- 1Department of Integrative Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Integrative Medicine, Shanghai Medical College, Fudan University, Shanghai, China
- 3Medical Department, Genecast Biotechnology Co., Ltd, Wuxi, China
Lung cancer treatment has transitioned fully into the era of immunotherapy, yielding substantial improvements in survival rate for patients with advanced non-small cell lung cancer (NSCLC). In this report, we present a case featuring a rare epidermal growth factor receptor (EGFR) mutation accompanied by high programmed death-ligand 1 (PD-L1) expression, demonstrating remarkable therapeutic efficacy through a combination of immunotherapy and chemotherapy. A 77-year-old male with no family history of cancer suffered from upper abdominal pain for more than half months in August 2020 and was diagnosed with stage IV (cT3N3M1c) lung squamous cell carcinoma (LUSC) harboring both a rare EGFR p.G719C mutation and high expression of PD-L1 (tumor proportion score [TPS] = 90%). Treatment with the second-generation targeted therapy drug Afatinib was initiated on September 25, 2020. However, resistance ensued after 1.5 months of treatment. On November 17, 2020, immunotherapy was combined with chemotherapy (Sintilimab + Albumin-bound paclitaxel + Cisplatin), and a CT scan conducted three months later revealed significant tumor regression with a favorable therapeutic effect. Subsequently, the patient received one year of maintenance therapy with Sintilimab, with follow-up CT scans demonstrating subtle tumor shrinkage (stable disease). This case provides evidence for the feasibility and efficacy of immunotherapy combined with chemotherapy in the treatment of EGFR-mutated and PD-L1 highly expressed LUSC.
1 Introduction
Lung cancer ranks as a foremost contributor to cancer-related mortality, standing among the most prevalent and lethal malignancies worldwide (1). Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancer cases, with lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) representing predominant subtypes. Specifically, LUSC comprises approximately 20-30% of NSCLC cases (2). The epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase that regulates cell growth and differentiation in normal cells (3). However, mutations in EGFR lead to its sustained activation, consequently promoting abnormal proliferation and survival of tumor cells (4). EGFR mutations predominantly manifest in LUAD, occurring in approximately 27% of cases. The common EGFR mutations include exon 19 deletions (comprising approximately 30-45% of EGFR mutations) and exon 21 L858R point mutations (comprising approximately 40-45% of EGFR mutations). Additionally, other EGFR mutations such as G719X (G719A, G719C, G719S), S768I, and L861Q are considered rare EGFR mutations, accounting for a lower proportion (5). In contrast, EGFR mutations in LUSC are relatively infrequent, accounting for merely about 7% or less of cases (6). Among the EGFR mutations detected in LUSC, reports on the specific mutation subtype EGFR G719C are extremely limited.
With the advancement of molecular biology in lung cancer, immunotherapy and targeted therapy have become the standard treatment approaches for advanced lung cancer (7). Studies have revealed significant survival benefits for stage II to IIIA NSCLC patients carrying common EGFR mutations (Ex19del or L858R) after complete resection (5-year survival rate of 5%). Additionally, NSCLC patients with stage II to IIIA EGFR mutation-positive tumors who received immunotherapy showed significantly higher median progression-free survival (PFS) compared to those who received chemotherapy alone (30.8 months vs. 19.8 months), along with a higher 3-year PFSrate (39.6% vs. 32.5%) (8). In a rare case of stage IB LUSC, the patient exhibited both EGFR p.L858R mutation and high programmed death-ligand 1 (PD-L1) expression. After two cycles of immunotherapy, the tumor significantly regressed, and subsequent right upper thoracoscopic lobectomy with mediastinal lymph node dissection led to complete pathological remission (9). The current consensus is that NSCLC patients with uncommon EGFR mutations typically exhibit resistance to EGFR tyrosine kinase inhibitors (EGFR-TKIs), resulting in overall poor benefits from first-generation EGFR-TKIs (5). However, studies have reported potential benefits from the second-generation EGFR-TKI drug, Afatinib, for patients carrying EGFR G719X mutations (10). A comprehensive meta-analysis of Afatinib revealed that patients with EGFR exon 20 insertions responded poorly to the drug, while those with G719X, S768I, and L861Q mutations responded well, with PFS outcomes comparable to common EGFR mutations (11). Another study found that a patient with G719D and 21 (L861Q) co-mutations in a poorly differentiated stage IVB NSCLC had a survival period of 13 months and was currently undergoing treatment with Amolertinib (12). Nevertheless, the simultaneous presence of EGFR driver gene mutations and PD-L1 overexpression in LUSC is extremely rare, and patients with PD-L1 negativity (<1%) often have longer median overall survival (OS) than PD-L1-positive patients (15.61 vs. 7.40 months, P=0.0138) (13, 14). Clinical trials have demonstrated the safety and efficacy of programmed cell death-1 (PD-1)/PD-L1 inhibitors in resectable NSCLC neoadjuvant therapy (15). Afatinib exhibits heterogeneity across different mutation genotypes, with TP53 mutation patients receiving less survival benefit than TP53 wild-type patients (16). Hence, further research into novel drugs and combination treatment strategies is crucial for expanding the clinical benefit population and improving the prognosis of patients with EGFR G719C uncommon mutation combined with high PD-L1 expression.
