- 1Center for Health Protection, National Institute of Public Health and the Environment (RIVM), Bilthoven, Netherlands
- 2Department of Research and Development, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, Netherlands
- 3Department of Health Technology and Services Research, Technical Medical Centre, University of Twente, Enschede, Netherlands
- 4Center for Nutrition, Prevention and Healthcare, National Institute of Public Health and the Environment (RIVM), Bilthoven, Netherlands
- 5Department of Health Sciences, Faculty of Science, Vrije Universiteit Amsterdam & Amsterdam Health Research Institute, Amsterdam, Netherlands
Background: During the COVID-19 pandemic cancer patients might have experienced delays in screening, diagnosis and/or treatment. A systematic review was conducted to give an overview of the effects of COVID-19 induced delays in oncological care on the physical and mental health outcomes of cancer patients.
Methods: MEDLINE and EMBASE databases were searched for articles on the effects of COVID-19 induced delays on physical and mental health outcomes.
Results: Out of 1333 papers, eighteen observational, and twelve modelling studies were included. In approximately half of the studies, tumor stage distribution differed during the pandemic compared to before the pandemic. Modelling studies predicted that the estimated increase in the number of deaths ranged from -0.04 to 30%, and the estimated reduction in survival ranged from 0.4 to 35%. Varying results on the impact on mental health, e.g. anxiety and depression, were seen.
Conclusions: Due to large methodological discrepancies between the studies and the varying results, the effect of COVID-19 induced delays on the physical and mental health outcomes of cancer patients remains uncertain. While modelling studies estimated an increase in mortality, observational studies suggest that mortality might not increase to a large extent. More longitudinal observational data from the pandemic period is needed for more conclusive results.
1 Introduction
The first COVID-19 cases were confirmed in December, 2019, in Wuhan, China (1). Thereafter, the virus quickly spread around the world. Many countries introduced social measures to reduce spreading of the virus. The World Health Organization for instance recommended to keep at least 1 meter (approximately 3.3 feet) distance from each other and to cancel social activities (2). Despite these recommendations, the number of hospitalized COVID-19 patients quickly increased in many countries at the start of the pandemic (3). This put an enormous pressure on the health care for non-COVID-19 patients. Specific measures in oncological care were therefore taken to 1) ensure safe and effective care for all cancer patients, 2) divert hospital resources and intensive care unit capacity towards COVID-19 patients, and 3) prevent infection of patients and health care staff. These last two measures led to the suspension of the national screening programs for breast, colorectal, and cervix cancer (4, 5). Moreover, all three measures caused the introduction of COVID-19 induced cancer-specific treatment guidelines. These guidelines recommended to alter, delay or cancel treatment, prerequisite this would not have an effect on the long-term outcomes of cancer patients (6). Both the suspension of the screening programs and the introduction of COVID-19 induced treatment guidelines resulted in a delay in screening, diagnosis, and treatment (5, 7–9).
Besides delays in diagnosis due to suspension of the screening programs, delays in diagnosis could also have occurred because patients with suspected cancer signs or symptoms delayed their visit to the general practitioner (GP) themselves. They might for instance have been concerned about contracting the virus and/or they did not want to overburden the health care system (10). In addition, patients experienced difficulties in gaining access to the GP at the start of the pandemic, which might also have led to a delay in diagnosis (11).
An emerging number of studies have investigated how delays in screening, diagnosis, and/or treatment have impacted the physical and mental health of cancer patients. However, a robust overview of the health effects of COVID-19 induced delays on cancer patients is lacking. Therefore, we conducted a systematic review to give a first overview of the physical and mental health outcomes of delays in oncological care due to the COVID-19 pandemic.
2 Methods
A systematic review of the physical and mental health outcomes of cancer patients affected by delay in cancer screening, diagnosis and/or treatment, due to the COVID-19 pandemic was conducted.
2.1 Search strategy
An extensive search strategy was developed in collaboration with a scientific librarian to retrieve relevant articles. During the COVID-19 pandemic, different terminology and synonyms were used. Therefore, literature was searched for the range of terms that were used to describe the topics of interest to ensure the capture of relevant articles. The applied search strategy included relevant oncological terms (e.g. cancer, oncology, neoplasm and carcinoma), and terms associated with delay (e.g. postponed, disrupted, lockdown and paused), and COVID-19 (e.g. corona and sars-cov-2). The searches were performed in the PubMed/MEDLINE and EMBASE databases on November 8th, 2021. Only studies that were published in English or Dutch between January 2020 - November 2021 (corresponding to the COVID-19 pandemic) were included. There was no restriction regarding status of publication, e.g. pre-print articles were acceptable. The full search strategy is listed in supplementary materials 1.
2.2 Study selection and extraction
Two independent researchers (EV and AE) screened the literature using Covidence (Veritas Health Innovation, Melbourne, Australia). The selection process consisted of two phases. In phase one all abstracts and titles were evaluated. The full text of the remaining eligible articles was then screened in phase two. Any conflicts between the reviewers were resolved by discussion. When no agreement could be made a third reviewer (AW) was solicited. Articles were included if they: 1) included information on cancer patients, 2) presented estimates of the effect of delay on health outcomes and related the delay to the COVID-19 pandemic. Relevant health outcomes had to be quantified with accepted metrics, e.g. survival or tumor staging, or measured with validated instruments, e.g. for mental health or quality of life. In order to get a general overview, to optimize comparability and to minimize the influence of COVID epicenters studies were excluded if they: 1) were single center studies, 2) included data only on patients who were not currently being treated for cancer (i.e. survivors, other diseases, etc.), and 3) were other types of articles than research papers (i.e. conference abstracts, protocols, reviews etc.). A uniform extraction template was used for data extraction. The following information was extracted from each study: the first author’s last name, country, study design, type of cancer, type of delay, types of health outcomes studied, study population and main results. All articles were extracted by two researchers and consensus was reached prior to the analysis. Several authors were contacted for further clarification or more detailed information. The most frequently reported health outcomes were selected for the synthesis of the results.
