
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 27 January 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.925233
This article is part of the Research TopicCase Reports in Radiation Oncology : 2022View all 17 articles
 Chenlu Zhang1†
Chenlu Zhang1† Wenshuai Liu2†
Wenshuai Liu2† Binliang Wang3
Binliang Wang3 Na Zhu4
Na Zhu4 Xi Guo1
Xi Guo1 Zhiming Wang1
Zhiming Wang1 Rongyuan Zhuang1
Rongyuan Zhuang1 Yang You1
Yang You1 Yong Zhang2
Yong Zhang2 Hanxing Tong2
Hanxing Tong2 Weiqi Lu2*
Weiqi Lu2* Yuhong Zhou1*
Yuhong Zhou1*Background: Liposarcomas (LPS) are mesenchymal malignancies with four principal subtypes presenting distinct molecular and clinical features. Pleomorphic liposarcoma (PLPS) is one of the rarest and most aggressive subtypes of LPS. Surgical resection is currently a preferred curative approach for localized PLPS. However, the prognosis of unresectable PLPS is extremely poor, and there is no standard treatment.
Case presentation: A 59-year-old Chinese woman was diagnosed with unresectable PLPS. The case was discussed and managed by specialists from a multidisciplinary team at Fudan Zhongshan Hospital. Preoperative radiotherapy (RT) of intensity-modulated radiation therapy (IMRT) at 50 Gy/25 Fx concurrently with the angiogenesis inhibitor anlotinib (8 mg, days 1–14, every 3 weeks) was prescribed to the patient. The dosage of anlotinib was increased to 10 mg after RT. After 6 months of treatment, the tumor had significantly shrunk and was successfully resected. Examination of the surgical specimens showed a pathological complete response (pCR). Until the latest follow-up (April 2022), no recurrence was observed, and disease-free survival has exceeded 14 months.
Conclusion: This case sheds light on the probability that perioperative RT combined with an angiogenesis inhibitor can be effectively used in PLPS, which is resistant to chemotherapy and usually considered to have a poor prognosis. Further studies with randomized controlled clinical trials will improve our knowledge of this preoperative treatment strategy.
Liposarcoma (LPS) is a heterogeneous soft tissue sarcoma. LPS is among the most common soft tissue sarcomas (STS) and accounts for approximately 15% to 20% of all STS (1). Pleomorphic liposarcoma (PLPS), a less frequent but more aggressive subtype with a 5-year survival rate of 57%, which is closer to that of other high-grade STS, accounts for only 5%–10% of LPS (2).
The LPS arising in the retroperitoneum and intra-abdomen is recommended to be evaluated and managed by a multidisciplinary team (MDT) according to the latest NCCN guideline. Surgery is currently the mainstay, but patients with unresectable disease—defined as tumors affecting important structures or causing unacceptable morbidity after excision—should consider perioperative treatment. Perioperative radiotherapy (RT) is one option, as it reduces tumor size and facilitates tumor resection (3). However, evidence regarding perioperative RT is limited. To date, the STRASS trial (NCT01344018) is the first randomized, phase III clinical trial to value the role of preoperative RT for localized retroperitoneal STS (RPS) (4). Unfortunately, this trial showed a negative result in that preoperative RT did not improve recurrence-free survival. A subgroup analysis of patients with LPS suggested a 10% increase in recurrence-free survival in the RT plus surgery group.
Angiogenesis inhibitors such as anlotinib have recently been proven to be effective in advanced and metastatic STS (5). Although angiogenesis inhibitors are not recommended as perioperative treatment in the guidelines, some recent early-phase clinical trials have explored the safety and efficacy of angiogenesis inhibitors in STS, mostly in combination with perioperative RT (6). These trials showed that the combination of antiangiogenesis therapy with perioperative RT is worth trying in individually selected patients.
Owing to the low incidence of PLPS and the lack of related investigations, the optimal perioperative treatment regimen is challenging. Here, we report a case of PLPS with a pathological complete response (pCR) after RT combined with an antiangiogenesis drug as perioperative therapy.