This study reports a case of LUSC in which the patient simultaneously exhibited EGFR G719X mutation and high PD-L1 expression, and received treatment with Afatinib. However, after one and a halfmonths of treatment, the patient developed drug resistance. Therefore, exploring potential resistance mechanisms is a challenging clinical issue that needs to be addressed. Through this research, we aim to provide insights into the treatment of LUSC patients with the rare EGFR G719X mutation and concurrent high PD-L1 expression.
2 Case presentation
A 77-year-old male with no family history of cancer suffered from upper abdominal pain for more than half a month in August 2020. An enhanced CT examination revealed suspicious malignant tumors in the left lung, left pleura, multiple lymph nodes (mediastinal, bilateral hilar, left clavicular area, hepatoduodenal ligament area, and retroperitoneum), as well as at the junction of the left and right lobes of the liver near the diaphragm apex and the right adrenal gland (Figure 1A). Additionally, the level of the serum tumor biomarkers CYFRA211, CEA, and CA724 were elevated above normal levels (Figure 1B). A liver biopsy performed on September 22, 2020, showed significant heterogeneity in tumor cells, indicating possible metastatic squamous cell carcinoma. Genetic testing of the liver metastasis tissue revealed EGFR p.G719C (mutation frequency 10.81%, number of supported reads 138; Figure 1C) and PIK3CA mutation. Immunohistochemical staining of the liver metastasis lesion showed high infiltration of CD8+ and PD-L1 in both the tumor area and stroma, with CD68+ macrophages including M1-type macrophages (CD68+CD163-) and M2-type macrophages (CD68+CD163+) having good infiltration in the tumor area. Additionally, a minimal number of exhausted T cells (CD8+PD1+) were observed in the tumor (Figures 2A–C). Based on the above data, the patient was diagnosed with the rare EGFR-mutated LUSC at stage cT3N3M1c with high PD-L1 expression (TPS [tumor proportion score] = 90%) and high tumor mutational burden (TMB, 13.63 mutations/mb). According to the National Comprehensive Cancer Network (NCCN) guidelines, the second-generation EGFR-TKI was administered as a once-daily oral dose of 40 mg on September 25, 2020.
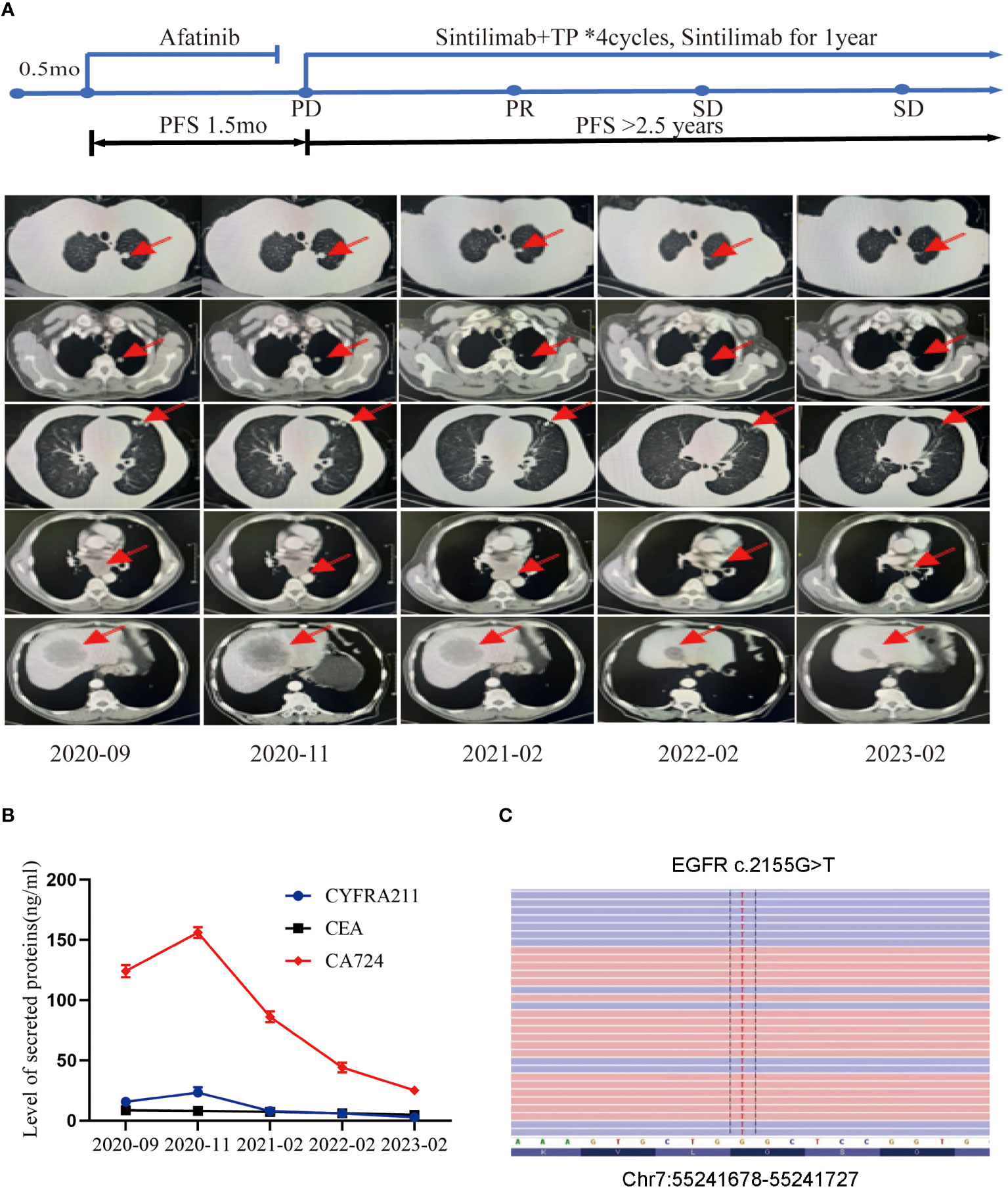
Figure 1 Tumor progression of the patient during the treatments. (A) The timeline of the therapies and imaging results of the computed tomography (CT) can during diagnosis and treatment at different time points. (B) The levels of secreted protein during treatment at different time points. (C) The site of EGFR mutation.
