
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 04 April 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.995637
This article is part of the Research Topic Precision Medicine in Gastrointestinal Cancers View all 24 articles
Background: The purpose of this study is to investigate the predictive significance of (platelet × albumin)/lymphocyte ratio (PALR) for lymph node metastasis (LNM) in patients with clinically node-negative colon cancer (cN0 CC).
Methods: Data from 800 patients with primary CC who underwent radical surgery between March 2016 and June 2021 were reviewed. The non-linear relationship between PALR and the risk of LNM was explored using a restricted cubic spline (RCS) function while a receiver operating characteristic (ROC) curve was developed to determine the predictive value of PALR. Patients were categorized into high- and low-PALR cohorts according to the optimum cut-off values derived from Youden’s index. Univariate and multivariate logistic regression analyses were used to identify the independent indicators of LNM. Sensitivity analysis was performed to repeat the main analyses with the quartile of PALR.
Results: A total of eligible 269 patients with primary cN0 CC were retrospectively selected. The value of the area under the ROC curve for PALR for predicting LNM was 0.607. RCS visualized the uptrend linear relationship between PALR and the risk of LNM (p-value for non-linearity > 0.05). PALR (odds ratio = 2.118, 95% confidence interval, 1.182-3.786, p = 0.011) was identified as an independent predictor of LNM in patients with cN0 CC. A nomogram incorporating PALR and other independent predictors was constructed with an internally validated concordance index of 0.637. The results of calibration plots and decision curve analysis supported a good performance ability and the sensitivity analysis further confirmed the robustness of our findings.
Conclusion: PALR has promising clinical applications for predicting LNM in patients with cN0 CC.
Colon cancer (CC) is a common malignancy of the gastrointestinal tract and the third leading cause of cancer-related mortality worldwide (1). A large number of studies have shown that lymph node metastasis (LNM) is an independent risk factor for the prognosis of patients with CC (2, 3). In addition, the scope of surgery for patients with CC should take full account of the preoperative lymph node status to avoid overtreatment (4). Furthermore, neoadjuvant chemotherapy is recommended for patients with stage cT1-4N+M0 CC, which can promote tumor regression and improve survival in patients with CC after borderline negative resection (5–8). Therefore, accurate preoperative prediction of LNM is essential for individualized treatment decisions and prognostic assessment (9). Although widely used in clinical practice, imaging techniques have not produced satisfactory results in the preoperative assessment of lymph node status in CC. It has been estimated that about 30% of lymph node involvement is missed by preoperative abdominal contrast-enhanced computed tomography (CECT) (10, 11). The clinical application of several new molecular biomarkers discovered for detecting LNM in CC, such as FXYD3 and miR-323a-3p, seems unrealistic due to their high cost and technical complexity (12, 13). Therefore, there is an urgent need for cost-effective and convenient preoperative biomarkers to accurately assess the LNM in CC. It has been clarified that preoperative inflammatory biomarkers, including platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), and albumin (ALB) can predict LNM in patients with CC (14–16). To our knowledge, the clinical significance of (platelet × albumin)/lymphocyte ratio (PALR), has not been assessed in cancers. The purpose of this study was to investigate the predictive value of PALR for LNM in patients with clinically nodal-negative CC (cN0 CC) and develop a nomogram to assist clinicians in formulating individualized treatments.
A total of eligible 269 patients with primary cN0 CC who underwent radical surgery between March 2016 and June 2021 in Yijishan Hospital were retrospectively selected. The inclusion criteria were as follows: [1] CC was confirmed by pathological examination; [2] patients underwent curative surgery (R0) and lymph node dissection; and [3] CECT was performed before operation in our hospital. Patients who met the following criteria were excluded: [1] LNM was detected by CECT before operation (cN+); [2] insufficient number of detected lymph nodes (< 12); [3] emergency admission; [4] a history of other malignancies or colectomy; [5] neoadjuvant chemotherapy; [6] with diseases affecting the blood system; and [7] incomplete data. Figure 1 demonstrates the detailed screening process.
