- 1Department of Breast Surgery, Tangshan People’s Hospital, Tangshan, China
- 2Department of Medical Imaging (Ultrasound), Tangshan People’s Hospital, Tangshan, China
Purpose: This aims to investigate the efficacy and safety of intercostal nerve anastomosis among breast cancer patients who undergo immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy.
Methods: From 2022 to 2023, female patients between the ages of 20 and 60 diagnosed with stage I–IIIA breast cancer, who required and were willing to undergo immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy, were screened and assigned to take the operation with (treatment group) or without (control group) intercostal nerve anastomosis (the nerves with appropriate length and thickness were selected from the 2nd-4th intercostal nerves, which were then dissociated and anastomosed to the posterior areola tissue). A radial incision at the surface projection of the tumor location was used. The patients’ breast local sensation was assessed using Semmes–Weinstein monofilaments before the operation as well as at 10 days, 3 months, and 6 months postoperatively. Furthermore, the patients’ quality of life was evaluated 6 months postoperatively using the EORTC QLQ-C30 questionnaire. Adverse events, operation duration, drainage volume, and the duration of drainage tube carrying time were also monitored and recorded.
Results: Compared to the pre-operative period, a significant decrease in local sensation was observed 10 days after surgery in both groups. However, the control group showed a significant reduction in sensation at 3 and 6 months postoperatively, while the treatment group showed noticeable recovery. A statistically significant difference (P < 0.001) in local sensation between the pre-operative and post-operative periods was observed at the final follow-up in the two groups. By the time of 3 and 6 months postoperatively, a significant difference was seen in the local sensation between the two groups. Intercostal nerve anastomosis was found to significantly improve the patients’ quality of life, including emotional (P = 0.01), physical (P = 0.04), and social functioning (P = 0.02) and pain (P = 0.04). There were no significant differences in general characteristics (such as age, BMI, and subtypes). Although intercostal nerve anastomosis increased the duration of operation by around 20 min (P < 0.001), it did not affect the volume or duration of postoperative drainage tube usage between the two groups.
Conclusion: This study indicated that intercostal nerve anastomosis improved the local sensation and quality of life of patients who underwent immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy.
Clinical Trial Registration: https://www.chictr.org.cn/showproj.html?proj=42487, identifier ChiCTR1900026340.
Introduction
Breast cancer, the most common malignancy in women, is seriously threatening the health of women all over the world (1). The defect of appearance post-operatively seriously affects the patients’ physical and mental health together with quality of life. The development of immediate prosthetic breast reconstruction after nipple–areola-sparing mastectomy (NSM) improved the patients’ appearance and quality of life after breast cancer surgery, without a significant impact on patients’ outcomes (2). However, the occurrence of postoperative sensory loss remains a significant concern, having a profound impact on the patients’ sexual functioning and body image perception, which remains challenging clinically.