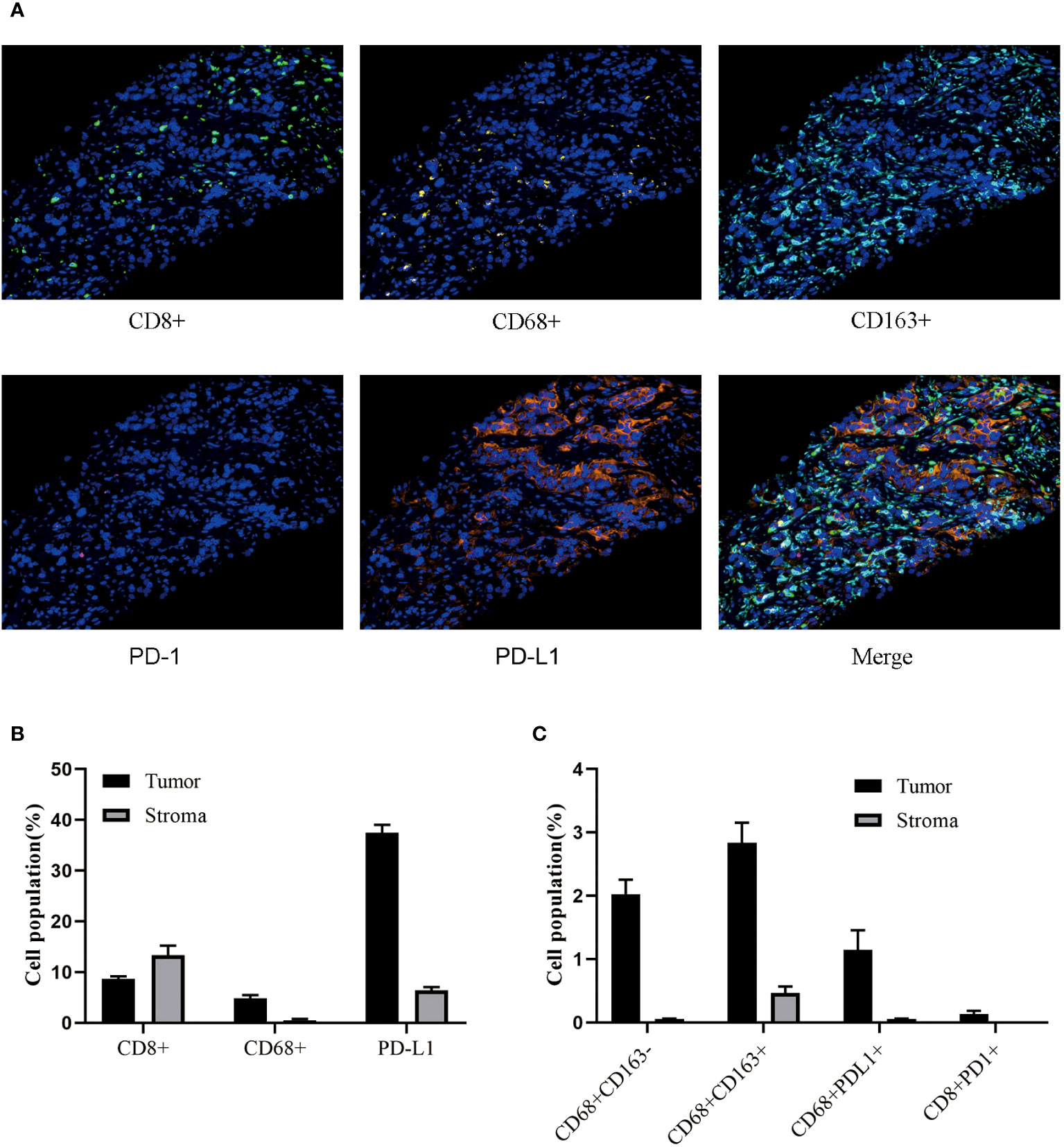
Figure 2 The Immune microenvironment status of liver metastases. (A) Multicolor analysis is used to detect the phenotypes of different cell populations. (B, C) Perform cluster analysis on various cell types in the stroma and tumor regions.
