
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 January 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.977160
This article is part of the Research Topic Advances in Imaging and Imaging-Directed Interventions in Hepatic Cancers View all 14 articles
Objective: To report the incidence of radiation pneumonitis after radioembolization.
Methods: In this retrospective study, from May 2009 to July 2021, 782 consecutive patients underwent radioembolization in two institutes. Medical internal radiation dose dosimetry and partition dosimetry were used for glass and resin Yttrium-90-labeled microspheres (90Y-microspheres), respectively. Medical records and radiological findings were retrospectively evaluated with emphasis on the symptomatic radiation pneumonitis.
Results: Of the 732 patients with lung shunt study and follow-up, 13 (1.8%) had symptomatic radiation pneumonitis and six patients died due to radiation pneumonitis. Of the 721 patients whose lung doses were calculated, 10 patients who were treated with glass (n = 5) and resin (n = 5) 90Y-microspheres had radiation pneumonitis. No significant statistical difference between glass and resin 90Y-microspheres (p = 0.304) was noted in terms of radiation pneumonitis incidence. Among the patients with radiation pneumonitis, all five patients treated with glass 90Y-microspheres had estimated lung doses > 29 Gy, whereas five patients treated with resin 90Y-microspheres had relatively wide range of lung dose reaching much lower value (13.21Gy).
Conclusion: The present study suggests that radiation pneumonitis after radioembolization may occur even though the manufacturer’s instructions are followed.
Radioembolization, using 90Y-microspheres, are used for the treatment of malignant liver tumors (e.g., hepatocellular carcinomas (HCCs) and colorectal liver metastases) (1). Radioactive 90Y-microspheres are small enough to pass through tumoral vessels in rare patients, resulting in radiation pneumonitis. For this reason, planning angiography and simulating 90Y-microsphere delivery by infusing 99mTc-macroaggregated albumin (99mTc-MAA) into the hepatic artery are used to measure the lung shunt fraction (LSF) and the estimated lung dose (2). The 25-Gy estimated lung dose by partition dosimetry is believed to be the safe upper limit for resin 90Y-microspheres (3, 4). However, for glass 90Y-microspheres, radioembolization is relatively contraindicated when the estimated lung dose is >30 Gy in a session and 50 Gy in a lifetime by medical internal radiation dose (MIRD) dosimetry (5).
Radiation pneumonitis is a rare but serious radioembolization complication that can occur 1–6 months after the procedure. Several case reports of radiation pneumonitis have been noted in the literature (6–8). This study, herein, reports the incidence ofradiation pneumonitis after radioembolization in the Asian population.
From May 2009 to July 2021, 782 consecutive patients underwent radioembolization in two institutes. The inclusion criteria were (a) patients who underwent planning angiography and 99mTc-MAA scan and (b) patients whose information regarding lung dose was available. Exclusion criteria were (a) no lung shunt study (n = 37) and (b) no follow-up imaging for at least 2 months (n = 13). Consequently, 732 patients (mean age, 62.8 ± 12.4 years [range, 21–92 years]), which comprised 592 men and 140 women, were included in the present study. Among these patients, 664 and 68 had hepatocellular carcinoma and other cancers, respectively (Table 1). Moreover, 493 and 239 patients were treated with glass and resin 90Y-microspheres, respectively. Furthermore, 36 patients received two sessions and 10 patients received three sessions of radioembolization.
Planning angiography includes celiac and superior mesenteric angiography, and depending on the need, right and/or left hepatic angiograms are also included. The operator advanced a microcatheter into the lobar artery supplying the primary target tumor, and 185 MBq of 99mTc-MAA was injected into the lobar hepatic artery. After injecting 99mTc-MAA, planar body scans that conjugated the anterior and posterior images were obtained for 10 min and were used to calculate the LSF.
Total liver volume, target volume, and tumor volume of each patient were measured from the most recent cross-sectional imaging study before treatment, including computed tomography (CT) scan and magnetic resonance imaging using Aquarius Intuition (Terarecon, Durham, NC).
For Therasphere, single compartment dosimetry (MIRD) was used to calculate the estimated lung dose as provided by the manufacturer, and the lung mass was set as 1,000g for all patients. For SIR-Spheres, partition dosimetry provided by the manufacturer was used, and the lung weight was set as 800 and 600 g for men and women, respectively.
Medical records and radiological findings were retrospectively evaluated. Radiation pneumonitis was diagnosed when patients presented with restrictive ventilatory dysfunction and typical bilateral lung infiltrates with exertional dyspnea and dry cough (9), and pathogens causing similar presentation such as pneumocystis carinii were not revealed. Severity was graded by Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The chi-square test was used to compare the radiation pneumonitis incidences between the groups. A p value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 25.0 software (SPSS, Inc., Chicago, IL, USA).