The nerves that innervate the nipple–areola complex are mainly the lateral and anterior cutaneous branches of the 4th intercostal nerves, while the 3rd and the 5th intercostal nerve play an auxiliary part (3). Previous studies showed that immediate targeted nipple–areola complex (NAC) re-innervation in immediate autologous and prepectoral implant breast reconstruction could lead to varying degrees of recovery of the NAC sensation (4–11). However, there is no data on the efficacy and safety of intercostal nerve anastomosis in immediate prosthetic breast reconstruction after nipple–areola-sparing mastectomy.
Therefore, we carried out this randomized, controlled, open-label clinical study to investigate this novel technique for intercostal nerve anastomosis in immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy.
Materials and methods
Participants’ eligibility
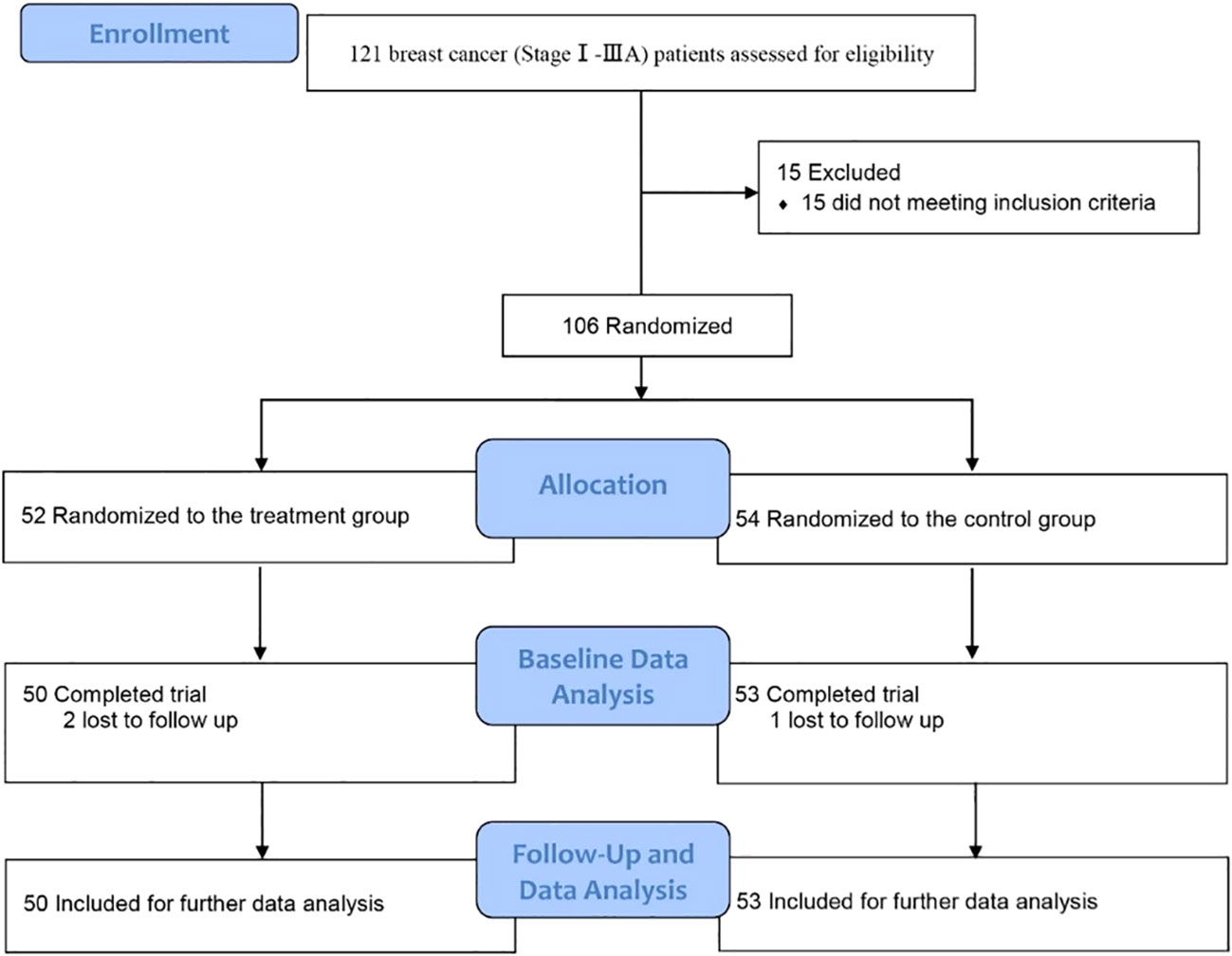
This clinical trial was approved by the Ethics Committee, Tangshan People’s Hospital, Tangshan, China (ethical approval number: RMYY-LL-2011-012) and registered in the Chinese Clinical Trial Registry [ChiCTR1900026340, https://www.chictr.org.cn/showproj.html?proj=42487]. From November 1, 2022 to February 23, 2023, breast cancer patients admitted in the Department of Breast Surgery, Tangshan People’s Hospital, were screened (Figure 1). The inclusion criteria were newly diagnosed stage I–IIIA breast cancer (according to AJCC 8th edition) patients who needed and were willing to choose immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy, aging between 20 and 60 years old, without immune system diseases, and who agreed to participate. Patients were excluded when they met any of the following criteria: refractory psoriasis, eczema, and other skin diseases that may cause skin damage (such as syphilis); diabetes and abnormal glucose tolerance; coronary heart disease, myocardial infarction, and poor heart function; organ failure or severe immunosuppression; in other clinical trials; severe liver and kidney disease; dementia (defined as MMSE ≤2) or Parkinson’s disease or other diseases leading to neurological dysfunction; patients with advanced or other malignant tumors; mental illness or a family history of mental illness; alcoholic or drug addicts; and refusal to or cannot cooperate with the treatment. Patients with postoperative wound infection or flap ischemic or disease progression or cannot continue to cooperate with the treatment would be excluded, too.
Type of surgery
NSM was performed by making a radial incision at the surface projection of the tumor location. All the implants were sub-pectoral combined with a titanized polypropylene mesh (TiLOOP®). Patients in the control group received the immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy. Patients in the treatment group received the immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy, and after that, the nerves with appropriate length and thickness were selected from the 2nd–4th intercostal nerves, which were coming off the lateral border of the pectoralis major muscle, dissected by the same breast surgeon, and separated sufficiently from the lateral to the medial margin, and the nerve branches were also separated until the appropriate length was reached (approximately 5–7 cm). If the nerve was relatively thin or the main nerve could not reach the required length, we chose to truncate its branches and anastomosis with the main nerve end to end, made sure that the nerves pass across the pectoralis major muscle and part of the nerves located on the surface of the pectoralis major muscle, and then anastomosed to suture the edge to the tissue of the nipple–areola complex (Figure 2, Supplementary Figure S1). While we did not need to anastomose blood vessels, we did not use magnification during the operation. The size of the implant was chosen based on both the measurement of the width, height, and convexity of the patient’s breast before surgery and the volume and weight of the breast measured with the drainage method together with the weighing method during the operation.