We recorded the serum levels of lymphocytes, platelets, ALB, and carcinoembryonic antigen (CEA) by performing routine blood tests on the day of admission. PLR and PALR were calculated according to the following formulas: PLR = Platelets (109/L)/Lymphocytes (109/L), PALR = (Platelets (109/L) × ALB (g/L))/(Lymphocytes (109/L)/1000). Postoperative pathology results including tumor site, size, grade, and the depth of tumor invasion (T stage) were reviewed by senior pathologists in our hospital. All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system (17).
Continuous variables were expressed as medians (interquartile range) and analyzed by Mann-Whitney U tests, while categorical variables were expressed as numbers and analyzed by the Chi-square test (18). Comparisons of the values of area under the receiver operating characteristic (ROC) curve (AUC) were carried out using the DeLong test (19, 20). Youden’s index was used to identify the optimum cut-off values of PALR and PLR based on the ROC curves (21). Independent predictors of LNM were obtained by combining univariate and multivariate logistic regression analyses. We used a restricted cubic spline (RCS) function with three knots at the 5th, 50th, and 95th centiles to develop a flexible model of the association of PALR with the risk of LNM (22). In terms of sensitivity analysis, we incorporated the quartile of PALR in the multivariate analysis. We used AUC, decision curve analysis (DCA), and the calibration curves to evaluate the nomogram as previously described (23–25). The internal validation of the model was performed via a bootstrap resample approach (1000 samples), together with the calculation of a corrected concordance index (C-index) (26). SPSS (Version 26.0), MedCalc (Version 15.2), and R (Version 4.0.2) were used for statistical analyses and graphics. All two-sided p values < 0.05 were considered significant.
We enrolled 269 eligible patients in our study with a 28.6% LNM rate. The optimum cut-off values of the PALR and PLR for LNM were set to 5.62, and 80.95, respectively. The patients were divided into high- and low- groups according to the optimum cut-off values. Young patients accounted for 52.0% and more than half of the patients were male (58.7%). The location of the tumor was mostly on the left side of the colon (61.7%). The majority of the patients were in T3 and T4 stages (81.0%) and almost all patients had highly or moderately differentiated primary tumors (96.3%). No significant differences were observed in terms of age (p = 0.110), tumor site (p = 0.491), and CEA (p = 0.693) between LNM-positive and LNM-negative groups. However, significant differences were observed between LNM-positive group and LNM-negative group while considering females (p = 0.048), and patients in a more advanced T stage (p = 0.023), with poorer differentiation (p = 0.025), smaller tumor size (p = 0.024), and higher PALR (p = 0.002), and PLR (p = 0.043). Table 1 provides detailed information regarding the different observations. When PALR was considered a continuous variable, patients in the LNM-negative group had lower PALR than those in the LNM-positive group (p = 0.006) (Figure 2).
Univariate analysis showed that sex, T stage, grade, and PALR were correlated with LNM in patients with cN0 CC. Multivariate analysis identified sex, T stage, and PALR as the independent predictors of LNM in cN0 CC (Table 2). The AUC value of PALR for predicting LNM was 0.607 (95% confidence interval [CI], 0.546 - 0.666), which was significantly better than that of PLR (0.568; P < 0.001). The diagnostic sensitivity and specificity of PALR were 41.2% and 77.1%, respectively. We used RCS with three knots at the 5th, 50th, and 95th centiles to develop a flexible model of the association of PALR with the risk of LNM based on multivariate analysis. In essence, the odds ratio (OR) curve exhibited an upward tendency, indicating a linear association between PALR and the risk of LNM (p for non-linearity > 0.05) and the risk of LNM increased with increasing PALR (Figure 3).