However, the CT scan conducted in November 2020 revealed an enlargement of the lung and liver lesions in comparison to the previous scan, leading to disease progression (PD) as per the Response Evaluation Criteria in Solid Tumors (RECIST) scoring criteria (Figure 1A). Additionally, the levels of tumor markers CYFRA211, CEA, and CA724 experienced further elevation (Figure 1B).
Upon developing resistance to Afatinib, a second mediastinal lymph node biopsy was performed, which revealed lung metastatic squamous cell carcinoma. We compared the results of genetic testing of liver metastatic tissue at the time of diagnosis and mediastinal lymph node metastatic tissue at the time of progression on afatinib therapy (Table 1). NGS sequencing of the mediastinal lymph node tissue identified three pathogenic/likely pathogenic mutations: EGFR p.G719C (14.62%), PIK3CA p.E545K (12.16%), and TP53 p.Q331* (23.08%). Immunogenomic analysis indicated a high tumor mutation burden (17.52/Mb) and 99% expression of PD-L1 in tumor cells. Given the patient’s age, physical condition, and in accordance with the NCCN guidelines, a combination of immunotherapy and chemotherapy was administered on November 17, 2020, for a total of four cycles. The treatment regimen included albumin-bound paclitaxel (200mg/m2, d1) + cisplatin (75mg/m2, d1) in combination with sintilimab (200mg, d0), with each cycle repeated every three weeks.
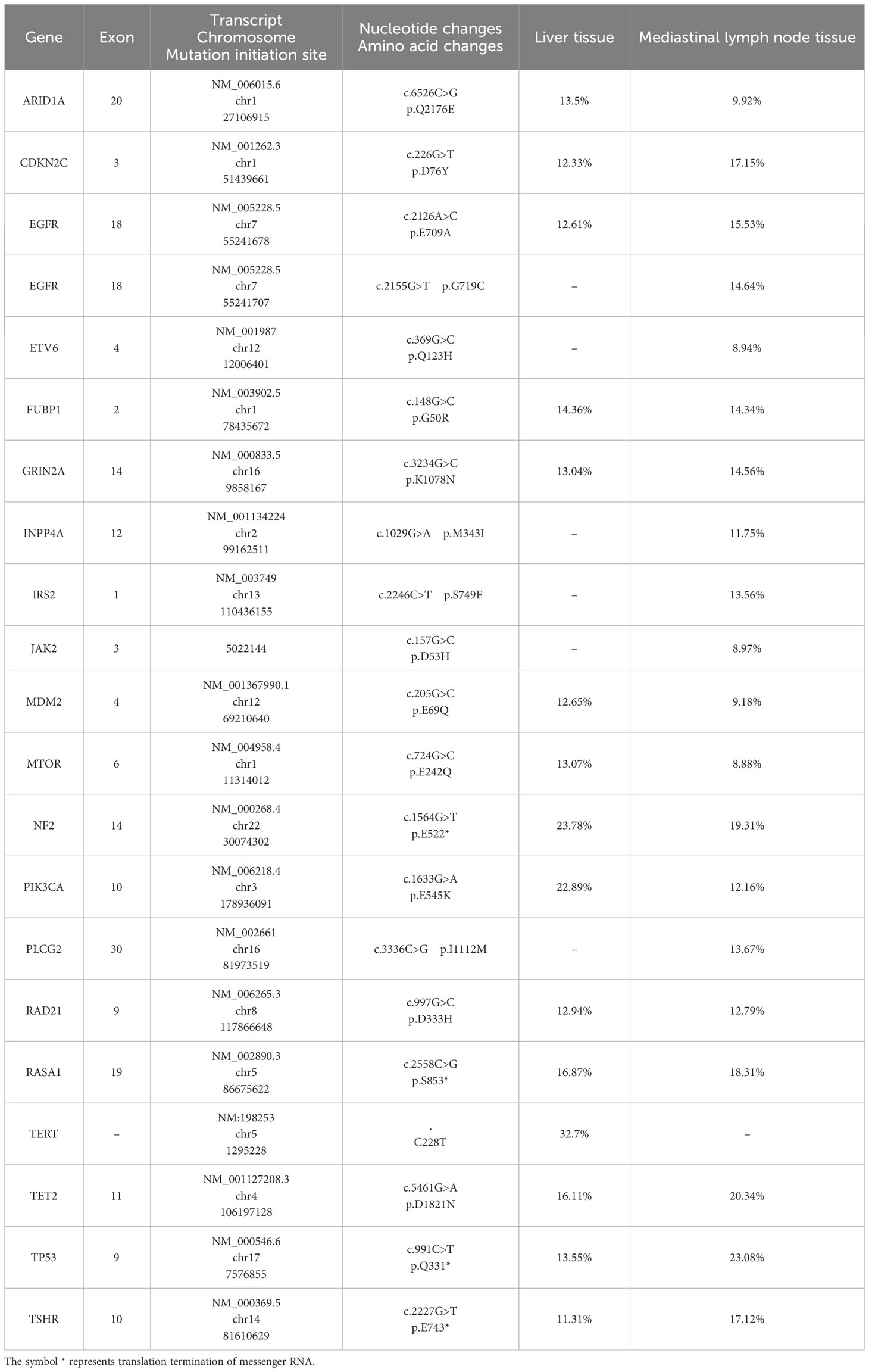
Table 1 Comparison of genetic testing results between liver metastasis tissue at diagnosis and mediastinal lymph node metastasis tissue during afatinib treatment.