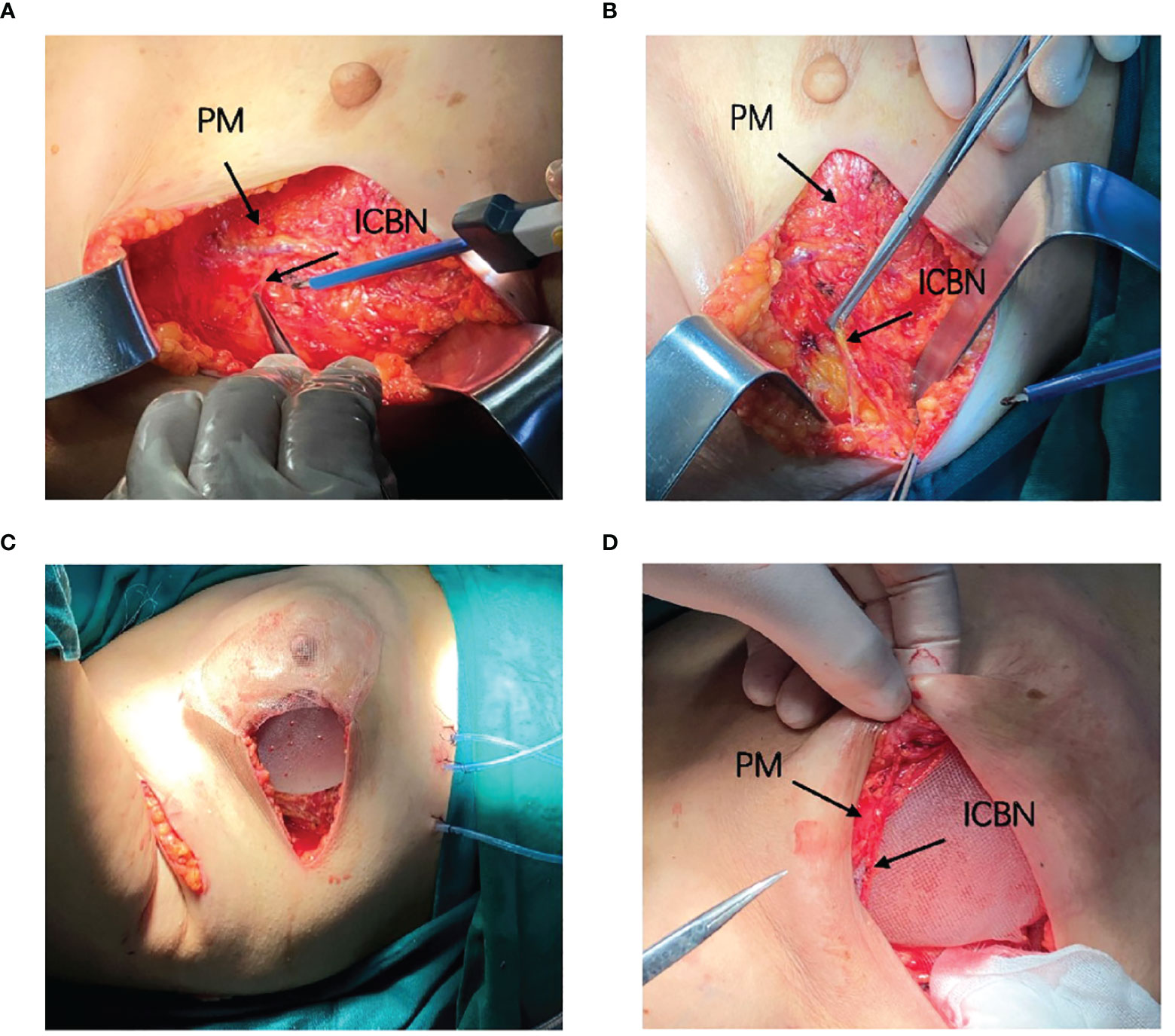
Figure 2 Surgical diagram. (A, B) Locate and dissociate the 4th intercostal nerve. (C) Implant the prosthesis and patch. (D) Anastomosis the nerve with the tissue behind the areola.
Outcome measures
Primary and secondary outcome
According to the division of the methods of breast sensory assessment (12), we employed Semmes–Weinstein monofilaments (6.65, 5.56, 4.31, 3.61, and 2.83 gs) to assess the sensation of the chosen nine points (the nipple, 12, 3, 6, and 9 o’clock axis of the areola, and 2 cm from the areola) pre-operatively and 10 days, 3 months, and 6 months postoperatively, respectively (Figure 3). The secondary outcomes were the duration of the operation, the patients’ quality of life (assessed using EORTC QLQ-C30), adverse events, and the volume and carrying time of the postoperative drainage tube of each group.
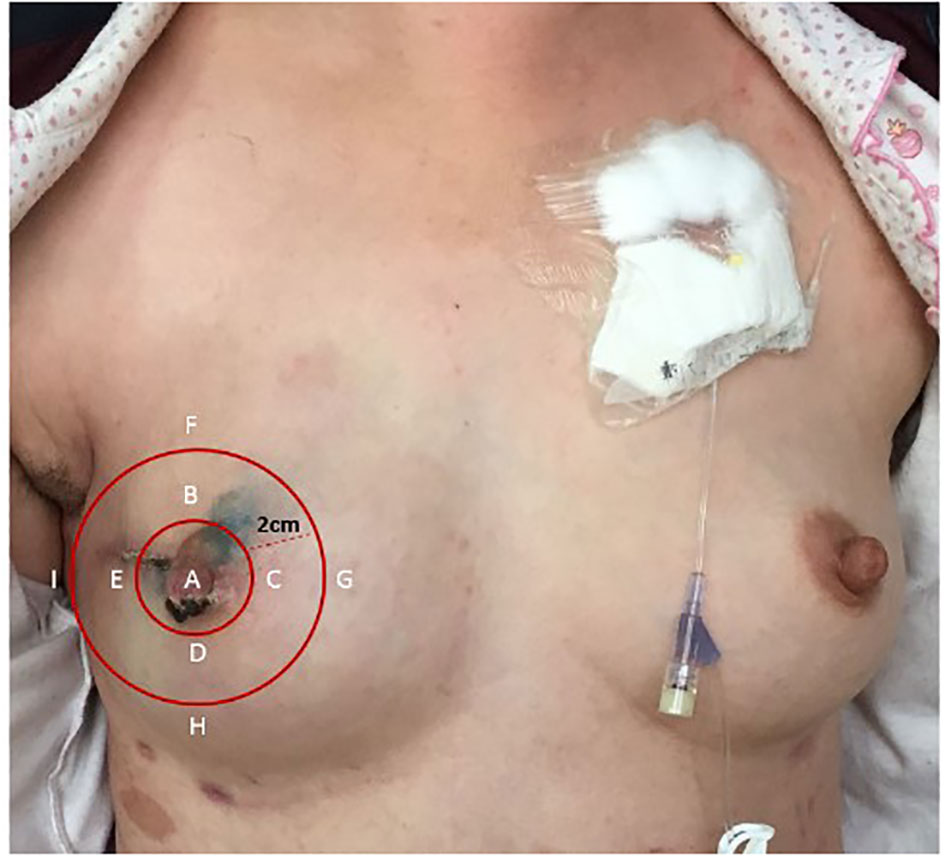
Figure 3 Nine points selected to assess the sensation. (A) The nipple. (B) 12 o’clock axis of the areola. (C) 3 o’clock axis of the areola. (D) 6 o’clock axis of the areola. (E) 9 o’clock axis of the areola. (F) 12 o’clock axis 2 cm from the areola. (G) 3 o’clock axis 2 cm from the areola. (H) 6 o’clock axis 2 cm from the areola. (I) 9 o’clock axis 2 cm from the areola.