3 Results
3.1 Selected studies
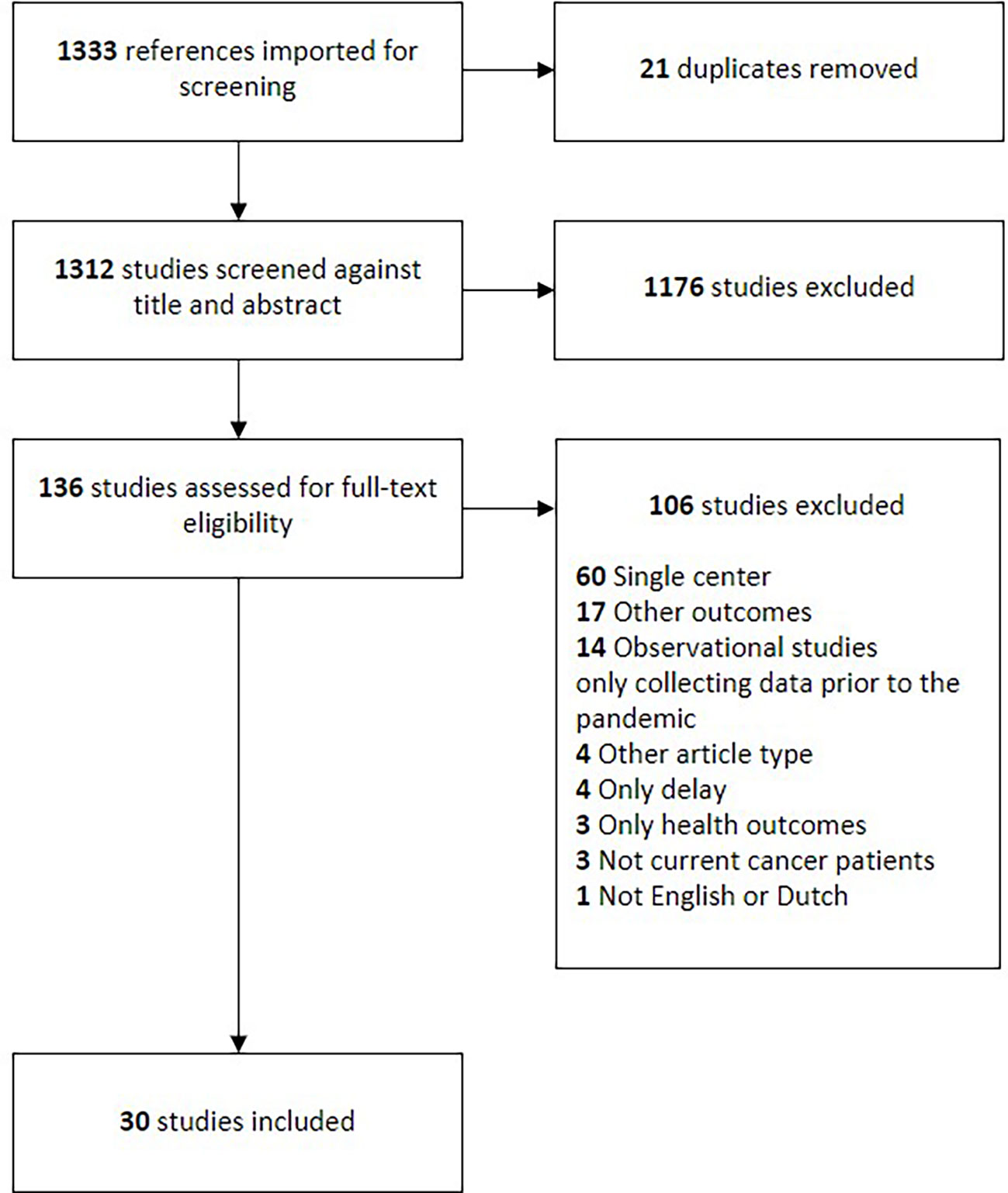
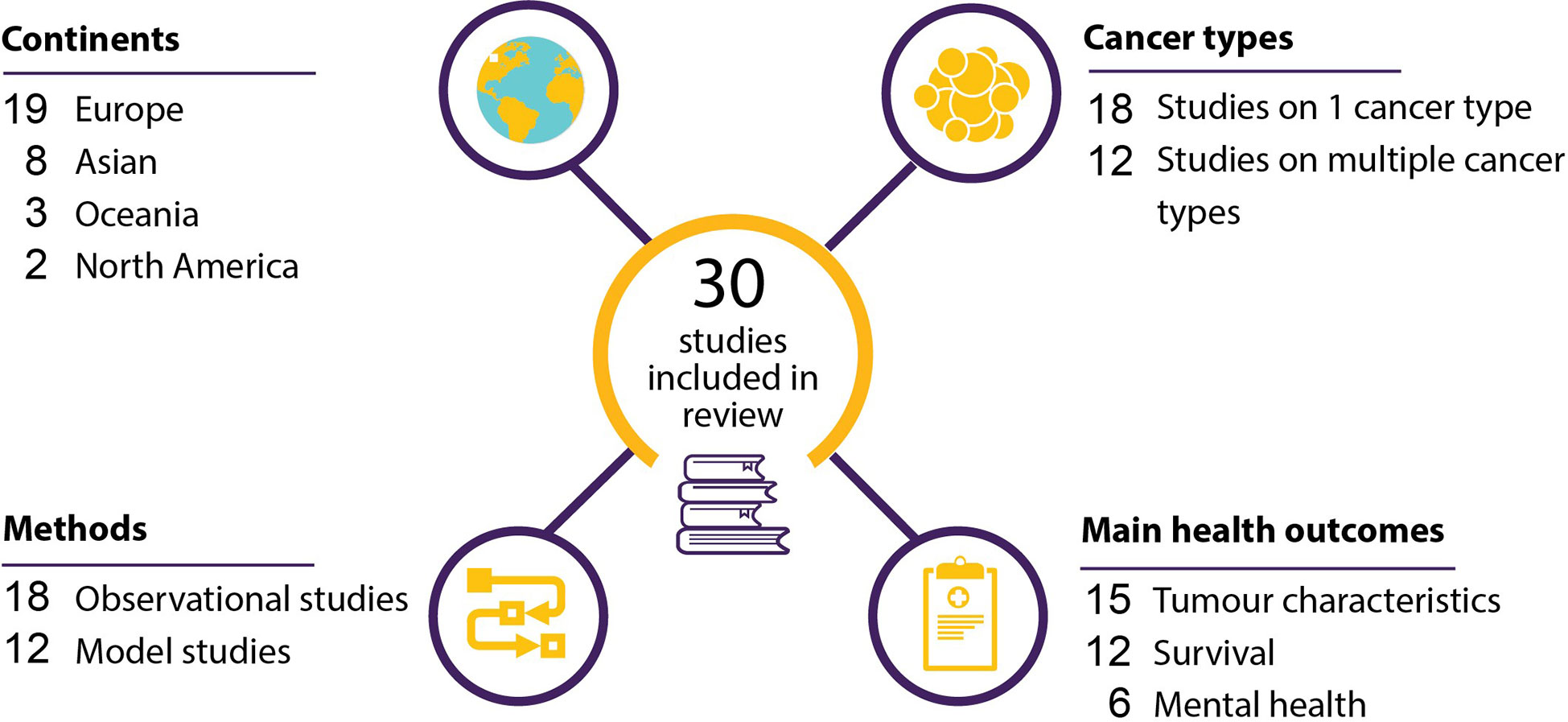
A total of 1333 studies resulted from the search strategy. Twenty-one studies were removed as duplicates. The remaining 1312 studies were screened on their titles and abstracts, after which 1176 articles were deemed irrelevant. This resulted in 136 articles for full-text assessment. One-hundred-and-six studies were excluded based on the full-text review, resulting in thirty articles included in the final analysis. The full flowchart of the screening process is shown in Figure 1.