By February 2021, the CT examination showed significant reduction in the size of the lesions in the left lung, liver, and adrenal gland, accompanied by a decrease in size in the mediastinal lymph nodes compared to the previous examination (Figure 1A). As per the RECIST scoring criteria, the assessment result indicated a partial response (PR). The levels of tumor marker levels also significantly decreased (Figure 1B).
The patient continued to receive maintenance therapy with sintilimab for one year. In February 2022, a follow-up CT scan indicated tumor stabilization (Figures 1A, B). Blood plasma circulating tumor DNA (ctDNA)-NGS tests were performed simultaneously in November 2020 and September 2022, revealing a substantial decrease in the number and frequency of gene mutations (Fishplot) (Figure 3A). Moreover, quantitative analysis of ctDNA levels demonstrated a significant reduction (17.72 vs. 10353.37 HGE/ml), consistent with the radiological evaluation results (Figure 3B).
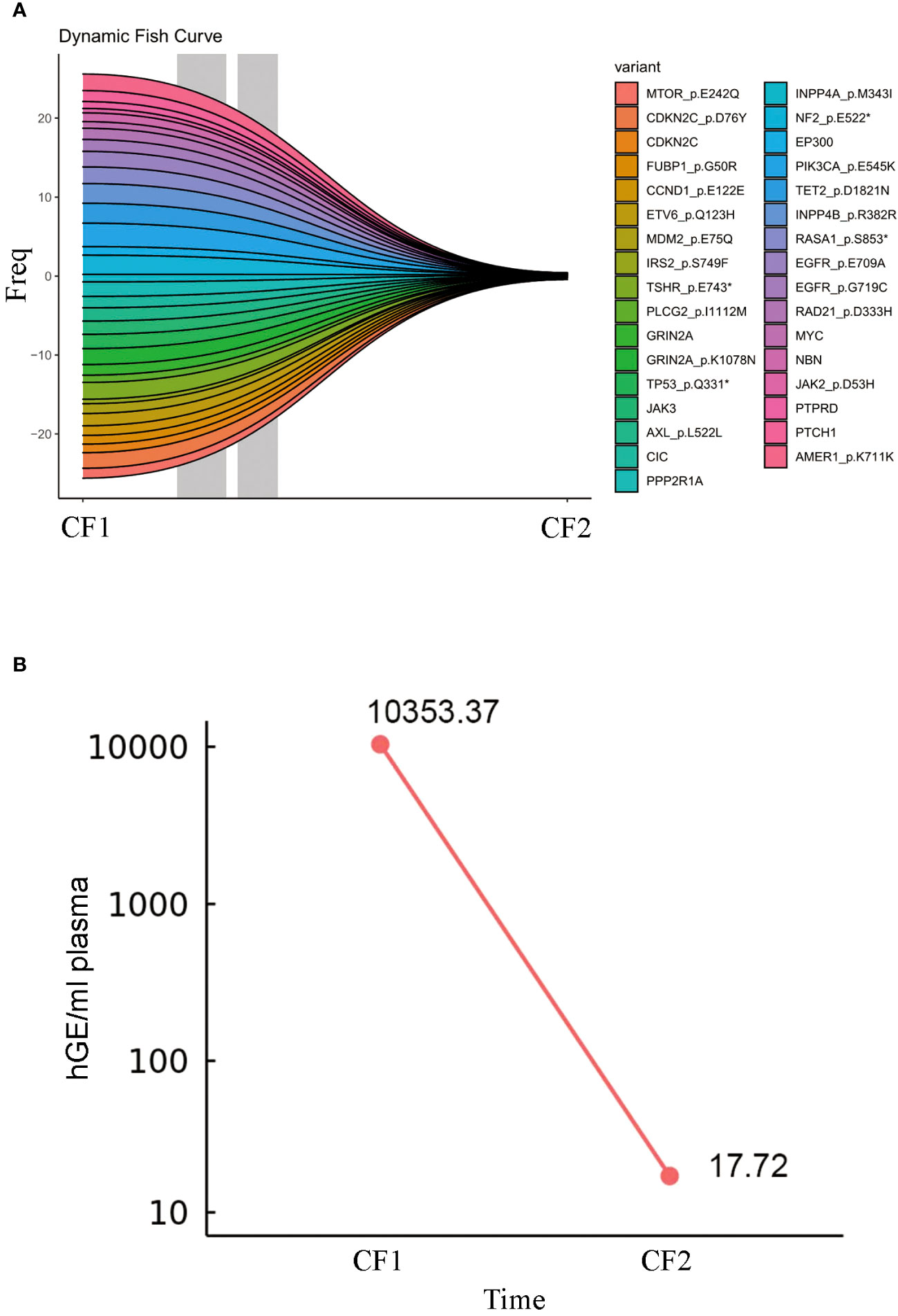
Figure 3 Circulating tumor DNA change during ICI treatments. (A) The fish plot illustrates the frequency and types of gene mutations. (B) The level of ctDNA.CF1: 2020.11, CF2:2022.9. The symbol * represents translation termination of messenger RNA.
