P, et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br J Cancer. (2021) 125(5):679–86. doi: 10.1038/s41416-021-01415-9
84. Liew DFL, Leung JLY, Liu B, Cebon J, Frauman AG, Buchanan RRC. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheuma Dis (2019) 22(2):297–302. doi: 10.1111/1756-185X.13444
85. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol (2016) 152(1):45. doi: 10.1001/jamadermatol.2015.2707
86. Vitorino M, Baptista de Almeida S, Alpuim Costa D, Faria A, Calhau C, Azambuja Braga S. Human microbiota and immunotherapy in breast cancer - A review of recent developments. Front Oncol (2022) 11:815772. doi: 10.3389/fonc.2021.815772
87. Alpuim Costa D, Nobre JG, Batista MV, Ribeiro C, Calle C, Cortes A, et al. Human microbiota and breast cancer—Is there any relevant link?—A literature review and new horizons toward personalised medicine. Front Microbiol (2021) 12:584332. doi: 10.3389/fmicb.2021.584332
88. Gately S. Human microbiota and personalized cancer treatments: role of commensal microbes in treatment outcomes for cancer patients. Precision Medicine in Cancer Therapy (2019) 178:253–64. doi: 10.1007/978-3-030-16391-4_10
Keywords: CDK4/6 inhibitor, abemaciclib, letrozole, breast cancer, orbit, metastases, case report, review
Citation: Rodrigues Alves N, Duarte AF, Ribeiro DF, Silva RS, Carvalho BA and Alpuim Costa D (2024) Successful management of bilateral orbital metastases from invasive lobular breast cancer with abemaciclib and letrozole: a case report and literature review. Front. Oncol. 14:1286910. doi: 10.3389/fonc.2024.1286910
Received: 31 August 2023; Accepted: 03 January 2024;
Published: 23 January 2024.
Edited by:
Deniz Can Guven, Hacettepe University, TürkiyeReviewed by:
Claudia Andreetta, Azienda Sanitaria Universitaria Friuli Centrale (ASU FC), ItalyPrince Kasongo Mwila, University of the Witwatersrand, South Africa
Copyright © 2024 Rodrigues Alves, Duarte, Ribeiro, Silva, Carvalho and Alpuim Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuno Rodrigues Alves, bnVuby5hbDExM0BnbWFpbC5jb20=; Diogo Alpuim Costa, ZGlvZ28uY29zdGFAY3VmLnB0
 Nuno Rodrigues Alves
Nuno Rodrigues Alves Ana Filipa Duarte
Ana Filipa Duarte David Fernandes Ribeiro
David Fernandes Ribeiro Rita Sousa Silva
Rita Sousa Silva Bruno Almeida Carvalho
Bruno Almeida Carvalho Diogo Alpuim Costa
Diogo Alpuim Costa