The case management is described below, and Figure 1A provides a detailed timeline. A 59-year-old Chinese woman presented with abdominal distension. The results of the blood test were normal, and the patient did not have a family history of malignancies. The performance status measured by the ECOG score was one. Computed tomography (CT) was performed in June 2020 and showed a soft tissue mass, sized 7.4 cm × 6.2 cm ×7.2 cm, in the left abdominal cavity with the superior mesenteric artery passing through (Figure 1B). A biopsy was performed, and histopathology revealed undifferentiated pleomorphic epithelioid or spindled cells, admixed with some cells presenting lipid vacuoles (Figure 1C). Lipoblasts could be found in the biopsy sections. The immunohistochemistry reading was ERG (−), CD34 (vessels+), CD117 (−), Fli-1 (−), OCT4 (−), SMA (−), Ki67 (20%+), CD31 (−), DOG1 (−), S100 (−), Des (−), and MDM2 (100%++). Fluorescence in situ hybridization (FISH) revealed no amplification of MDM2 and CDK4 and no rearrangement of DDIT3 (Figure 1D). The diagnosis was pleomorphic liposarcoma, staging cT2N0M0G2, IIIA based on the above information and pathological evaluation of the biopsy. Furthermore, 3D reconstruction imaging confirmed that the first branch of the superior mesenteric artery was surrounded by the tumor (Figure 1E). The careful preoperative imaging helped the MDT to comprehensively evaluate the case. At that time, immediate surgery would necessitate a resection of the entire small bowel to completely remove the tumor, and the patient would need lifelong parenteral nutrition afterward. Consequently, preoperative RT combined with an antiangiogenesis drug was suggested, and the patient agreed with it. From July 2020 to August 2020, the patient received intensity-modulated radiation therapy (IMRT) of 50 Gy/25 Fx concurrently with anlotinib (8 mg, days 1–14, every 3 weeks). The anlotinib dosage was increased to 10 mg after RT. The therapy was well tolerated, and the main adverse event was G1 fatigue. Follow-up CT scans were performed in October and December 2020, indicating notable tumor shrinkage to 2.8 cm × 1.9 cm (Figure 2A). On 29 January 2021, the patient underwent radical resection of the lesion and partial resection of the superior mesenteric artery and small intestines (Figure 2B). The surgical margins showed no evidence of tumor involvement. The pathology of surgical specimens displayed adipose tissue composed of large areas of hyperplastic collagen tissue, scattered small blood vessels, histocytes, and chronic inflammatory cells (Figure 2C). The diagnosis results revealed a pCR. After surgery, the patient underwent CT and blood tests every 3 months. At the last follow-up in April 2022, 14 months postsurgery, the patient was in good condition, and no suspicious recurrence was detected.
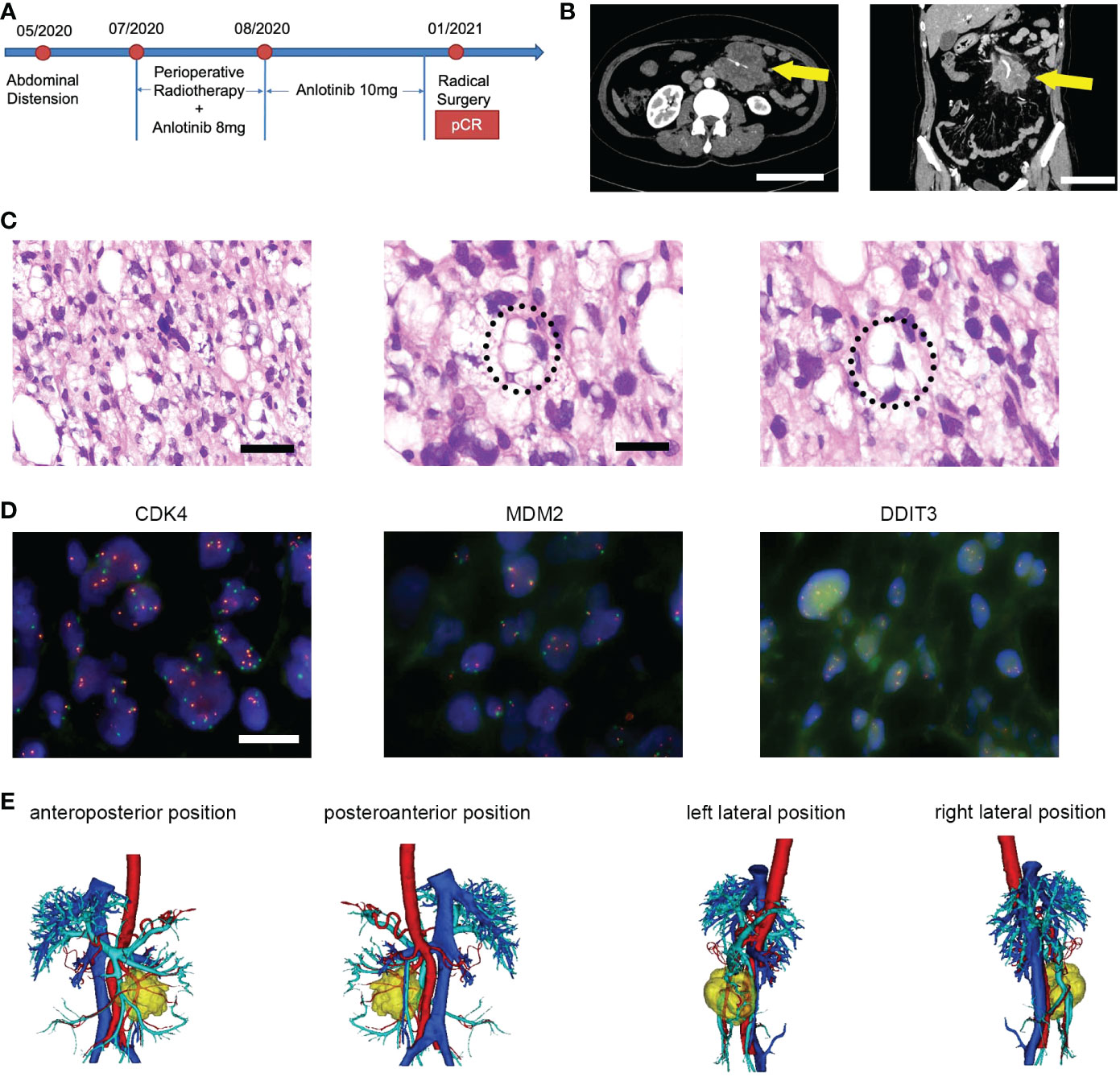