3 Discussion
In LUAD, the most common mutated genes are KRAS and EGFR, while in LUSC, common mutated genes include TP53 and CDKN2A. The EGFR G719C mutation is extremely rare in LUSC (6). The frequency of EGFR genomic alterations (including mutations and amplifications) and ALK rearrangements in the Chinese NSCLC population is significantly higher than in Western populations, at 50.1% and 7.8%, respectively. Among them, approximately 7.4% of patients carry concurrent activating and uncommon mutations, while 11.6% of patients carry only uncommon EGFR mutations, with uncommon EGFR mutations often co-occurring with genomic alterations in ALK, CDKN2A, NTRK3, TSC2, and KRAS (17). Recent research reported a case of LUSC with an EGFR G719X mutation. The patient underwent EGFR-TKI treatment and achieved an overall survival duration of 25.4 months (18). Mutations in KRAS and EGFR are usually mutually exclusive, but when coexistent, KRAS mutations may lead to resistance to EGFR inhibitors (19). Among 101 patients with EGFR mutations, strong positive PD-L1 expression significantly reduced objective response rate and PFS compared to weak positive or negative PD-L1 expression. Moreover, higher PD-L1 expression was observed in primary resistance patients to EGFR-TKIs (20). Based on the CT results and tumor biomarkers, this study indicated the development of resistance to Afatinib as early as September 2020, consistent with previous findings suggesting that positive PD-L1 expression mainly occurs in patients with primary resistance to EGFR-TKIs. Another study found that patients with PD-L1 ≥1% exhibited a higher rate of primary resistance to EGFR-TKI treatment. Regarding PFS, the median for patients with PD-L1≥1%, PD-L1≥25%, and PD-L1≥50% were 2.1 months, 1.8 months, and 1.6 months, respectively (21). These results suggest that high PD-L1 expression may be a contributing factor to TKI resistance and rapid progression in LUSC.
Furthermore, we detected mutations in PIK3CA p.E545K and TP53 p.Q331*. TP53 mutations may lead to impaired immune recognition and antigen presentation of tumor cells, making it difficult for the immune system to recognize and attack tumor cells (22). PIK3CA mutations may interfere with the function and metabolism of immune cells, disrupting the tumor immune surveillance mechanism (23). This state of immune evasion may contribute to the development of resistance to EGFR-TKIs in tumor cells. Additionally, TP53 and PIK3CA mutations may interact with other oncogenic signaling pathways, such as RAS/MAPK and PI3K/AKT, further enhancing the survival and proliferation capabilities of tumor cells, thereby reducing the effectiveness of EGFR-TKIs (24).
PD-1 is a protein localized on the surface of immune cells, while PD-L1 is a protein expressed on the surface of cancer cells. Immune checkpoint inhibitors (ICI) can block the binding between PD-1 and PD-L1, thereby restoring the immune system’s ability to attack cancer cells (25). In this study, while the high PD-L1 expression suggested a favorable response to monotherapy, the decision to combine immunotherapy with chemotherapy was guided by several factors including the patient’s age, overall physical condition, and the desire for a comprehensive and aggressive treatment approach. The rationale behind the combination therapy was to optimize treatment efficacy while minimizing potential risks and maximizing the chances of a robust response given the advanced stage of the disease. The patient was old, though in good health. More importantly, this patient had a large tumor load with liver metastases and mediastinal lymphoma metastasis, and combination chemotherapy and immunotherapy was chosen to reduce the tumor load more rapidly. On the other hand, the patient combined multiple tumor drivers, including high PDL1 expression combined with G719X mutation and also squamous carcinoma, and there may be the influence of factors other than immunity, so the combination therapy was used in order to comprehensively inhibit tumor growth. In addition, based on the characteristics of driver gene-positive NSCLC, immunotherapy alone may have a risk of hyperprogression, so combination therapy is more conducive to comprehensive control of tumor development. A clinical study called ORIENT-12 compared the efficacy and safety of sintilimab (ICI) combined with platinum-based drugs and gemcitabine (GP) versus GP alone in patients with locally advanced or metastatic LUSC. The results showed that after a median follow-up of 12.9 months, the PFS of the sintilimab combined with the GP group was significantly better than that of the placebo combined with the GP group, and the incidence of treatment-related adverse events leading to death in the two groups was 4.5% and 6.7%, respectively (26). In this study, the use of sintilimab combined with chemotherapy also achieved favorable immunotherapy outcomes in LUSC patients. This may be due to the high TMB increasing the number of tumor-specific antigens on the surface of tumor cells. These antigens can be recognized by the patient’s immune system as “foreign” substances, thereby stimulating immune cells to attack tumor cells. Additionally, antigens released by tumor cells with high TMB can more effectively activate T cells, leading to a stronger immune response against tumor cells (27). The characteristics of immune-inflammatory liver metastases include a large number of immune cell infiltrations. High levels of PD-L1 and CD8+ infiltrations in the tumor area and stroma are typical features of positive indicators in liver metastases. This suggests that liver metastases may be influenced by T cell immune surveillance and play an important role in the immune-inflammatory microenvironment (28). M1-type macrophages (CD68+CD163-) are typically associated with anti-tumor immune responses, while M2-type macrophages (CD68+CD163+) are more likely to promote tumor growth and metastasis (29). In this study, both M1 and M2-type macrophages showed good infiltration in the tumor area, suggesting that the immune-inflammatory microenvironment of liver metastases may involve complex interactions between different macrophage subtypes. The presence of very few exhausted T cells may also impact the immune response to immunotherapy in patients with positive indicators of liver metastases, warranting further investigation (30). Therefore, the immune-inflammatory microenvironment is often considered as a predictive factor for a patient’s sensitivity to immunotherapy (31). In an immune-inflammatory microenvironment, immunotherapy may be more likely to stimulate immune cells to attack tumor cells, leading to better treatment outcomes (32).