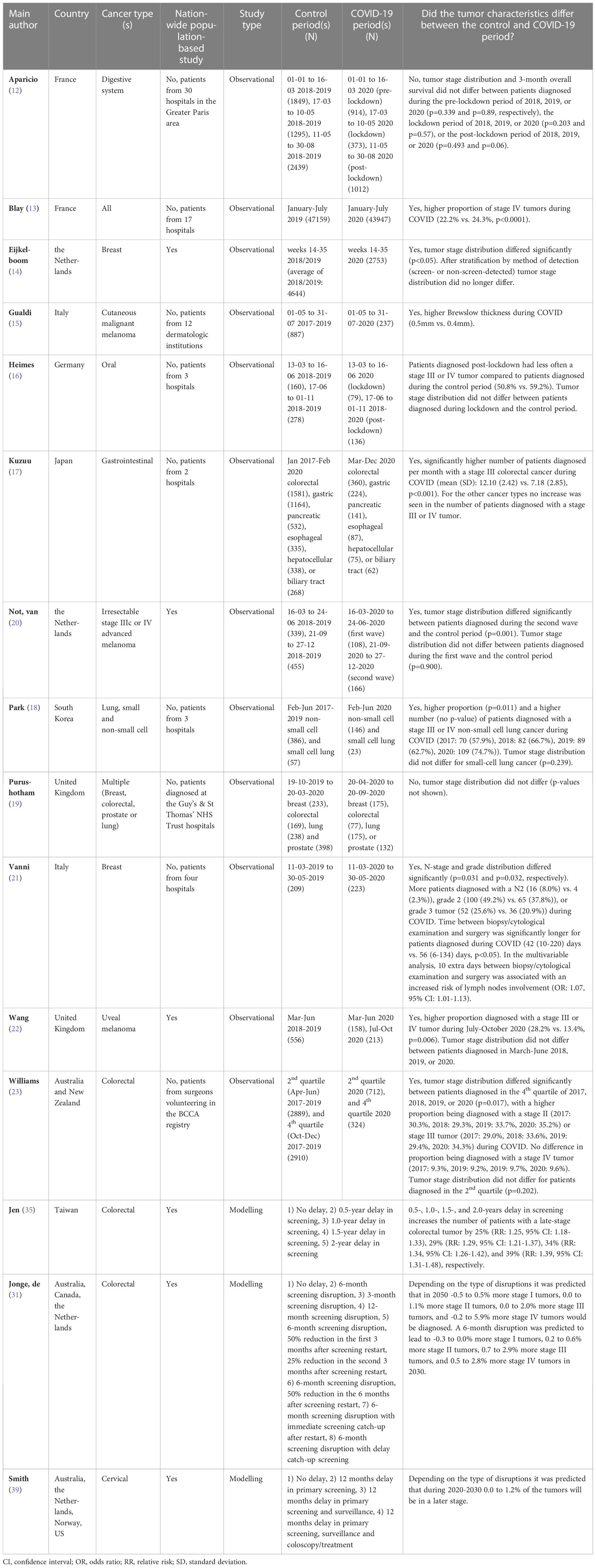
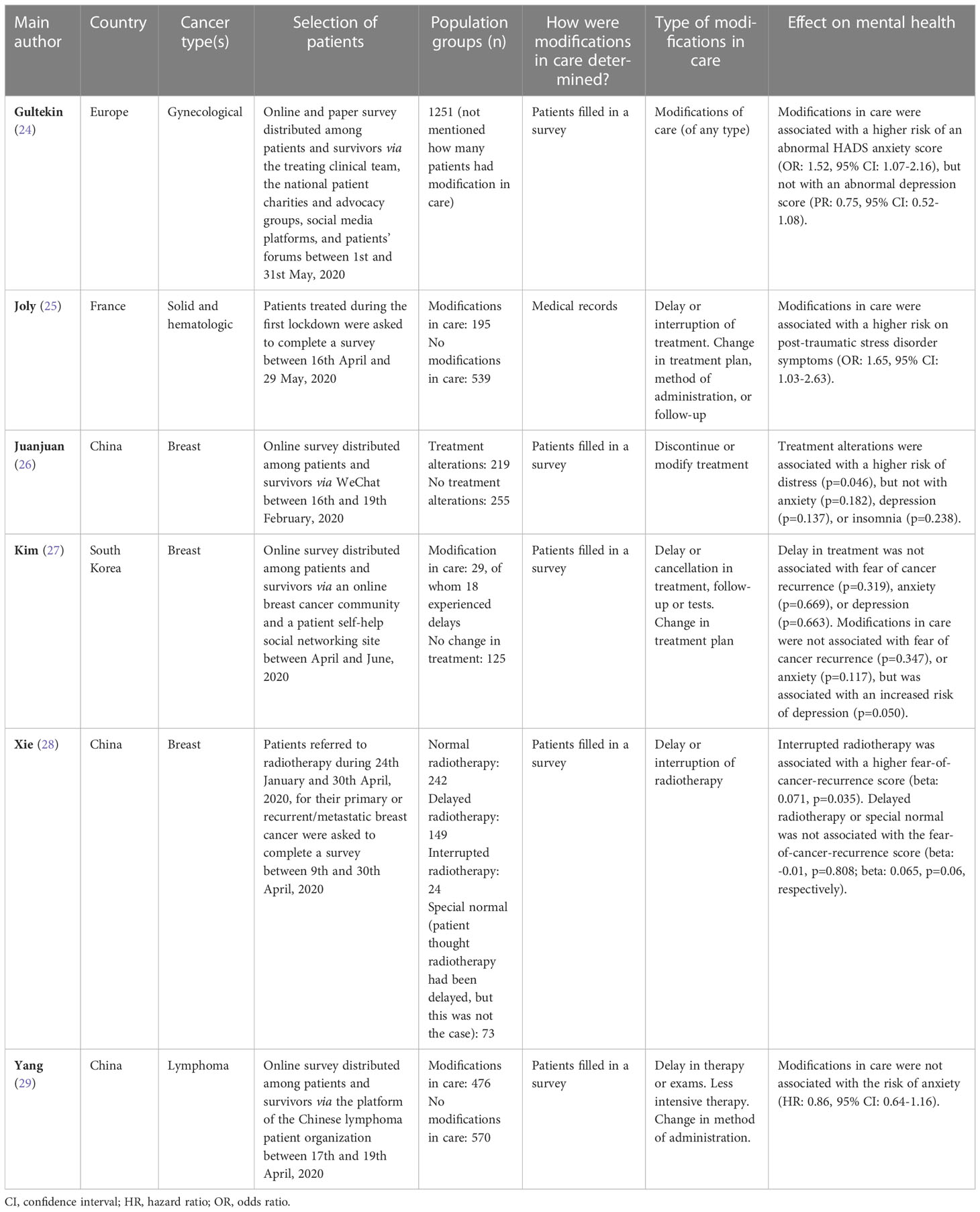
Twelve of the included studies compared the characteristics of tumors diagnosed during and before the pandemic (12–23), six were cross-sectional studies (24–29), and twelve were modelling studies (30–41). Nineteen of the studies were performed in Europe (12–16, 19–22, 24, 25, 31, 33, 36–41), three in Oceania (23, 31, 32), eight in Asia (17, 18, 26–29, 34, 35), and two in North-America (30, 31). The included studies investigated the effect of delays in oncological care on 36 different tumor types or groups of tumors. Tumor characteristics was the main health outcome in fifteen studies (Table 1) (12–23), survival in nine (Tables 2, 3) (30, 32–34, 36–38, 40, 41), tumor characteristics and survival in three (31, 35, 39) and mental health in six (Table 4) (24–29). Results are discussed based on the type of health outcome studied: tumor characteristics, survival or mental health. The included studies are shown graphically in Figure 2.