Of the 732 patients, 13 (1.8%) had symptomatic radiation pneumonitis and were treated with steroids (Table 2) (Figure 1). All 13 patients did not receive systemic chemotherapy. All 13 patients had HCCs which were treated with glass (n = 6) and resin (n = 7) 90Y-microspheres. Dyspnea (n = 12) and cough (n = 6) were the common symptoms. The time interval between radioembolization and symptom onset ranged from 1.1 to 6.7 months (mean, 3.5 months; median, 3.3 months). Six patients died due to respiratory failure without tumor progression. Only one patient (patient no. 12) had radiation pneumonitis after second session of radioembolization, among the 46 patients who received multiple sessions.
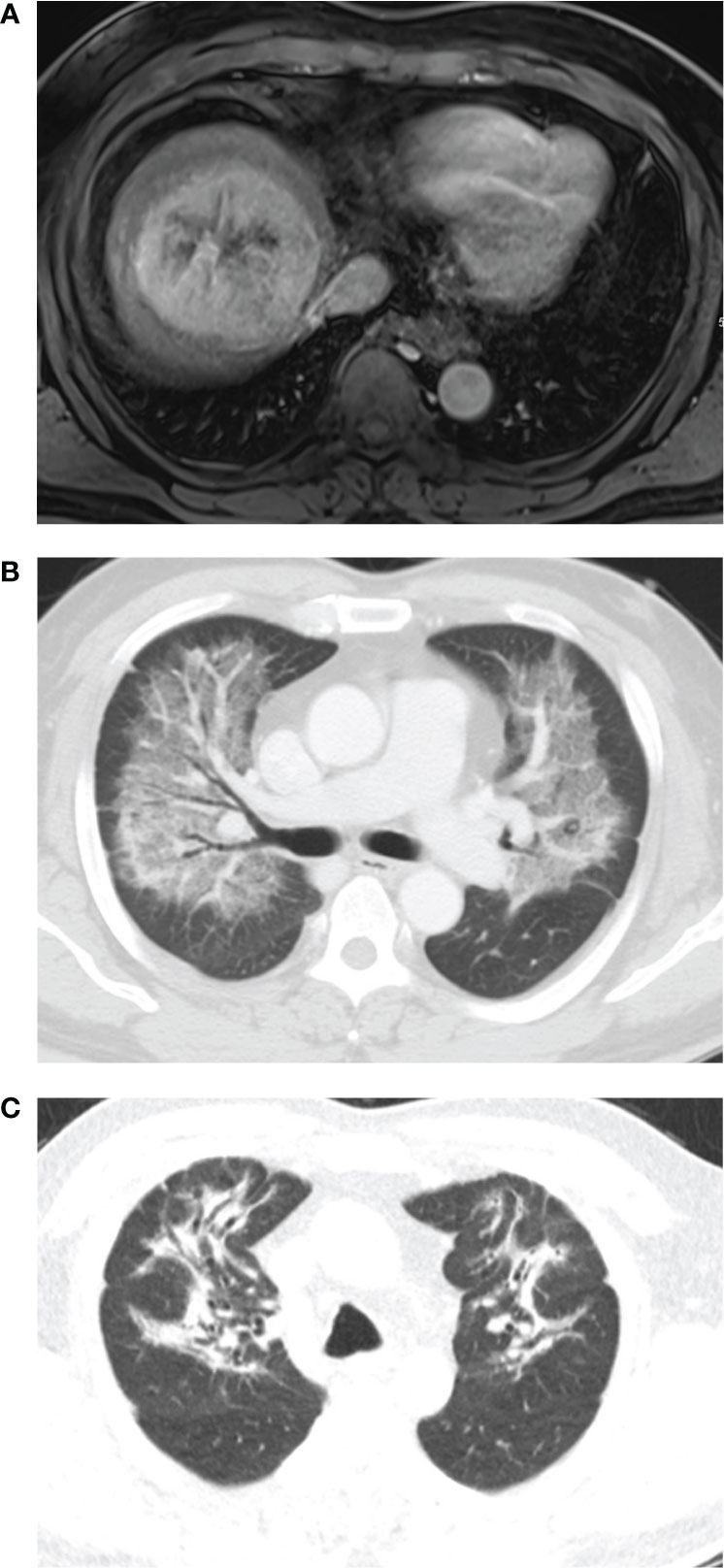
Figure 1 A 65-year-old man had a 9cm single mass in the right lobe. The lung shunt fraction was 14.1%, and 2.4 GBq of resin 90Y-microspheres were delivered into the right hepatic artery. The estimated lung dose was 21.01 Gy. (A) Magnetic resonance shows a mass in right lobe. (B) Chest CT scan 3.8 months after radioembolization shows diffuse consolidation and ground-glass opacity in both the lungs with subpleural sparing. (C) Chest CT scan 4 months after steroid therapy shows fibrotic changes in both the lungs.