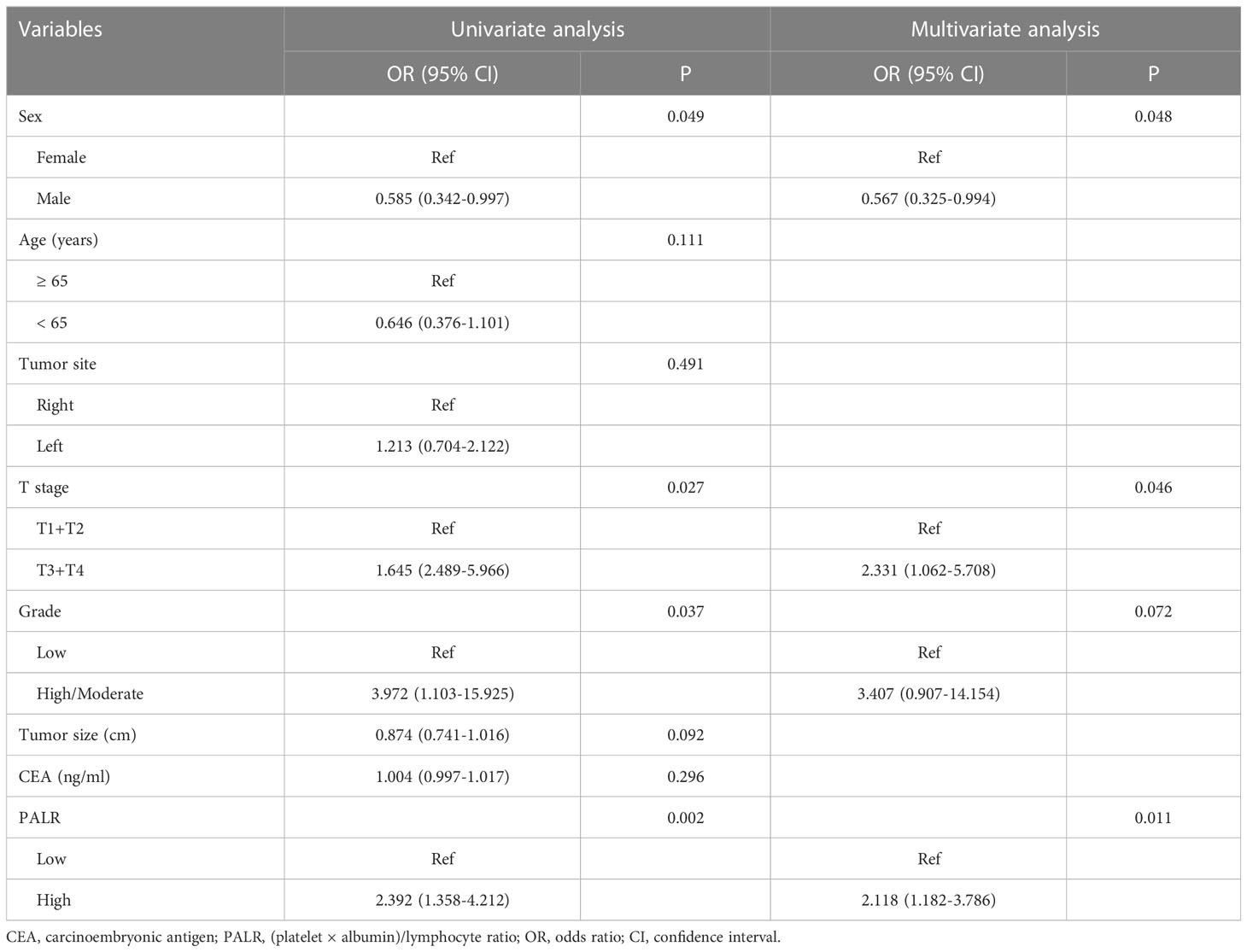
Table 2 Logistic analyses the predictors of lymph node metastasis in patients with cN0 colon cancer.
Of the 269 patients, 193 were categorized as the ‘low PALR’ group while the remainder as the ‘high PALR’ group according to the optimum cut-off values. The results showed a significant association between higher PALR and the parameters including right-sided CC, larger tumor size, higher PLR, and higher LNM rate, while sex, age, T stage, grade, and CEA were not statistically correlated with higher PALR (Table 3). The distribution of PALR among different clinicopathological variables is shown in Figure 4. A higher PALR was observed in patients with right-sided CC with deeper invasion.