3.1 Tumor characteristics
Out of the fifteen studies comparing the characteristics of tumors diagnosed during and before the COVID-19 pandemic twelve were observational studies (12–23, 31, 35, 39) and three were modelling studies (Table 1) (31, 35, 39). The observational studies will be discussed first.
All studies gathered their data during 2020. Three studies were nationwide population-based studies (14, 20, 22). In nine studies, the total number of tumors diagnosed during compared to before the pandemic was lower (12–15, 17, 19, 20, 22, 23). Out of these nine studies, three showed that, in percentage terms, the decrease in the number of diagnosed stage 0, I, and/or II tumors was higher than the decrease in the number of diagnosed stage III and IV tumors (14, 17, 23). In seven of the studies, tumor stage or grade distribution was different during compared to before the pandemic (13, 14, 18, 20–23), with a higher proportion of patients being diagnosed with a stage III or IV, or grade 3 tumor during the pandemic (13, 14, 18, 21–23). Out of these seven studies three studies only showed this difference in distribution in patients diagnosed in July-October, 2020, or October-December, 2020, but not in patients diagnosed earlier during the pandemic (i.e., March-June 2020 or April-June, 2020) (20, 22, 23). In one study tumor stage distribution did no longer differ between breast cancer patients diagnosed before and during the pandemic after stratification by method of detection (screen- or non-screen-detected) (14). Two studies found no difference in tumor stage distribution during compared to before the pandemic (12, 19). One study found a lower proportion of late-stage (stage III or IV) oral tumors diagnosed during the COVID-19 pandemic (16). Another study showed a higher median Brewslow thickness of cutaneous malignant melanomas diagnosed post lockdown (15).
A statistically significant higher number of people were diagnosed per month with a stage III colorectal tumor during compared to before the pandemic in the study of Kuzuu et al. (17). The study of Park et al. and Vanni et al. showed that a higher number of patients were diagnosed with a late-stage non-small-cell lung tumor or a N2, grade 2, or grade 3 breast tumor during compared to before the pandemic, respectively (18, 21). However, it was not tested whether this was statistically significant. One study found a higher number of patients diagnosed with a late-stage uveal melanoma during July-October 2020, but not during March-June 2020 (22).
Of the fifteen studies looking at tumor characteristics, three were modelling studies (31, 35, 39). One study predicted that, depending on the type of screening disruption, 0 to 1.2% of the cervical tumors will be detected at a later stage (39). The other two studies predicted that a six-month delay in screening would increase the estimated number of patients with a late-stage colorectal tumor with 18 to 33% (35), or with 1.2 to 5.7% (31).
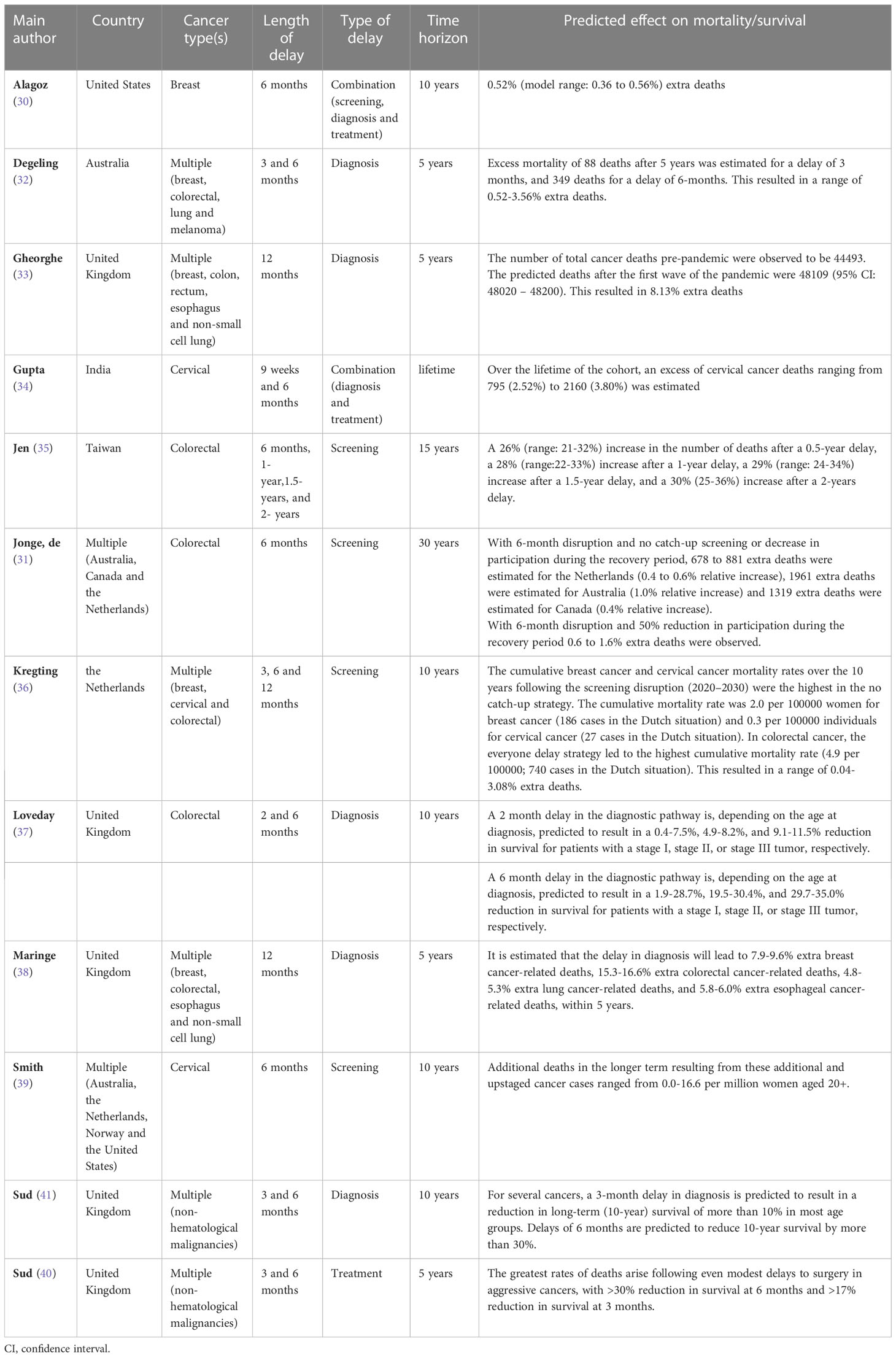
3.3 Survival
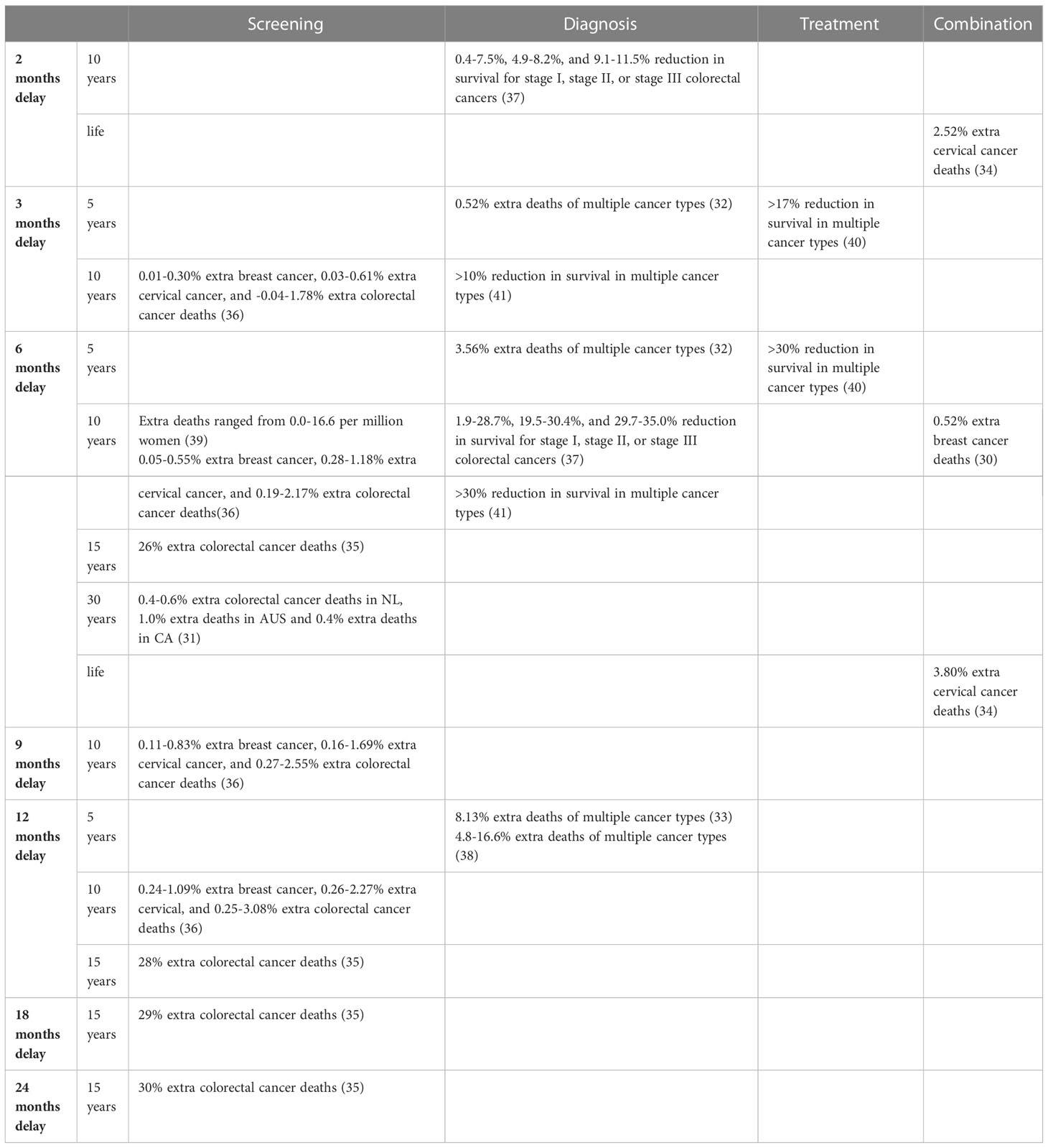
Twelve modelling studies investigated the impact of delay due to COVID-19 on the survival of cancer patients (Table 2). Studies used different time horizons, ranging from five-year time horizons to lifetime. Five studies modelled the impact of a delay in diagnosis (32, 33, 37, 38, 41), four studies modelled delays in cancer screening (31, 35, 36, 39), two studies modelled combinations of types of delay (30, 34), and one study modelled the effect of treatment delay (40). There was also variation in the length of delay, ranging from studies using two months and other studies using up to two years of delay.