Lung dose was not accurately calculated in 11 patients (three and eight with and without radiation pneumonitis, respectively). Radioembolization was performed by placing a balloon catheter in the right hepatic vein to reduce lung shunt in eight of 719 patients without radiation pneumonitis. Lung dose was not precisely estimated in the following three patients with radiation pneumonitis. Patient no. 11 had HCC, which was supplied by both the hepatic and right inferior phrenic artery. 99mTc-MAA was injected into the right hepatic artery, and the LSF was 7.65%. The right inferior phrenic angiogram showed tumor blush and pulmonary shunt, and 90Y-microspheres were injected into both the right hepatic and right inferior phrenic arteries without arteriovenous shunt embolization. Patient no. 12 had received two radioembolization sessions. The LSF was 14.38% at the first radioembolization session. The second radioembolization session was performed 6 months after the first session, and the LSF was assumed as 14.38% without repeating the lung shunt study. Patient no.13 had a large arteriovenous shunt, and the LSF was 49.32%. 90Y-microspheres were injected into the hepatic artery after complete arteriovenous shunt embolization.
The lung dose could be calculated in 721 patients, which ranged from 0.013 to 38.14 Gy (Table 3). The estimated lung dose was >30 and >25 Gy in 24 and 20 patients who were treated with glass and resin 90Y-microspheres, respectively. Of the 721 patients, 10 who were treated with glass (n = 5) and resin (n = 5) 90Y-microspheres had radiation pneumonitis. No significant statistical difference between glass and resin 90Y-microspheres (p = 0.304) was noted in terms of radiation pneumonitis incidence. For glass 90Y-microspheres, radiation pneumonitis was more frequent in patients whose lung dose was >30 Gy than in patients whose lung dose was ≤30 Gy (p = 0.001). The same trend was obtained for resin 90Y-microsphere, though the difference was not statistically significant (p = 0.061) probably for an insufficient number of patients. Notably, all five radiation pneumonitis patients treated with glass 90Y-microspheres had estimated lung dose >29 Gy (29.12 ~ 38.14Gy). In contrast, five radiation pneumonitis patients treated with resin 90Y-microspheres had relatively wide range of lung dose reaching much lower level (13.21Gy).
Radiation pneumonitis is rare but could be a fatal complication after radioembolization (6–8). Exertional dyspnea and dry cough are common clinical manifestations. CT scan commonly shows bilateral symmetric ground-glass opacity and consolidation with relative peripheral/hilar sparing, which is the so-called “bat wing appearance.” In addition, steroid therapy is the treatment mainstay.
MIRD dosimetry for glass 90Y-microspheres is used, and 30 Gy of lung dose is considered as an upper limit. Salem et al. reported no case of radiation pneumonitis in 403 patients treated with glass 90Y-microspheres (10) even if 18 and 58 patients had >30 and >30 Gy of single and cumulative lung doses, respectively. The estimated lung dose in patients with radiation pneumonitis ranged from 29.12 to 38.14 Gy in this study. The 30 Gy cutoff value should be reconsidered. For resin 90Y-microspheres, body surface area (BSA) method and partition dosimetry are commonly used in western and in eastern countries, respectively. In BSA method, LSF >20% is an absolute contraindication and dose reductions of 20% and 40% are recommended if LSF exceeds 10% or 15%, respectively (3, 4). In partition dosimetry, 25 Gy of the estimated lung dose is considered as an upper limit. Lung mass was previously regarded as 1,000 g. Lung mass was set as 800 and 600 g for men and women, respectively, for the Asian population since 2019 as recommended by the manufacturer (4). In all patients treated with resin 90Y-microspheres in this study, lung dose was recalculated with reduced lung mass (800 and 600 g for men and women, respectively). Three patients had radiation pneumonitis with a low estimated lung dose (13.21–21.01 Gy). These three patients did not have any underlying lung disease such as emphysema and did not undergo chemotherapy.
Six patients had estimated lung dose above the recommended lung dose limit in our series. In early study period, some patients were treated using glass 90Y-microsphere with predicted lung dose above 30 Gy, referring to Salem’s article (10) which had reported the safety of radioembolization with high predicted lung dose (patient no. 3, 4 and 5). In late study period, the recommended lung dose limit has been strictly followed. Two patients (patient no. 9 and 10) were treated using resin 90Y-microsphere before the revised recommendation by the manufacturer was applied, when the suggested lung dose limit was 30Gy and lung mass was set as 1,000g. Patient no. 13 was treated after embolization of arteriovenous shunt, as mentioned above.