Randomization and masking
A researcher who was not involved in data management or statistical analyses performed randomization with a 1:1 ratio using SPSS 22.0 software. The randomized numbers were sealed in an envelope and stored until the study ended. The enrolled patients received immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy with or without intercostal nerve anastomosis by the same breast surgeon according to the assignment. During the study, the researcher was responsible for the follow-up. The physicians who were involved in the patients’ care and all patients were blinded. If any unexpected things happened to the enrolled patients, the physician could unmask the treatment assignment or remove the patient from the study.
Statistical analysis
To compare the outcomes, data analyses were performed using SPSS statistical software version 22.0 (IBM, Chicago, IL, USA). All variables with multi-time points were analyzed with analysis of variance (ANOVA) followed by Scheffe’s Test. The normally distributed data (such as age, BMI, quality of life, and the volume and carrying time of the postoperative drainage tube) were compared using an unpaired t-test. Those data which were not normally distributed (such as subtype) were compared using chi-square analysis. A two-tailed P less than 0.05 was considered to be of statistical significance.
Results
Characteristics of the patients studied
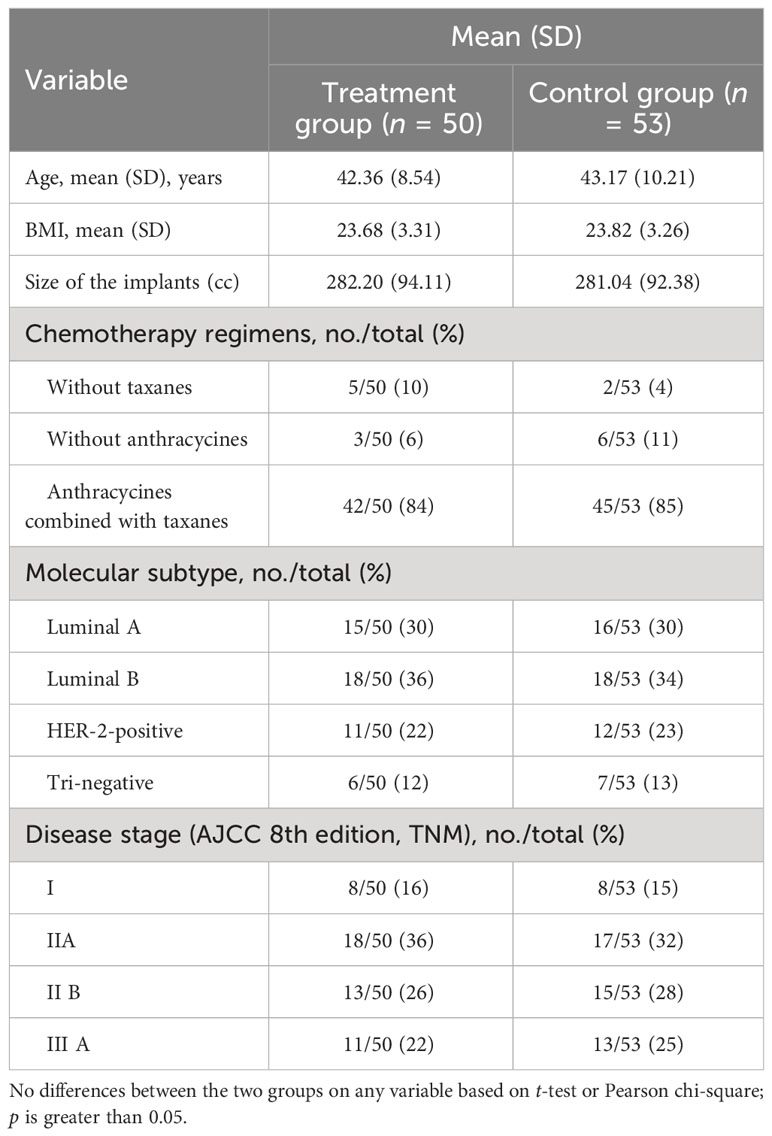
Between November 1, 2022 and February 23, 2023, a total of 121 newly diagnosed breast cancer (stage I–IIIA according to AJCC 8th edition) patients were screened. Among them, 15 patients were excluded because of diabetes. A total of 106 patients were enrolled and randomly assigned to take immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy with (treatment group) or without (control group) intercostal nerve anastomosis. There were no significant differences between the two groups in molecular subtypes, clinical stage, the application of chemotherapy regiments, target therapy, endocrine therapy, as well as postoperative radiotherapy. All patients enrolled in the study underwent postoperative adjuvant chemotherapy. Among them, seven patients received chemotherapy regimens without taxanes (five in the treatment group and two in the control group), nine patients received chemotherapy regimens without anthracycines (three in the treatment group and six in the control group), and 87 patients received chemotherapy regimens with anthracycines combined with taxanes (42 in the treatment group and 45 in the control group). Furthermore, all patients with hormone receptor-positive breast cancer received endocrine therapy following chemotherapy, while those with HER-2-positive breast cancer received targeted therapy as part of their treatment plan. Additionally, a total of 35 patients underwent postoperative radiotherapy, with 16 in the treatment group and 19 in the control group. None of the patients enrolled received pre-operative chemotherapy, radiotherapy, or hormone therapy. The size of the implant ranged between 155 to 510 cc, and no significant difference was seen between the two groups (P = 0.95). The general and clinical information of patients enrolled are shown in Table 1. Ultimately, a total of 103 patients (50 in the treatment group and 53 in the control group) finished the study. There were three patients lost to follow-up due to COVID-19. Further analysis included all baseline and follow-up data of the 103 patients.