As a consequence, the effect of delay on survival showed large variation between studies. Studies estimated a 0.4 to 35% reduction in survival and a -0.04 to 30% increase in the number of deaths, depending on the type and lengths of delays. A delay of two months in diagnosis, or a combination of diagnosis and treatment was estimated to decrease survival by 0.4 to 11.5% (37), and to increase the number of cancer deaths with 2.52% (34), respectively. A delay of three months in screening, diagnosis or treatment was estimated to decrease survival by >10 to >17% (40, 41) and to increase the number of deaths by -0.04 to 1.78% (32, 36). Delays of six months in screening, diagnosis, treatment, or a combination of screening, diagnosis and treatment decreased survival with an estimated 1.9 to 35.0% and increased the number of deaths with an estimated 0.05 to 26% (30–32, 34–37, 39–41). A delay of nine months in screening was estimated to increase the number of deaths by 0.11 to 2.55% (36) A delay of twelve months in screening or diagnosis was estimated to increase the number of deaths by 0.24 to 28% (33, 35, 36, 38). In the study that modelled the longest delay, i.e., a delay of 18 and 24 months in screening, a 29% and 30% increase in the number of deaths was estimated, respectively (35).
Delay in the cancer screening programs was estimated to increase the number of deaths with -0.04 to 30% (31, 35, 36, 39). Delays in diagnosis were estimated to reduce survival with 0.4 to 35.0% and to increase the number of deaths with 0.52 to 16.6% (32, 33, 37, 38, 41). Treatment delays were estimated to be associated with a >17 to >30% lower survival (40). Delays in combinations of types of delay increased the number of estimated deaths with 0.52 to 3.80% (30, 34).
Based on the modelled time horizons in the studies, delays were estimated to decrease survival with >17 to >30% and to increase the number of deaths with 0.52 to 16.6%, over a 5-year time horizon (32, 33, 38, 40). Delays were estimated to decrease survival with 0.4 to 35.0%, and to increase the number of deaths with -0.04 to 3.08% over a ten-year time horizon (30, 36, 37, 39, 41), to increase the number of deaths with 26 to 30% over a fifteen-year time horizon (35), to increase the number of deaths with 0.4 to 1.0% over a thirty-year time horizon (31), and to increase the number of deaths with 2.52 to 3.80% over a lifetime time horizon (34).
The modelling assumptions varied between the studies. Five of the studies assumed disruptions within the United Kingdom’s urgent 2-week-wait referral pathways. The remaining seven studies each have unique assumptions. A table showcasing the estimated effects on survival per type of delay, time horizon and place within the cancer care can be found in Table 3.
3.4 Mental health
Six cross-sectional studies investigated the impact of modifications in care due to the COVID-19 pandemic on the mental health of cancer patients (Table 4) (24–29). Modifications in care included delays, interruptions, or cancellations of treatments, tests, or follow-up visits, and changes in the treatment plan, method of treatment administration, or follow-up. Four studies included both cancer patients and survivors (24, 25, 27, 29). Five of the studies performed their study between April to June, 2020 (24, 25, 27–29), and one in February, 2020 (26). The latter was performed in the Hubei region, the region first affected by the pandemic. In one study medical records were used to determine whether patients experienced modification in care (25), in the other five studies patients filled in a survey to detect modifications in care (24, 26–29).