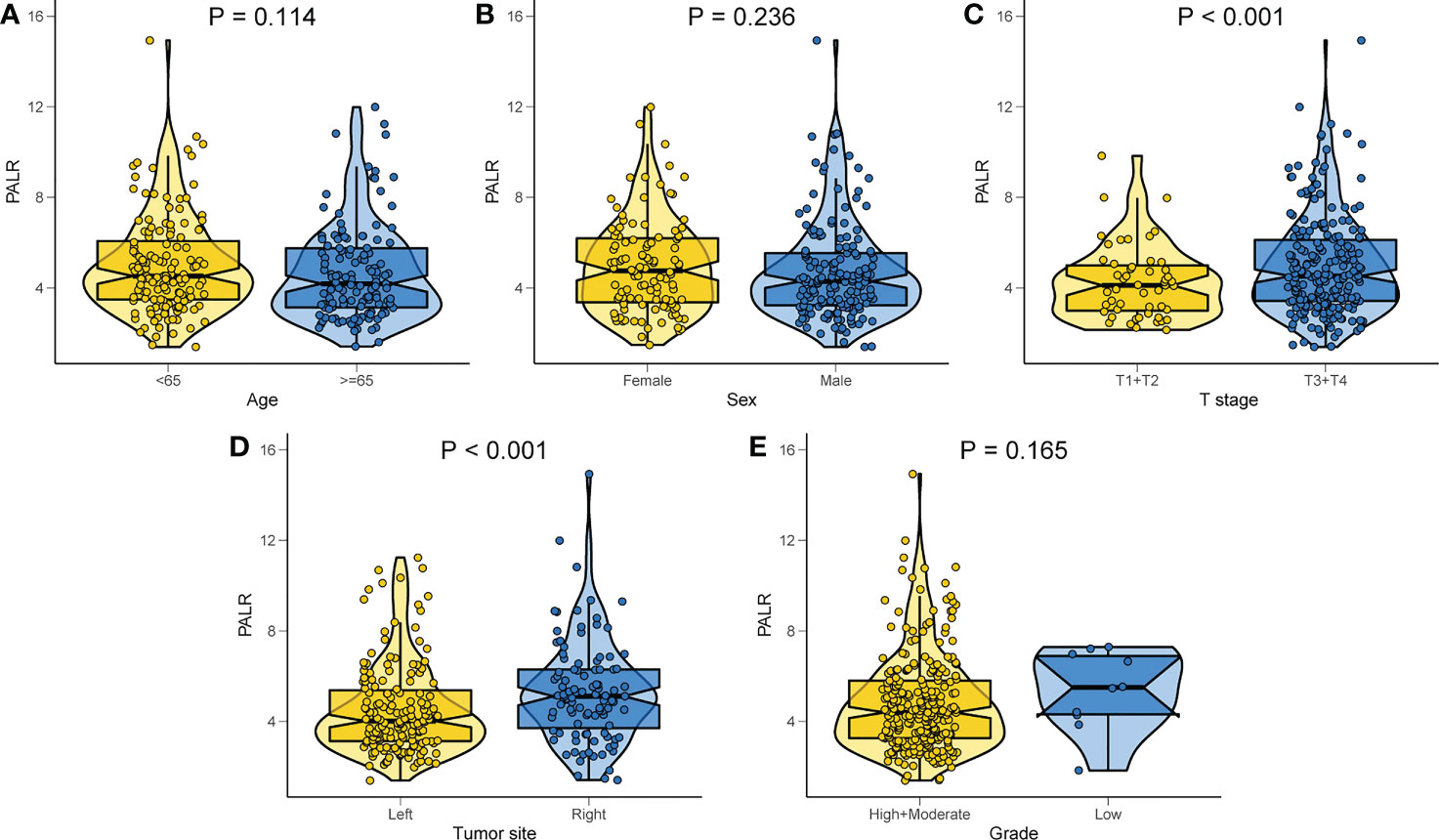
Figure 4 Distribution of PALR values among different clinicopathological variables. (A) Age, (B) Sex, (C) T stage, (D) Tumor site, (E) Grade.
Higher PALR quartiles are independently correlated with the risk of LNM in cN0 CC after adjustments for sex, T stage, and grade (p for trend = 0.041). The adjusted OR for the highest PALR versus the lowest quartile was 2.328 (95% CI, 1.060 - 5.291) for LNM (Supplementary Table 1).