Intercostal nerve anastomosis improved the local sensation of patients
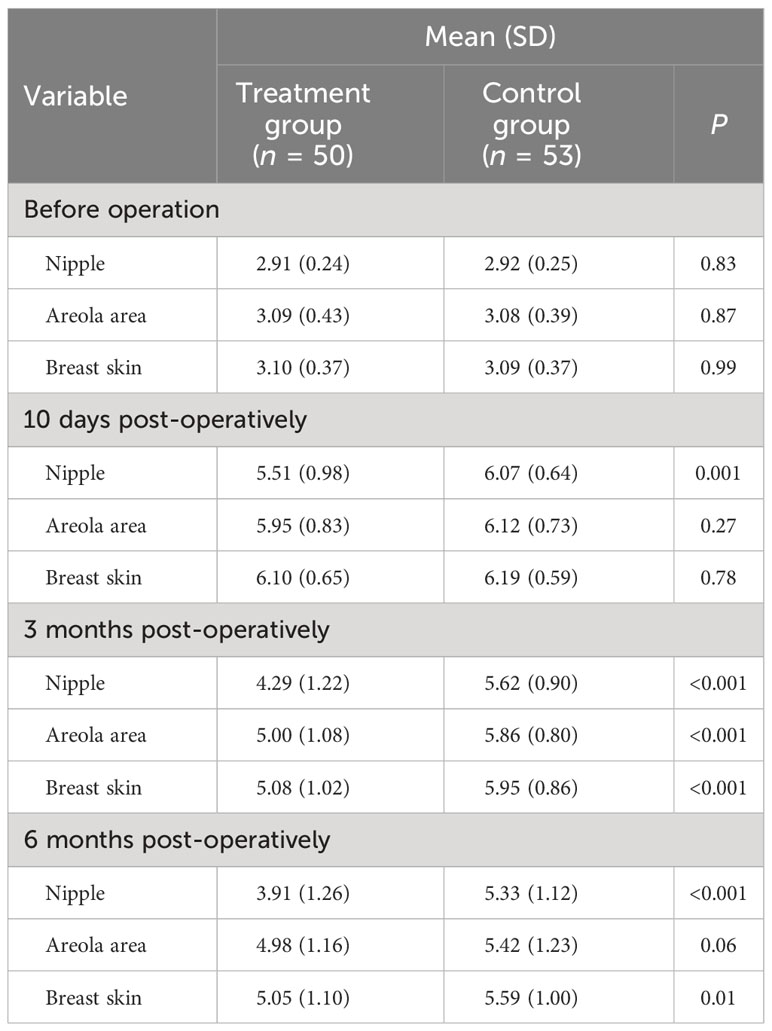
The local sensation was compared using the mean lowest monofilament weight detected. The patients in the two groups held similar nipple sensation (P = 0.83), areola area sensation (P = 0.87), and breast skin sensation (P = 0.99) pre-operatively. Though the local sensation all showed a marked decline 10 days after the operation in both of the two groups (P < 0.001), intercostal nerve anastomosis significantly improved the nipple sensation (P = 0.001) and slightly improved the areola area sensation (P = 0.27) and breast skin sensation (P = 0.78). By the time of 3 months after operation, patients in the treatment group showed better recovery than the control group in the nipple sensation (P < 0.001), areola area sensation (P < 0.001), and breast skin sensation (P < 0.001). The nipple sensation (P < 0.001), areola area sensation (P = 0.06), and breast skin sensation (P = 0.01) recovered more better in the treatment group than the control group at 6 months post-operatively, although both of the groups showed a significant reduction (P < 0.001) on the local sensation between the pre-operative and post-operative periods at final follow-up. As time goes by, intercostal nerve anastomosis improved the local sensation of patients who underwent immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy (Table 2).