Studies showed dissimilar results concerning the effects of modifications in care on the mental health of cancer patients/survivors. A positive association was seen between modifications in care and post-traumatic stress disorder symptoms (25) or levels of distress (26). One study found a positive association with fear of cancer recurrence in patients with interrupted radiotherapy, but not in patients with delayed radiotherapy (28), another study found no association with fear of cancer recurrence (27). A positive association with depression was found in one study (27), but not in two others (24, 26). One study found a positive association with anxiety (24), while no association with anxiety was found in three other studies (26, 27, 29).
4 Discussion
To our knowledge, this is the first systematic review that aims to give an overview of the impact of delays in screening, diagnosis and/or treatment caused by the COVID-19 pandemic on the physical and mental health outcomes of cancer patients. Overall, there was a large discrepancy between both the methods and results of the studies.
4.1 Tumor characteristics
Nine studies showed a decrease in the number of tumors diagnosed during compared to before the pandemic (12–15, 17, 19, 20, 22, 23). Three of these studies showed that, in percentage terms, the decrease in the number of diagnosed stage 0, I, and II tumors was higher than the decrease in the number of diagnosed stage III and IV tumors (14, 17, 23). There may be two possible complementary explanations for this larger decrease in the number of early-stage tumor diagnoses. The first may be that early-stage tumors cause mild symptoms, for which a visit to the GP might be postponed, while late-stage tumors often cause severe symptoms for which care is sought, even during a pandemic. Second, in many countries the national breast and colorectal cancer screening program was temporarily suspended during the pandemic. Tumors detected at the breast cancer screening program mainly consist of stage 0, I, or II (42). Colorectal cancer screening aims to detect pre-malignant lesions and malignancies at an early stage, mainly stage I (43). Hence, suspension of the screening program would mainly have led to a decrease in the diagnosis of adenomas and stage I tumors. The large decrease in the number of early-stage tumors causes a relative increase in the proportion of patients being diagnosed with a late-stage tumor (stage III or IV), without necessarily an actual increase in the absolute number of patients being diagnosed with a late-stage tumor.
One study showed a statistically significant increase in the number of diagnosed stage III colorectal tumors (17). However, the results of this study should be interpreted with caution as this study only included two hospitals and the other two studies including colorectal cancer patients showed a decrease in the number of patients diagnosed with a stage III tumor during the pandemic (19, 23). The results of the study of Kuzuu et al. are however in accordance with one of the two modelling studies which predicted a 25% increase in the number of late-stage tumors due to a six-month delay in screening (17, 35). Three other studies also showed an increase in the number of stage III or IV, or grade 3 tumors. However, these studies did not test whether this was significant and they only included a small number of patients (between the 146 and 223 patients diagnosed during the COVID-periods) (18, 21, 22). Therefore, these results should also be interpreted with care.
The three observational studies including colorectal cancer patients all showed a decrease in the total number of colorectal cancer diagnoses (17, 19, 23). However, this can probably not be solely attributed to the COVID-19 pandemic. Many countries implemented colorectal cancer screening programmes to detect premalignant colorectal lesions. This resulted in a decreasing trend in the number of colorectal cancer diagnoses observed in the years following the introduction of the screening programs (44).
4.2 Survival
Overall, the twelve included modelling studies estimated a decreased survival. However, the estimated reductions in survival varied widely. The models based their parameters on pre-COVID data, which might not be an accurate reflection of the effects caused by COVID-19 delay. The observational studies included in this review gathered data during the COVID-19 pandemic. Compared to modelling studies, observational studies are more likely to showcase a realistic effect of the pandemic with all its complexities and unique circumstances like the applied mitigation strategies and prioritizing based on urgency.
The large variance in estimates might also be explained by the differences between the models in terms of assumptions, input data and modelling choices. All of these affect the outcomes of the model, making it difficult to compare the results. It should also be noted that all models by definition have some form of selection bias due to the inherent fact that no model is built when no effect on the outcomes is expected. The historical observational data chosen for the input parameters showcased an effect between delay and survival and therefore the models reproduce that effect as well, possibly leading to a self-fulfilling prophecy. However, the outcomes of the included studies do give an indication of the range in which COVID delays might have affected survival of cancer patients.
4.3 Mental health
Studies included in the current review provided dissimilar results about the effect of delays and modifications in care on the mental health of cancer patients. A systematic review concluded that the COVID-19 pandemic negatively impacted the mental health of cancer patients (7). In addition, it is known that being diagnosed with cancer is an important risk factor for depression and anxiety (45). It might be possible that the COVID-19 pandemic and the diagnosis of cancer already increased the levels of depression and anxiety to such an extent that factors such as modifications in care did not further increase the levels of depression and anxiety. Moreover, good mental support and information provision by the treating clinical team might also have prevented an increase in mental health problems. However, five of the included studies did not mention whether this social support was available (24–28). One study showed that good social services were indeed associated with lower levels of anxiety (29). Finally, studies might have been too small to show an association between modifications in care and mental health.
A limitation of five of the included studies is that they distributed their survey online (26–29) or mostly online (24), thereby limiting the generalizability of the results. Furthermore, five of the included studies did not adjust for or stratified by tumor stage (24, 26–29). One study showed that patients with a late-stage tumor had higher levels of concern due to COVID-19 compared to patients with an early-stage tumor (8), suggesting that the urge of being treated timely was related to the stage. Finally, all included studies were performed at the start of the pandemic and were therefore focusing on the early onset of mental health problems, while mental health problems could disappear later in time or, conversely, develop later in time.
4.4 Limitations
This systematic review has several limitations. Firstly, there was large heterogeneity between the included papers (e.g. heterogeneity in cancer types, assumptions, degree of modelled delay, study period), making it difficult to observe patterns and draw conclusions. Comparability might also be hampered due to dissimilarities between countries. In addition, four of the studies on mental health did not mention what the COVID-19 induced delays entailed for patients in terms of delay or alterations in care (25–27, 29), which also complicates the comparability between studies. Secondly, we considered using a quality assessment tool to assess the quality of the included papers. However, there was a large discrepancy between the applied methods of the papers. None of the available quality assessment tools fully complied with the papers, making the overall scoring of the quality impossible. Therefore, we omitted the use of such a tool. Third, the COVID-19 pandemic is still ongoing. Long-term effects can only be predicted for now using models and historical data. The upcoming years will likely show the real outcomes of this unique period, when the follow-up period of cohorts experiencing delay will gradually increase.
5 Conclusion
The effect on health outcomes due to delays caused by COVID-19 remain uncertain. Observational studies describing data up till 2020 did not provide evidence for an increase in the absolute numbers and incidence of late-stage tumors, suggesting that mortality might not increase to a large extent. The modelling studies estimated that COVID-19 induced delays in screening, diagnosis, and/or treatment will lead to a lower survival of cancer patients, but the estimates varied widely and were based on selective literature showing health effects of delay. The observational studies showed varying results concerning the effect on mental health. This may be related to the fact that the studies available regarding mental health only report on a relatively short period of data collection, ranging from 3 days to 3 months. More observational studies, with a longer follow-up period, are needed to give more conclusive results about the effects of modifications in oncological care due to the COVID-19 pandemic on the physical and mental health outcomes of cancer patients. Multiple of these studies have emerged during 2022. An update of this review in the near future and a comparison of new results with our findings from 2020 and 2021 will be needed to confirm our findings, and to shed light on the role of a longer follow-up period on possible detrimental health effects of delays in cancer care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
EV and GW conceived the idea of a review article on this topic. EV and AE conducted the research and wrote the article. All authors contributed to the article and approved the submitted version.