To facilitate clinicians to calculate the risk of LNM in the individual patient with cN0 CC, we constructed a nomogram incorporating PALR. After taking into account previous research results and the biology of CC (27, 28), tumor grade was still included in our prediction model, although it was not an independent factor of LNM in our study. The indicators, including sex, T stage, grade, and PALR were selected in this model, as shown in Figure 5A. The adjusted C-index of nomogram after 1000 times of bootstrap resampling was 0.637. The calibration plots showed that the calibration prediction curve fits well with the ideal curve (Hosmer-Lemeshow test: p = 0.993) (Figure 5B). The ROC and DCA curves showed that the nomogram had a higher predictive value and net benefit compared with PALR only, indicating that this model could benefit patients in predicting the risk of LNM (Figures 5C, D).
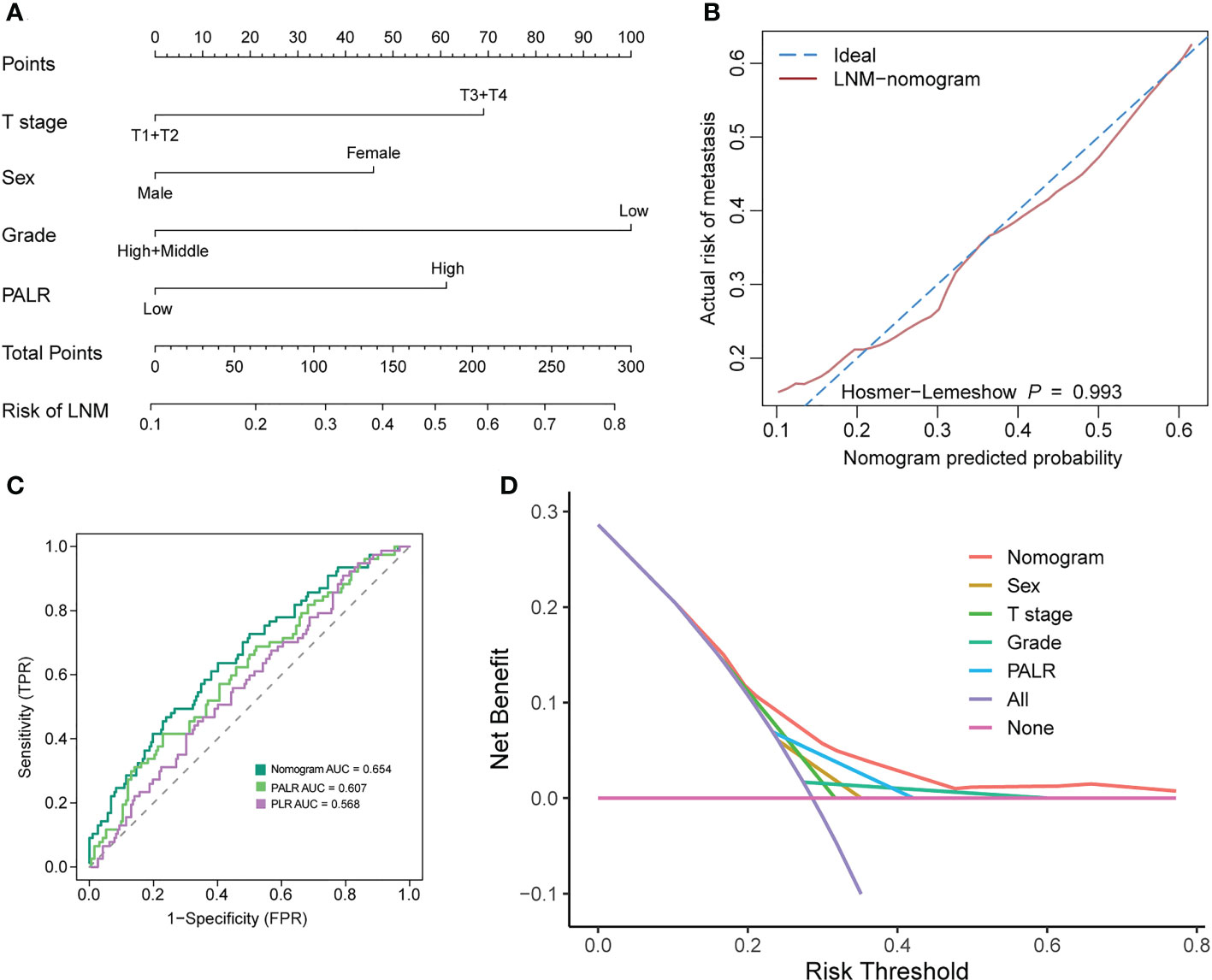
Figure 5 Developing and evaluating a nomogram for predicting LNM in patients with cN0 colon cancer. (A) nomogram, (B) calibration curves, (C) ROC curves, (D) Decision curve analysis.