Intercostal nerve anastomosis improved the patients’ quality of life
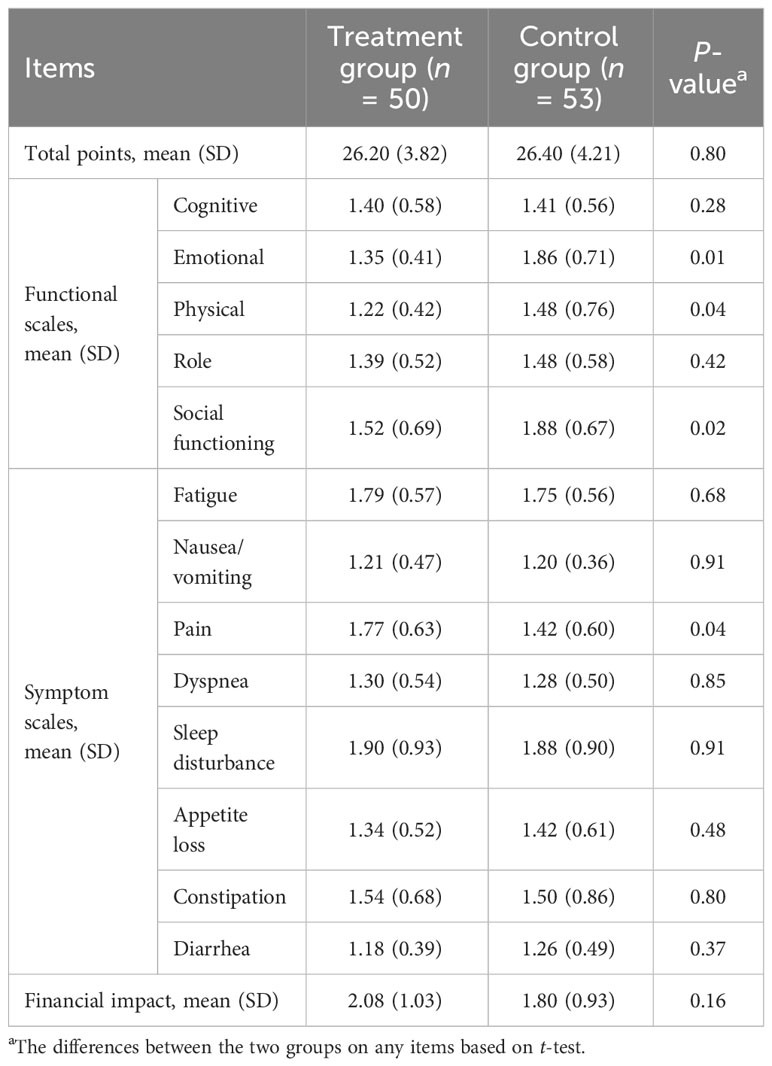
Compared to the control group, intercostal nerve anastomosis improved the patients’ quality of life; the improved allover domain included three functional scales [emotional (P = 0.01), physical (P = 0.04), and social functioning (P = 0.02)] and one symptom scale [pain (P = 0.04)], although there was no significant difference between the two groups at total points of EORTC QLQ-C30 (Table 3).
Intercostal nerve anastomosis did not bring obvious adverse reactions
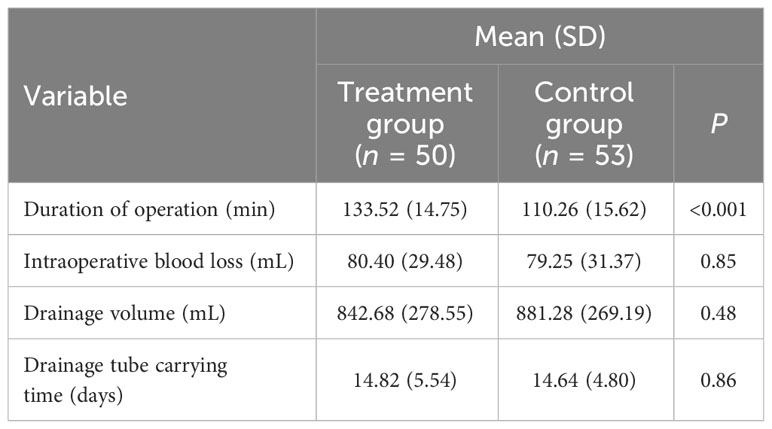
Compared to the control group, the duration of operation increased by around 20 min, which had a significant difference (P < 0.001). This operation did not increase the patients’ intraoperative blood loss (P = 0.85) or drainage volume (P = 0.48), and the patients in the two groups had a similar carrying time of the postoperative drainage tube (P = 0.86) (Table 4).
Discussion
Constantly improved surgical methods have kept the outlooks of breast cancer patients (13). With similar complications and revisions, immediate prosthetic breast reconstruction after nipple–areola-sparing mastectomy is emerging as a preferred method of breast reconstruction (14). However, the missing of sensation after operation still remains challenging clinically (15). Previous research have shown that re-innervation in immediate autologous breast reconstruction could lead to varying degrees of recovery of the NAC sensation (4–10); however, while there were relatively few studies with a small sample size and a lack of high-quality randomized controlled studies or meta-analyses, together with different types of breast reconstruction (such as TRAM flaps, DIEP flaps, LD flaps, and free gluteal flaps) as well as the different reinnervation patterns described—from the center to the periphery in innervated flaps and from the periphery to the center in non-innervated flaps (16–18)—no conclusions could be drawn yet. There was also one study which showed that nerve preservation and allografting in NSM followed by immediate, direct-to-implant, prepectoral implant reconstruction could provide 90% rate of preserved sensation (11). With a lack of relevant studies, especially data on subpectoral prosthesis reconstruction, there is still a long way to go. In our randomized trial conducted among patients with stage I–IIIA breast cancer, the addition of intercostal nerve anastomosis improved the recovery of local sensation of patients who underwent immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy and improved the patients’ quality of life, without increasing the patients’ intraoperative blood loss and the volume or carrying time of the postoperative drainage tube. Throughout our study, no patient suffered from a flap-related complication which might lead to an effect on the outcome. Although the addition of intercostal nerve anastomosis increased the duration of operation by around 20 min, considering our results, we believe that this is acceptable.