Funding
AE and SS were (partially) funded by The Netherlands Organization for Health Research and Development (ZonMw), project number: 10430022010014 and are employees of the Netherlands Comprehensive Cancer Organization (IKNL) and the University of Twente. EV, AG and GW are employees of the National Institute of Public Health and the Environment (RIVM). RIVM received a grant from the Dutch Ministry of Health, Welfare and Sports for the COVID-19 research program, with which this study was partially funded.
Acknowledgments
The authors would like to thank Rob van Spronsen and Adriënne Rotteveel for their involvement in developing the search strategy. The authors acknowledge the members of the COVID and Cancer-NL Consortium (ZonMW: projectnumber 10430022010014). Prof. dr. S. Siesling, department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht and Technical Medical Centre, department of Health Technology and Services Research, Twente University, Enschede, Dr. J.C. van Hoeve, department of research and development, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, prof. dr. M.A.W. Merkx, department of research and development, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht and IQ healthcare, Radboud University Nijmegen Medical Centre, Nijmegen, prof. dr. N.J. de Wit, department of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht; Dr. C.W. Helsper, department of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, M.Sc. I. Dingemans, Dutch Federation of Cancer Patient Organizations (NFK), Utrecht, prof. dr. I.D. Nagtegaal, department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, on behalf of the Automated Pathology Archive (PALGA), Drs. M. van der Schaaf, department of Insight and Innovation, Dutch Hospital Data (DHD), Utrecht, Prof. dr. C.H. van Gils, department of Clinical Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, prof. dr. H.C.P.M. van Weert, department of General Practice, Amsterdam Public Health, Amsterdam UMC location AMC, Amsterdam, prof. dr. M. Verheij, department of Radiation Oncology, Radboud University Medical Center, Nijmegen, on behalf of SONCOS (Dutch Multidisciplinary Oncology Foundation), all the Netherlands.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.998940/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China. New Engl J Med (2020) 382(8):727–33. doi: 10.1056/NEJMoa2001017
2. World Health Organization. COVID-19: physical distancing. Available at: https://www.who.int/westernpacific/emergencies/covid-19/information/physical-distancing#:~:text=Physical%20distancing%20helps%20limit%20the,Protect%20yourself%20and%20others (Accessed 15-03-2022).
3. Worldometer. COVID-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/ (Accessed February 24, 2021).
4. Alkatout I, Biebl M, Momenimovahed Z, Giovannucci E, Hadavandsiri F, Salehiniya H, et al. Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.675038
5. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: A systematic review. J Gastrointestinal Cancer (2022) 53(3):730–44. doi: 10.1007/s12029-021-00679-x
6. Zaniboni A, Ghidini M, Grossi F, Indini A, Trevisan F, Iaculli A, et al. A review of clinical practice guidelines and treatment recommendations for cancer care in the COVID-19 pandemic. Cancers. (2020) 12(9):2452. doi: 10.3390/cancers12092452
7. Dhada S, Stewart D, Cheema E, Hadi MA, Paudyal V. Cancer services during the covid-19 pandemic: Systematic review of patient’s and caregiver’s experiences. Cancer Manage Res (2021) 13:5875–87. doi: 10.2147/CMAR.S318115
8. Rodriguez GM, Ferguson JM, Kurian A, Bondy M, Patel MI. The impact of COVID-19 on patients with cancer: A national study of patient experiences. Am J Clin Oncol (2021) 44(11):580. doi: 10.1097/COC.0000000000000865
9. van de Poll-Franse LV, de Rooij BH, Horevoorts NJE, May AM, Vink GR, Koopman M, et al. Perceived care and well-being of patients with cancer and matched norm participants in the COVID-19 crisis: Results of a survey of participants in the Dutch PROFILES registry. JAMA Oncol (2021) 7(2):279–84. doi: 10.1001/jamaoncol.2020.6093
10. Quinn-Scoggins HD, Cannings-John R, Moriarty Y, Whitelock V, Whitaker KL, Grozeva D, et al. Cancer symptom experience and help-seeking behaviour during the COVID-19 pandemic in the UK: A cross-sectional population survey. BMJ Open (2021) 11(9):e053095. doi: 10.1136/bmjopen-2021-053095
11. Rimmer A. Patients have struggled to access general practice during the pandemic, healthwatch reports. Br Med J Publishing Group (2021) 372:n798. doi: 10.1136/bmj.n798
12. Aparicio T, Layese R, Hemery F, Tournigand C, Paillaud E, De Angelis N, et al. Effect of lockdown on digestive system cancer care amongst older patients during the first wave of COVID-19: The CADIGCOVAGE multicentre cohort study. Digestive Liver Dis (2021) 54(1):10–8. doi: 10.1016/j.dld.2021.09.017
13. Blay JY, Boucher S, Le Vu B, Cropet C, Chabaud S, Perol D, et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open (2021) 6(3):100134. doi: 10.1016/j.esmoop.2021.100134
14. Eijkelboom AH, de Munck L, Lobbes MBI, van Gils CH, Wesseling J, Westenend PJ, et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med (2021) 151:106602. doi: 10.1016/j.ypmed.2021.106602
15. Gualdi G, Porreca A, Amoruso GF, Atzori L, Calzavara-Pinton P, De Tursi M, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clinics Dermatol (2021) 39(5):911–9. doi: 10.1016/j.clindermatol.2021.05.015
16. Heimes D, Müller LK, Schellin A, Naujokat H, Graetz C, Schwendicke F, et al. Consequences of the COVID-19 pandemic and governmental containment policies on the detection and therapy of oral malignant lesions–a retrospective, multicenter cohort study from germany. Cancers (2021) 13(12):2892. doi: 10.3390/cancers13122892
17. Kuzuu K, Misawa N, Ashikari K, Kessoku T, Kato S, Hosono K, et al. Gastrointestinal cancer stage at diagnosis before and during the COVID-19 pandemic in Japan. JAMA Network Open (2021) 4(9):e2126334. doi: 10.1001/jamanetworkopen.2021.26334
18. Park JY, Lee YJ, Kim T, Lee CY, Kim HI, Kim JH, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer (2020) 20(1):1040. doi: 10.1186/s12885-020-07544-3
19. Purushotham A, Roberts G, Haire K, Dodkins J, Harvey-Jones E, Han L, et al. The impact of national non-pharmaceutical interventions ('lockdowns') on the presentation of cancer patients. ecancermedicalscience (2021) 15:1180. doi: 10.3332/ecancer.2021.1180
20. van Not OJ, van Breeschoten J, van den Eertwegh AJM, Hilarius DL, De Meza MM, Haanen JB, et al. The unfavorable effects of COVID-19 on Dutch advanced melanoma care. Int J Cancer (2021) 150(5):816–24. doi: 10.1200/JCO.2021.39.15_suppl.e21502