In this study, we reported a novel preoperative biomarker, PALR, an independent predictor of LNM in patients with cN0 CC, which had a linear association with the risk of LNM. In addition, a nomogram based on PALR and clinicopathological parameters was constructed, which exhibited good performance in predicting the individual risk of LNM.
Previous studies have clarified that the systemic inflammatory response and nutritional status play important roles in tumor development and progression (29, 30). High levels of platelets are capable of promoting tumor progression and metastasis by increasing angiogenesis through the production of the vascular endothelial growth factor (31). Further, the platelet-derived growth factors have been suggested to be lymphangiogenic factors, which may either alone or jointly promote lymphatic metastasis (32). A recent study reported by Kundaktepe et al. showed a positive association of platelet counts with LNM in patients with CC (33). On the other hand, lymphocytes play a pivotal part in antitumor response by inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration (34, 35). Several studies have consistently shown that tumor-infiltrating lymphocytes can kill CC cells via the Fas/FasL pathway (36, 37). Moreover, high platelet and low lymphocyte counts are associated with tumor progression and high PLR levels may contribute to unfavorable anti-tumor function (38).
ALB is the most abundant serum protein that reflects the nutritional status and inflammatory responses (39). Jiang et al. reported preoperative hypoalbuminemia as a risk factor for a high proportion of LNM in patients with CC (40). Inflammation or nutritional index as independent predictors of LNM have been reported in many types of tumors. In the previous studies of CC and medullary thyroid carcinoma, PLR was independently correlated with LNM (14, 41). ALB was also confirmed as a predictive biomarker of LNM in patients with gastric neuroendocrine tumor (42). Chen et al. identified that the prognostic nutritional index had an independent correlation with LNM of patients with non-small cell lung cancer (43). Our focus on the role of inflammation and nutrition in tumor progression prompted us to evaluate PALR as a novel biomarker for predicting LNM in patients with cN0 CC, which has the potential to reflect the balance between systemic inflammation and nutritional status.
The current study has several limitations. First, this study is a single-center retrospective study, and selectivity bias is inevitable. Second, due to the relatively small sample size, the persuasiveness of our findings will be compromised to some extent. Therefore, future validation studies having a large-sample size with adequate representative groups from other centers are necessary to promote the clinical application of PALR. Third, the indicators of grade and the T stage were obtained from the postoperative pathological analysis. Although the T stage and grade can be obtained preoperatively through imaging techniques and puncture, respectively, there is a possibility that the preoperative diagnosis may differ from the postoperative pathological findings (44, 45). Fourth, further follow-up of patients is warranted to investigate the relationship between PALR and overall survival.