In our study, the recovery of the local sensation in the control group was slightly worse than those reported in previous studies (10, 19). We supposed that the reasons might be that the relatively thinner layer of subcutaneous fat in our operation (less than 5 mm) made it harder to retain nerves. The recovery of the patients’ local sensation in our treatment group was also not as good as those of previous studies (10). We think that the possible reasons are as follows: firstly, unlike previous studies which were autologous reconstruction or immediate pre-pectoral prosthesis reconstruction, we performed immediate sub-pectoral prosthesis reconstruction. Secondly, we used a scalpel instead of an electrotome to free the flap, which led to a relatively thinner flap. Lastly, we used only autogenous nerves, and the length and thickness of the nerves were not choosable, which may have an impact on nerve recovery. In order to avoid any effect of the different ways of different surgeons, all operations were performed by the same surgeon.
Previous research confirmed that the medial and lateral cutaneous branches of the 3rd to 5th intercostal nerves were the origin of the sensory nerves of the breast, mostly exited from under the 4th rib (20). During our operations, we noticed that the 4th lateral intercostal nerves coming off the lateral border of the pectoralis major muscle were relatively thin and short, which might have made it harder for the sensation to recover. The 3rd lateral intercostal nerves coming off the lateral border of the pectoralis major muscle seemed to be longer than the 4th, which might have made the intercostal nerve anastomosis easier and led to better recovery of local sensation. Therefore, we plan to verify it in the next study. An end-to-end anastomosis of the nerve will result in better recovery (21). However, it was hard to find the end of the nerve after we skeletonized the tissue behind the nipple (Figure 4).
As we know, taxanes, platinum derivatives, and vinca alkaloids are the compounds most commonly associated with chemotherapy-induced peripheral neuropathy when used alone or as combined therapies (22). Most of our patients received chemotherapy regimen that included taxanes, which might affect postoperative local nerve sensation. By the time of 3 and 6 months after operation, respectively, a significant difference was seen in the recovery of local sensation between patients in the two groups. While the symptoms of chemotherapy-induced peripheral neuropathy may persist for 6 months and longer after chemotherapy in about 30% of patients (23), we suspect that patients in the treatment group might probably have recovered better by the time the symptoms of chemotherapy-induced peripheral neuropathy disappear.
Our study had some limitations: Firstly, this was a single-center study with a relatively small sample size; this may not represent the whole patient population. Secondly, the scale that we employed was EORTC QLQ-C30. It may not reflect the patients’ quality of life as good as the breast reconstruction module of Breast-QTM (24); the results obtained may not be comprehensive. Thirdly, the lack of a long-term follow-up of patients may lead to inaccuracy in the patients’ prognosis.
In conclusion, we demonstrated that intercostal nerve anastomosis improved the local sensation of patients with immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy, thus improving the patients’ quality of life. This study can be considered as a “proof of concept” one, and hence the effectiveness of this therapy needs to be evaluated further in future trial(s) with a large sample size before it can be introduced for routine clinical use.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Tangshan People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZJ: Conceptualization, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Y-PL: Conceptualization, Formal analysis, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. J-LS: Investigation, Supervision, Writing – original draft, Writing – review & editing. HD: Investigation, Software, Supervision, Writing – review & editing. YZ: Methodology, Resources, Writing – review & editing. D-SY: Resources, Supervision, Writing – review & editing. R-XJ: Methodology, Writing – review & editing. H-FC: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the participating patients and their families for their cooperation during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1261936/full#supplementary-material
Abbreviations
NSM, nipple–areola-sparing mastectomy; NAC, nipple–areola complex; AEs, adverse events; ISPBR, immediate subpectoral prosthetic breast reconstruction; BMI, body mass index; MMSE, Mini-Mental State Examination; AJCC, American Joint Committee on Cancer; Luminal A, Estrogen receptor-positive [ER(+)] and/or progesterone receptor-positive [PR(+)], human epidermal growth factor receptor-2-negative [HER-2(-)], and Ki-67 ≤20%; Luminal B, Estrogen receptor-positive [ER(+)] and/or progesterone receptor-positive [PR(+)], human epidermal growth factor receptor-2-negative [HER-2(-)], Ki-67 >20%, or estrogen receptor-positive [ER(+)], and/or progesterone receptor positive [PR(+)]; HER-2 (+), HER-2-positive, estrogen receptor-negative [ER(-)], progesterone receptor negative [PR(-)], human epidermal growth factor receptor-2-positive [HER-2(+)]; Tri-Negative, Estrogen receptor-negative [ER(-)], progesterone receptor-negative [PR(-)], human epidermal growth factor receptor-2-negative [HER-2 (-)]; TNM, Tumor size (T), nodal status (N), and metastasis (M).
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Weber WP, Haug M, Kurzeder C, Bjelic-Radisic V, Koller R, Reitsamer R, et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res Treat (2018) 172(3):523–37. doi: 10.1007/s10549-018-4937-1
3. Zhou SL, Luo SK, Wang HB, Sun ZS, Xiang XU, Chen GZ, et al. Adult female nipple and areola nerve complex anatomical characteristics and clinical significance. Chin J Clin Anat (2013) 1(1):19–21.