Related studies have discussed the use of ctDNA as a biomarker in patients with advanced cancer undergoing immunotherapy. One study collected 316 plasma samples from 94 patients, including baseline and sampling every three cycles, and analyzed them using specific ctDNA detection methods. The results showed that the concentration of ctDNA at baseline was correlated with PFS, overall survival, clinical response, and clinical benefit; and this correlation became more significant as treatment progressed (33). Another study analyzed ctDNA samples from 978 patients with 16 types of advanced tumors before treatment and during treatment (171 samples). The results indicated that pretreatment ctDNA levels appeared to have prognostic value in a broad analysis of immune checkpoint blockade, while dynamic changes in ctDNA during treatment could predict treatment benefit (34). In this study, it was found that the level of ctDNA in the blood plasma significantly decreased after treatment compared to before treatment, which was consistent with the radiological evaluation, further supporting the effectiveness of the treatment.
4 Conclusion
In conclusion, we have presented a case report of stage IV (cT3N3M1c) LUSC patient who harbored both a rare EGFR p.G719C mutation and high expression of PD-L1 (TPS=99%). Through the combined approach of immunotherapy and chemotherapy, we observed favorable therapeutic outcomes, with evident tumor reduction. This case provides valuable evidence for the feasibility and efficacy of immunotherapy combined with chemotherapy in managing LUSC with a rare EGFR mutation and high PD-L1 expression. Such efforts will contribute to a more comprehensive evaluation of this treatment strategy’s effectiveness and offer more efficient therapeutic choices for lung cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of the Zhongshan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-fZ: Formal analysis, Writing – original draft, Writing – review & editing. X-xB: Data curation, Software, Writing – review & editing. H-yS: Data curation, Formal analysis, Investigation, Writing – review & editing. X-xG: Data curation, Formal analysis, Funding acquisition, Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by (National Natural Science Foundation of China) (Grant number 82104450).
Acknowledgments
The authors thank all members involved in this work, as well as the National Nature Foundation, which was critical to completing this study.
Conflict of interest
Author H-yS was employed by the company Genecast Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am (2019) 103(3):463–73. doi: 10.1016/j.mcna.2018.12.006
2. Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF pathway inhibition: A promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol (2021) 16(2):205–15. doi: 10.1016/j.jtho.2020.10.006
3. Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol (2020) 61:167–79. doi: 10.1016/j.semcancer.2019.09.015
4. Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li X, et al. Immune checkpoint inhibitors in EGFR-mutated NSCLC: dusk or dawn? J Thorac Oncol (2021) 16(8):1267–88. doi: 10.1016/j.jtho.2021.04.003
5. Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol (2018) 29(suppl_1):i3–9. doi: 10.1093/annonc/mdx702
6. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553(7689):446–54. doi: 10.1038/nature25183
7. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol (2022) 40(6):586–97. doi: 10.1200/jco.21.01497
8. Planchard D. Adjuvant osimertinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med (2020) 383(18):1780–2. doi: 10.1056/NEJMe2029532
9. Xu X, Shi Z, Fu D, Huang D, Ma Z. EGFR mutations and high PD-L1 expression of lung squamous cell carcinoma patients achieving pCR following neoadjuvant immuno-chemotherapy: Case report. Front Oncol (2022) 12:1008932. doi: 10.3389/fonc.2022.1008932
10. Floc’h N, Lim S, Bickerton S, Ahmed A, Orme J, Urosevic J, et al. Osimertinib, an irreversible next-generation EGFR tyrosine kinase inhibitor, exerts antitumor activity in various preclinical NSCLC models harboring the uncommon EGFR mutations G719X or L861Q or S768I. Mol Cancer Ther (2020) 19(11):2298–307. doi: 10.1158/1535-7163.mct-20-0103
11. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16(2):141–51. doi: 10.1016/s1470-2045(14)71173-8
12. Li L. Two non-small cell lung cancer (NSCLC) patients with brain metastasis harboring epidermal growth factor receptor (EGFR) G719X and L861Q mutations benefited from aumolertinib: two cases report and review of the literature. Heliyon (2022) 8(9):e10407. doi: 10.1016/j.heliyon.2022.e10407
13. Zhao F, Wang M, Zhu J. Hypoxia-related lncRNAs to build prognostic classifier and reveal the immune characteristics of EGFR wild type and low expression of PD-L1 squamous and adenocarcinoma NSCLC. Cancer Med (2021) 10:931718(17). doi: 10.3389/fimmu.2022.931718