21. Vanni G, Tazzioli G, Pellicciaro M, Materazzo M, Paolo O, Cattadori F, et al. Delay in breast cancer treatments during the first COVID-19 lockdown. a multicentric analysis of 432 patients. Anticancer Res (2020) 40(12):7119–25. doi: 10.21873/anticanres.14741
22. Wang H, Elsheikh M, Gilmour K, Cohen V, Sagoo MS, Damato B, et al. Impact of COVID-19 pandemic on eye cancer care in united kingdom. Br J Cancer. (2021) 124(8):1357–60. doi: 10.1038/s41416-021-01274-4
23. Williams E, Kong JC, Singh P, Prabhakaran S, Warrier SK, Bell S. The impact of the COVID-19 pandemic on colorectal cancer diagnosis and management; a binational colorectal cancer audit study. ANZ J Surg (2021) 91(10):2091–6. doi: 10.1111/ans.17071
24. Gultekin M, Ak S, Ayhan A, Strojna A, Pletnev A, Fagotti A, et al. Perspectives, fears and expectations of patients with gynaecological cancers during the COVID-19 pandemic: A pan-European study of the European network of gynaecological cancer advocacy groups (ENGAGe). Cancer Med (2021) 10(1):208–19. doi: 10.1002/cam4.3605
25. Joly F, Rigal O, Guittet L, Lefèvre-Arbogast S, Grellard JM, Binarelli G, et al. Post-traumatic stress symptomatology and adjustment of medical oncology practice during the COVID-19 pandemic among adult patients with cancer in a day care hospital. Cancer (2021) 127(24):4636–45. doi: 10.1002/cncr.33856
26. Juanjuan L, Santa-Maria CA, Hongfang F, Lingcheng W, Pengcheng Z, Yuanbing X, et al. Patient-reported outcomes of patients with breast cancer during the COVID-19 outbreak in the epicenter of China: A cross-sectional survey study. Clin Breast Cancer. (2020) 20(5):e651–e62. doi: 10.1016/j.clbc.2020.06.003
27. Kim SY, Kim S. Do COVID-19-Related treatment changes influence fear of cancer recurrence, anxiety, and depression in breast cancer patients? Cancer Nurs (2021) 45(2):E628–38. doi: 10.1097/NCC.0000000000000937
28. Xie J, Qi W, Cao L, Tan Y, Huang J, Gu X, et al. Predictors for fear of cancer recurrence in breast cancer patients referred to radiation therapy during the COVID-19 pandemic: A multi-center cross-section survey. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.650766
29. Yang S, Dong D, Gu H, Gale RP, Ma J, Huang X. Impact of stopping therapy during the SARS-CoV-2 pandemic in persons with lymphoma. J Cancer Res Clin Oncol (2021) 147(5):1469–79. doi: 10.1007/s00432-020-03426-0
30. Alagoz O, Lowry KP, Kurian AW, Mandelblatt JS, Ergun MA, Huang H, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: Estimates from collaborative simulation modeling. J Natl Cancer Institute (2021) 113(11):1484–94. doi: 10.1093/jnci/djab097
31. de Jonge L, Worthington J, van Wifferen F, Iragorri N, Peterse EFP, Lew JB, et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatology. (2021) 6(4):304–14. doi: 10.1016/S2468-1253(21)00003-0
32. Degeling K, Baxter NN, Emery J, Jenkins MA, Franchini F, Gibbs P, et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia-Pacific J Clin Oncol (2021) 17(4):359–67. doi: 10.1111/ajco.13505
33. Gheorghe A, Maringe C, Spice J, Purushotham A, Chalkidou K, Rachet B, et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: A national population-based modelling study in England, UK. Eur J Cancer. (2021) 152:233–42. doi: 10.1016/j.ejca.2021.04.019
34. Gupta N, Chauhan AS, Prinja S, Pandey AK. Impact of covid-19 on outcomes for patients with cervical cancer in india. JCO Global Oncol (2021) 7:716–25. doi: 10.1200/GO.20.00654
35. Jen GHH, Yen AMF, Hsu CY, Chiu HM, Chen SLS, Chen THH. Modelling the impacts of COVID-19 pandemic on the quality of population-based colorectal cancer screening. Prev Med (2021) 151:106597. doi: 10.1016/j.ypmed.2021.106597
36. Kregting LM, Kaljouw S, de Jonge L, Jansen EEL, Peterse EFP, Heijnsdijk EAM, et al. Effects of cancer screening restart strategies after COVID-19 disruption. Br J Cancer. (2021) 124(9):1516–23. doi: 10.1038/s41416-021-01261-9
37. Loveday C, Sud A, Jones ME, Broggio J, Scott S, Gronthound F, et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: A UK modelling study. Gut. (2021) 70(6):1053–60. doi: 10.1136/gutjnl-2020-321650
38. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol (2020) 21(8):1023–34. doi: 10.1016/S1470-2045(20)30388-0
39. Smith MA, Burger EA, Castanon A, de Kok IMCM, Hanley SJB, Rebolj M, et al. Impact of disruptions and recovery for established cervical screening programs across a range of high-income country program designs, using COVID-19 as an example: A modelled analysis. Prev Med (2021) 151:106623. doi: 10.1016/j.ypmed.2021.106623
40. Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol (2020) 31(8):1065–74. doi: 10.1016/j.annonc.2020.05.009
41. Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol (2020) 21(8):1035–44. doi: 10.1016/S1470-2045(20)30392-2
42. de Munck L, Siesling S, Fracheboud J, den Heeten GJ, Broeders MJ, de Bock GH. Impact of mammographic screening and advanced cancer definition on the percentage of advanced-stage cancers in a steady-state breast screening programme in the Netherlands. Br J Cancer. (2020) 123(7):1191–7. doi: 10.1038/s41416-020-0968-6
43. Toes-Zoutendijk E, Kooyker AI, Elferink MA, Spaander MC, Dekker E, de Koning HJ, et al. Stage distribution of screen-detected colorectal cancers in the Netherlands. Gut. (2018) 67(9):1745–6. doi: 10.1136/gutjnl-2017-315111
44. Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol (2021) 22(7):1002–13. doi: 10.1016/S1470-2045(21)00199-6
Keywords: COVID-19, cancer, systematic review, delay, mental health, stage, survival, mortality
Citation: van Vliet ED, Eijkelboom AH, van Giessen A, Siesling S and de Wit GA (2023) Physical and mental health outcomes of COVID-19 induced delay in oncological care: A systematic review. Front. Oncol. 13:998940. doi: 10.3389/fonc.2023.998940
Received: 20 July 2022; Accepted: 16 January 2023;
Published: 27 January 2023.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Marta Makara-Studzińska, Jagiellonian University Medical College, PolandChao-Hui Sylvia Huang, University of Alabama at Birmingham, United States
Copyright © 2023 van Vliet, Eijkelboom, van Giessen, Siesling and de Wit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ella D. van Vliet, ZWxsYS52YW4udmxpZXRAcml2bS5ubA==
†These authors have contributed equally to this work and share first authorship
 Ella D. van Vliet
Ella D. van Vliet Anouk H. Eijkelboom
Anouk H. Eijkelboom Anoukh van Giessen4
Anoukh van Giessen4