Current dosimetry has limitations; planar scintigraphy using 99mTc-MAA is not adequate to simulate biodistribution of 90Y-microsphere, especially in lung (11). Individual lung mass is not taken into account in these models. In addition, the lung dose limitations proposed by manufacturers are not validated with proper methodology.Thus, radiation pneumonitis after radioembolization seems to be able to occur, even though the lung dose limitation suggested by the manufacturers was followed in most patients. Consequently, the authors modified the lung dose cutoff value (i.e., 25 Gy for men and 20 Gy for women with glass 90Y-microspheres and 20 Gy with resin 90Y-microspheres).
This study has several limitations. First, the dosimetry between glass and resin 90Y-microspheres is different. Lung mass was considered as 1,000 and 600–800 g for glass and resin 90Y-microspheres, respectively. Second, superselective radioembolization via multiple target vessels is a common form of daily clinical practice. However, 99mTc-MAA was injected into the lobar hepatic artery in most cases. Thus, the different injection sites of radioactive 90Y-microspheres and 99mTc-MAA may affect the lung dose.
In conclusion, the present study suggests that radiation pneumonitis after radioembolization may occur even though the manufacturer’s instructions are followed, and the recommended cutoff value of the estimated lung dose may be adjusted to a slightly lower value.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board, Severance Hospital, Yonsei University Health System. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Guarantor of integrity of the entire study: GK. Study concepts and design: H-CK. Literature research: H-CK, GK. Clinical studies: H-CK, GK. Data analysis: H-CK, GK. Manuscript preparation: H-CK. Manuscript editing: GK. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. EJNMMI (2022) 49(5):1682–99. doi: 10.1007/s00259-021-05600-z
2. Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: A state-of-the-Art brachytherapy treatment for primary and secondary liver malignancies. part 1: Technical and methodologic considerations. J Vasc Interv Radiol (2006) 17(8):1251–78. doi: 10.1097/01.RVI.0000233785.75257.9A
3. Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TW, et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys (2012) 82(1):401–7. doi: 10.1016/j.ijrobp.2010.08.015
4. Ltd SMP. Package inserted for sir-spheres® y-90 resin microspheres (2019). Available at: https://www.sirtex.com/media/169278/pi-ec-13-spheres-ifu-eu-row.pdf.
5. Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther (2016) 33(5):699–714. doi: 10.1007/s12325-016-0324-7
6. Lin M. Radiation pneumonitis caused by yttrium-90 microspheres: Radiologic findings. AJR Am J Roentgenol (1994) 162(6):1300–2. doi: 10.2214/ajr.162.6.8191985
7. Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys (1995) 33(4):919–24. doi: 10.1016/0360-3016(95)00039-3
8. Wright CL, Werner JD, Tran JM, Gates VL, Rikabi AA, Shah MH, et al. Radiation pneumonitis following yttrium-90 radioembolization: Case report and literature review. J Vasc Interv Radiol (2012) 23(5):669–74. doi: 10.1016/j.jvir.2012.01.059
9. Sangro B, Martinez-Urbistondo D, Bester L, Bilbao JI, Coldwell DM, Flamen P, et al. Prevention and treatment of complications of selective internal radiation therapy: expert guidance and systemic review. Hepatology (2017) 66(3):969–82. doi: 10.1002/hep.29207
10. Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol (2008) 31(5):431–8. doi: 10.1097/COC.0b013e318168ef65
11. Chiesa C, Sjogreen-Gleisner K, Walrand S, Strigari L, Flux G, Gear J, et al. EANM dosimetry committee series on standard operational procedures: a unified methodology for 99mTc-MAA pre- and 90Y peri-therapy dosimetry in liver radioembolization with 90Y microspheres. EJNMMI (2021) 8(1):77–120. doi: 10.1186/s40658-021-00394-3
Keywords: hepatocellular carcinoma, radioembolization, radiation pneumonitis, dosimetry, dyspnea
Citation: Kim H-C and Kim GM (2023) Radiation pneumonitis following Yttrium-90 radioembolization: A Korean multicenter study. Front. Oncol. 13:977160. doi: 10.3389/fonc.2023.977160
Received: 24 June 2022; Accepted: 04 January 2023;
Published: 16 January 2023.
Edited by:
Xiankai Sun, University of Texas Southwestern Medical Center, United StatesReviewed by:
William Dezarn, Wake Forest University, United StatesCopyright © 2023 Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyoung Min Kim, a2ltZ21AeW9uc2VpLmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.