14. Chen Q, Shang X, Liu N, Ma X, Han W, Wang X, et al. Features of patients with advanced EGFR-mutated non-small cell lung cancer benefiting from immune checkpoint inhibitors. Front Immunol (2022) 13:931718. doi: 10.3389/fimmu.2022.931718
15. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
16. Pang LL, Gan JD, Tan JR, Huang YH, Liao J, Liang WT, et al. Efficacy and potential resistance mechanisms of afatinib in advanced non-small cell lung cancer patients with EGFR G719X/L861Q/S768I. Cancer (2022) 128(21):3804–14. doi: 10.1002/cncr.34451
17. Wen S, Dai L, Wang L, Wang W, Wu D, Wang K, et al. Genomic signature of driver genes identified by target next-generation sequencing in chinese non-small cell lung cancer. Oncologist (2019) 24(11):e1070–e81. doi: 10.1634/theoncologist.2018-0572
18. Bi H, Ren D, Wu J, Ding X, Guo C, Miura S, et al. Lung squamous cell carcinoma with rare epidermal growth factor receptor mutation G719X: a case report and literature review. Ann Transl Med (2021) 9(24):1805. doi: 10.21037/atm-21-6653
19. Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PloS Med (2005) 2(1):e17. doi: 10.1371/journal.pmed.0020017
20. Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J, et al. Strong programmed death ligand 1 expression predicts poor response and de novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol (2018) 13(11):1668–75. doi: 10.1016/j.jtho.2018.07.016
21. Hsu KH, Huang YH, Tseng JS, Chen KC, Ku WH, Su KY, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naïve advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer (2019) 127:37–43. doi: 10.1016/j.lungcan.2018.11.021
22. AlGabbani Q. Mutations in TP53 and PIK3CA genes in hepatocellular carcinoma patients are associated with chronic Schistosomiasis. Saudi J Biol Sci (2022) 29(2):848–53. doi: 10.1016/j.sjbs.2021.10.022
23. Adderley H, Rack S, Hapuarachi B, Feeney L, Morgan D, Hussell T, et al. The utility of TP53 and PIK3CA mutations as prognostic biomarkers in salivary adenoid cystic carcinoma. Oral Oncol (2021) 113:105095. doi: 10.1016/j.oraloncology.2020.105095
24. Patel SJ, Trivedi GL, Darie CC, Clarkson BD. The possible roles of B-cell novel protein-1 (BCNP1) in cellular signalling pathways and in cancer. J Cell Mol Med (2017) 21(3):456–66. doi: 10.1111/jcmm.12989
25. Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist (2019) 24(Suppl 1):S31–s41. doi: 10.1634/theoncologist.2019-IO-S1-s05
26. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol (2021) 16(9):1501–11. doi: 10.1016/j.jtho.2021.04.011
27. McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol (2021) 32(5):661–72. doi: 10.1016/j.annonc.2021.02.006
28. Liu Z, Wang T, She Y, Wu K, Gu S, Li L, et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer (2021) 20(1):105. doi: 10.1186/s12943-021-01398-4
29. Jia K, Chen Y, Sun Y, Hu Y, Jiao L, Ma J, et al. Multiplex immunohistochemistry defines the tumor immune microenvironment and immunotherapeutic outcome in CLDN18.2-positive gastric cancer. BMC Med (2022) 20(1):223. doi: 10.1186/s12916-022-02421-1
30. Wang D, Fang J, Wen S, Li Q, Wang J, Yang L, et al. A comprehensive profile of TCF1(+) progenitor and TCF1(-) terminally exhausted PD-1(+)CD8(+) T cells in head and neck squamous cell carcinoma: implications for prognosis and immunotherapy. Int J Oral Sci (2022) 14(1):8. doi: 10.1038/s41368-022-00160-w
31. Dancsok AR, Gao D, Lee AF, Steigen SE, Blay JY, Thomas DM, et al. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology (2020) 9(1):1747340. doi: 10.1080/2162402x.2020.1747340
32. Jeremiasen M, Borg D, Hedner C, Svensson M, Nodin B, Leandersson K, et al. Tumor-associated CD68(+), CD163(+), and MARCO(+) macrophages as prognostic biomarkers in patients with treatment-naïve gastroesophageal adenocarcinoma. Front Oncol (2020) 10:534761. doi: 10.3389/fonc.2020.534761
33. Bratman SV, Yang SYC, Iafolla MAJ, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer (2020) 1(9):873–81. doi: 10.1038/s43018-020-0096-5
Keywords: lung squamous cell carcinoma, EGFR G719X mutation, anti-PD1 therapy, afatinib, inflammatory microenvironment
Citation: Zhu Z-f, Bao X-x, Shi H-y and Gu X-x (2024) Case report: A lung squamous cell carcinoma patient with a rare EGFR G719X mutation and high PD-L1 expression showed a good response to anti-PD1 therapy. Front. Oncol. 14:1283008. doi: 10.3389/fonc.2024.1283008
Received: 25 August 2023; Accepted: 08 January 2024;
Published: 31 January 2024.
Edited by:
Sannula Kesavardhana, Indian Institute of Science (IISc), IndiaReviewed by:
Wantao Wu, Central South University, ChinaXiaomin Niu, Shanghai Jiao Tong University, China
Copyright © 2024 Zhu, Bao, Shi and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi-xi Gu, Z3V4aXhpQHNpbmEuY29t
 Zhen-feng Zhu
Zhen-feng Zhu Xu-xia Bao
Xu-xia Bao Hong-yan Shi
Hong-yan Shi Xi-xi Gu
Xi-xi Gu