PALR is a novel promising inflammation-nutrition biomarker to predict the LNM in patients with cN0 CC. Large-scale prospective studies are required to validate our results in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Yijishan Hospital of Wannan Medical College. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WW designed the research. WD, CH and WW performed the study and analyzed the data. WD and WW wrote the paper and interpreted the data. WW help to revised manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.995637/full#supplementary-material
Supplementary Table 1 | Multivariate logistic analysis the predictors of LNM according to the quartile of PALR in patients with cN0 colon cancer.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Ishii K, Watanabe J, Goto K, Suwa Y, Nakagawa K, Suwa H, et al. The prognostic significance of apical lymph node metastasis in patients with high-risk stage III colon cancer. Sci Rep (2022) 12(1):2059. doi: 10.1038/s41598-022-06054-5
3. Suzuki O, Sekishita Y, Shiono T, Ono K, Fujimori M, Kondo S. Number of lymph node metastases is better predictor of prognosis than level of lymph node metastasis in patients with node-positive colon cancer. J Am Coll Surg (2006) 202(5):732–6. doi: 10.1016/j.jamcollsurg
4. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol (2020) 25(1):1–42. doi: 10.1007/s10147-019-01485-z
5. Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum (2022) 65(2):148–77. doi: 10.1097/DCR.0000000000002323
6. Chinese Society of Clinical Oncology Colorectal Cancer Expert Committee. Consensus of Chinese experts in perioperative treatment of resectable advanced colorectal cancer (2019). Zhonghua Wei Chang Wai Ke Za Zhi (2019) 22(8):701–10. doi: 10.3760/cma.j.issn.1671-0274.2019.08.001
7. Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review and meta-analysis. Int J Colorectal Dis (2021) 36(10):2063–70. doi: 10.1007/s00384-021-03945-3
8. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
9. Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, et al. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med (2020) 18(1):46. doi: 10.1186/s12967-020-02215-0
10. Nerad E, Lahaye MJ, Maas M, Nelemans P, Bakers FCH, Beets GL, et al. Diagnostic accuracy of CT for local staging of colon cancer: A systematic review and meta-analysis. Am J Roentgenology (2016) 207(5):984–95. doi: 10.2214/AJR.15.15785
11. Rollven E, Abraham-Nordling M, Holm T, Blomqvist L. Assessment and diagnostic accuracy of lymph node status to predict stage III colon cancer using computed tomography. Cancer Imaging (2017) 17(1):3. doi: 10.1186/s40644-016-0104-2
12. Meding S, Balluff B, Elsner M, Schone C, Rauser S, Nitsche U, et al. Tissue-based proteomics reveals FXYD3, S100A11 and GSTM3 as novel markers for regional lymph node metastasis in colon cancer. J Pathol (2012) 228(4):459–70. doi: 10.1002/path.4021
13. Lee IH, Kim G, Kwak SG, Baek DW, Kang BW, Kim HJ, et al. Predictive value of circulating miRNAs in lymph node metastasis for colon cancer. Genes (2021) 12(2):176. doi: 10.3390/genes12020176
14. Catal O, Ozer B, Sit M. Prediction of lymph node metastasis in colon cancer via platelet to lymphocyte ratio and platelet count. J Coll Physicians Surg Pak (2020) 30(3):250–3. doi: 10.29271/jcpsp.2020.03.250
15. Turhan VB, Unsal A, Gok HF, Ozturk B, Ozturk D, Simsek GG, et al. Predictive value of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in determining the stage of colon tumors. Cureus (2021) 13(9):e18381. doi: 10.7759/cureus.18381
16. Yao J, Chen Q, Deng Y, Zhao J, Bi X, Li Z, et al. Nomograms predicting primary lymph node metastases and prognosis for synchronous colorectal liver metastasis with simultaneous resection of colorectal cancer and liver metastases. Ann Palliat Med (2021) 10(4):4220–31. doi: 10.21037/apm-20-2303
17. Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
18. du Prel JB, Rohrig B, Hommel G, Blettner M. Choosing statistical tests: part 12 of a series on evaluation of scientific publications. Dtsch Arztebl Int (2010) 107(19):343–8. doi: 10.3238/arztebl.2010.0343
19. Zhou X-H. Comparing correlated areas under the ROC curves of two diagnostic tests in the presence of verification bias. Biometrics (1998) 54(2):453–70. doi: 10.2307/3109755
20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics (1988) 44(3):837–45. doi: 10.2307/2531595
21. Fluss R, Faraggi D, Reiser B. Estimation of the youden index and its associated cutoff point. Biom J (2005) 47(4):458–72. doi: 10.1002/bimj.200410135