4. Spiegel AJ, Menn ZK, Eldor L, Kaufman Y, Dellon AL. Breast reinnervation: DIEP neurotization using the third anterior intercostal nerve. Plast Reconstr Surg Glob Open (2013) 1(8):e72. doi: 10.1097/GOX.0000000000000008
5. Beugels J, Cornelissen AJM, van Kuijk SMJ, Lataster A, Heuts EM, Piatkowski A, et al. Sensory Recovery of the breast following innervated and noninnervated DIEP flap breast reconstruction. Plast Reconstr Surg (2019) 144(2):178e–88e. doi: 10.1097/PRS.0000000000005802
6. Beugels J, Cornelissen AJM, Spiegel AJ, Heuts EM, Piatkowski A, van der Hulst RRWJ, et al. Sensory recovery of the breast after innervated and non-innervated autologous breast reconstructions: A systematic review. J Plast Reconstr Aesthet Surg (2017) 70(9):1229–41. doi: 10.1016/j.bjps.2017.05.001
7. Petit JY, Veronesi U, Orecchia R, Rey P, Martella S, Didier F, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat (2009) 117(2):333–8. doi: 10.1007/s10549-008-0304-y
8. Wagner JL, Fearmonti R, Hunt KK, Hwang RF, Meric-Bernstam F, Kuerer HM, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol (2012) 19(4):1137–44. doi: 10.1245/s10434-011-2099-z
9. Yueh JH, Houlihan MJ, Slavin SA, Lee BT, Pories SE, Morris DJ. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg (2009) 62(5):586–90. doi: 10.1097/SAP.0b013e31819fb1ac
10. Tevlin R, Brazio P, Tran N, Nguyen D. Immediate targeted nipple-areolar complex re-innervation: Improving outcomes in immediate autologous breast reconstruction. J Plast Reconstr Aesthet Surg (2021) 74(7):1503–7. doi: 10.1016/j.bjps.2020.11.021
11. Anne WP, Ziv MP. Nerve preservation and allografting for sensory innervation following immediate implant breast reconstruction. Plast Reconstr Surg Glob Open (2019) 7(7):e2332. doi: 10.1097/GOX.0000000000002332
12. Blondeel PN, Demuynck M, Mete D, Monstrey SJ, Van Landuyt K, Matton G, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg (1999) 52(1):37–44. doi: 10.1054/bjps.1998.3011
13. Peled AW, Amara D, Piper ML, Klassen AF, Tsangaris E, Pusic AL. Development and validation of a nipple-specific scale for the BREAST-Q to assess patient-reported outcomes following nipple-sparing mastectomy. Plast Reconstr Surg (2019) 143(4):1010–7. doi: 10.1097/PRS.0000000000005426
14. Colwell AS, Christensen JM. Nipple-sparing mastectomy and direct-to-implant breast reconstruction. Plast Reconstr Surg (2017) 140(null):44S–50S. doi: 10.1097/PRS.0000000000003949
16. Santanelli F, Longo B, Angelini M, Laporta R, Paolini G. Prospective computerized analyses of sensibility in breast reconstruction with non-reinnervated DIEP flap. Plast Reconstr Surg (2011) 127(1):1790–5. doi: 10.1097/PRS.0b013e31820cf1c6
17. Yap LH, Whiten SC, Forster A, Stevenson HJ. Sensory recovery in the sensate free transverse rectus abdominis myocutaneous flap. Plast Reconstr Surg (2005) 115(5):1280–8. doi: 10.1097/01.PRS.0000156988.78391.D6
18. Temple CLF, Tse R, Bettger-Hahn M, MacDermid J, Gan BS, Ross DC. Sensibility following innervated free TRAM flap for breast reconstruction. Plast Reconstr Surg (2006) 117(7):2119–2127 [discussion 2128-2130]. doi: 10.1097/01.prs.0000218268.59024.cc
19. Rochlin DH, Brazio P, Wapnir I, Nguyen D. Immediate targeted nipple-areolar complex reinnervation: improving outcomes in gender-affirming mastectomy. Plast Reconstr Surg Glob Open (2020) 8(3):e2719. doi: 10.1097/GOX.0000000000002719
20. Knackstedt R, Gatherwright J, Cakmakoglu C, Djohan M, Djohan R. Predictable location of breast sensory nerves for breast reinnervation. Plast Reconstr Surg (2019) 143(2):393–6. doi: 10.1097/PRS.0000000000005199
21. Charlotte J, Nathalie B, Caroline L. Nerve transfers in the forearm: potential use in spastic conditions. Surg Radiol Anat (2022) 44(8):1091–9. doi: 10.1007/s00276-022-02990-z
22. Stubblefield MD, Burstein HJ, Burton AW, Custodio CM, Deng GE, Ho M, et al. NCCN task force report: management of neuropathy in cancer. J Natl Compr Canc Netw (2009), S1–S26. doi: 10.6004/jnccn.2009.0078
23. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain (2014) 155(12):2461–70. doi: 10.1016/j.pain.2014.09.020
Keywords: intercostal nerve anastomosis, immediate subpectoral prosthetic breast reconstruction (ISPBR), nipple-areola-sparing mastectomy (NSM), breast cancer, local sensation
Citation: Juan Z, Liang Y-P, Shen J-L, Dai H, Zhang Y, Yao D-S, Jiang R-X and Cai H-F (2024) Efficacy and safety of intercostal nerve anastomosis in immediate subpectoral prosthetic breast reconstruction after nipple–areola-sparing mastectomy: a randomized, controlled, open-label clinical study. Front. Oncol. 14:1261936. doi: 10.3389/fonc.2024.1261936
Received: 19 July 2023; Accepted: 03 January 2024;
Published: 26 January 2024.
Edited by:
Jian Yin, Tianjin Medical University Cancer Institute and Hospital, ChinaReviewed by:
Piotr Pluta, Polish Mother’s Memorial Hospital Research Institute, PolandLipi Shukla, St Vincent’s Hospital (Melbourne), Australia
Copyright © 2024 Juan, Liang, Shen, Dai, Zhang, Yao, Jiang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Feng Cai, MTMzMDMwNTAwMDVAMTYzLmNvbQ==
 Zhang Juan
Zhang Juan Yong-Ping Liang
Yong-Ping Liang Jiang-Lun Shen1
Jiang-Lun Shen1