22. Harrell FE Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst (1988) 80(15):1198–202. doi: 10.1093/jnci/80.15.1198
23. Vickers AJ, Holland F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J (2021) 21(10):1643–8. doi: 10.1016/j.spinee.2021.02.024
24. Austin PC, Steyerberg EW. Bootstrap confidence intervals for loess-based calibration curves. Stat Med (2014) 33(15):2699–700. doi: 10.1002/sim.6167
25. Tong C, Wang W, Xia Y, He C. A potential novel biomarker in predicting lymph node metastasis of gastric signet ring cell carcinoma: A derived monocyte to lymphocyte ratio. Am J Surg (2022) 223(6):1144–50. doi: 10.1016/j.amjsurg.2021.10.026
26. Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models. J Clin Epidemiol (2001) 54(8):774–81. doi: 10.1016/s0895-4356(01)00341-9
27. Yan Y, Liu H, Mao K, Zhang M, Zhou Q, Yu W, et al. Novel nomograms to predict lymph node metastasis and liver metastasis in patients with early colon carcinoma. J Transl Med (2019) 17(1):193. doi: 10.1186/s12967-019-1940-1
28. Xu Y, Chen Y, Long C, Zhong H, Liang F, Huang LX, et al. Preoperative predictors of lymph node metastasis in colon cancer. Front Oncol (2021) 11:667477. doi: 10.3389/fonc.2021.667477
29. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol (2017) 18(8):843–50. doi: 10.1038/ni.3754
30. Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
31. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer (2011) 11(2):123–34. doi: 10.1038/nrc3004
32. Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. BioMed Pharmacother (2007) 61(9):534–9. doi: 10.1016/j.biopha.2007.08.009
33. Papila Kundaktepe B, Papila C. The clinical significance of preoperative plasma fibrinogen levels and platelet counts in resectable colon cancer. World J Surg Oncol (2021) 19(1):69. doi: 10.1186/s12957-021-02180-y
34. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
35. Grizzi F, Basso G, Borroni EM, Cavalleri T, Bianchi P, Stifter S, et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflammation Res (2018) 67(5):375–89. doi: 10.1007/s00011-017-1128-1
36. O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci (2000) 910:178–92. doi: 10.1111/j.1749-6632.2000.tb06708.x
37. Zhu Q, Liu JY, Xu HW, Yang CM, Zhang AZ, Cui Y, et al. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol (2005) 11(39):6125–9. doi: 10.3748/wjg.v11.i39.6125
38. Li Z, Xu Z, Huang Y, Zhao R, Cui Y, Zhou Y, et al. Prognostic values of preoperative platelet-to-lymphocyte ratio, albumin and hemoglobin in patients with non-metastatic colon cancer. Cancer Manag Res (2019) 11:3265–74. doi: 10.2147/CMAR.S191432
39. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr (2001) 20(3):271–3. doi: 10.1054/clnu.2001.0439
40. Jiang Z, Li Y, Han G, Zhang J, Li Z, Wang D, et al. Association of serum albumin level with clinicopathologic features and prognosis in colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi (2016) 19(1):80–3. doi: 10.3760/cma.j.issn.1671-0274.2016.01.018
41. Jiang K, Lei J, Chen W, Gong Y, Luo H, Li Z, et al. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Med (Baltimore) (2016) 95(40):e5079. doi: 10.1097/MD.0000000000005079
42. Li X, Shao L, Lu X, Yang Z, Ai S, Sun F, et al. Risk factors for lymph node metastasis in gastric neuroendocrine tumor: a retrospective study. BMC Surg (2021) 21(1):174. doi: 10.1186/s12893-021-01174-7
43. Chen M, Yang Y, He C, Chen L, Cheng J. Nomogram based on prognostic nutrition index and chest CT imaging signs predicts lymph node metastasis in NSCLC patients. J Xray Sci Technol (2022) 30(3):599–612. doi: 10.3233/XST-211080
44. Castro-Pocas FM, Dinis-Ribeiro M, Rocha A, Santos M, Araujo T, Pedroto I. Colon carcinoma staging by endoscopic ultrasonography miniprobes. Endosc Ultrasound (2017) 6(4):245–51. doi: 10.4103/2303-9027.190921
Keywords: colon cancer, lymph node metastasis, (platelet × albumin)/lymphocyte ratio, restricted cubic spline, sensitivity analysis
Citation: Duan W, Wang W and He C (2023) A novel potential inflammation-nutrition biomarker for predicting lymph node metastasis in clinically node-negative colon cancer. Front. Oncol. 13:995637. doi: 10.3389/fonc.2023.995637
Received: 16 July 2022; Accepted: 20 March 2023;
Published: 04 April 2023.
Edited by:
Afsaneh Barzi, City of Hope National Medical Center, United StatesReviewed by:
E. Ramsay Camp, Baylor College of Medicine, United StatesCopyright © 2023 Duan, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d3d3eUB3bm1jLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.