Figure 1 (A) Timeline of therapy in a patient with unresectable PLPS who received preoperative RT plus anlotinib and achieved pCR after the surgery. (B) The initial CT scan revealed a soft tissue mass in the left abdominal cavity with the superior mesenteric artery passing through, measuring 7.4 cm × 6.2 cm × 7.2 cm in diameter. Scale bar = 10 cm. (C) Micrographs of hematoxylin and eosin (HE) staining. Scale bar = 50 μm. The latter two images showed the lip blasts in a circle. Scale bar = 25 μm. (D) The FISH image showed negative results labeled by CDK4, MDM3, and DDIT3 probes, respectively. Scale bar = 10 μm. (E) 3D reconstruction imaging displayed the relationship between tumors and vessels. Yellow, tumor; red, artery; blue, vein.

Figure 2 (A) The CT scan showed that the tumor shrank remarkably after the treatment of perioperative RT plus anlotinib, measuring 2.8 cm × 1.9 cm. (B) The surgical specimens of the tumor, partial small intestines, mesentery, and superior mesenteric artery. (C) Micrographs of HE staining showed collagen tissue with no viable tumor cells. The images displayed blood vessels, histiocytes, and chronic inflammatory cells, which were pointed with arrowheads.
PLPS is the rarest and most aggressive subtype of LPS, and there is currently no standardized treatment yet. Complete resection with clear surgical margins is the preferred curative option for localized PLPS (7). However, local recurrence of PLPS is approximately 30%–50% (8), which is the reason for the predominant failure of surgical treatment. Perioperative treatment may resolve this issue by reducing local recurrence. In this case, although the follow-up of 14 months was rather short, the patient did not develop a local or distant recurrence during this time.
The results of a systematic review and meta-analysis indicated that external beam radiation therapy could reduce local recurrence in RPS (odds ratio (OR) = 0.47, p <0.0001), which was less frequent in the preoperative RT group than the postoperative group (OR = 0.03, p = 0.02) (9). Another study showed that patients with intermediate or high-grade RPS could benefit from the treatment with preoperative RT plus complete resection, with 5-year local recurrence-free survival (RFS) of 60%, disease-free survival (DFS) of 46%, and overall survival (OS) rates of 61% (10). Additionally, retrospective studies have revealed that using perioperative RT in combination with surgery for RPS could also improve OS. The analysis showed that the median OS was significantly improved in the perioperative RT plus surgery group compared to the surgery-only group, which were 110 and 66 months, respectively (hazard ratio = 0·70, 95% confidence interval = 0·59–0·82; p < 0.0001) (11).
However, in contrast to extremity STS (3, 12), the role of preoperative RT in RPS still lacks relevant high-grade clinical evidence. The STRASS trial is a prominent randomized clinical trial that aimed to evaluate the effect of preoperative RT on RPS (4). There was no improvement in abdominal recurrence-free survival (ARFS) in patients receiving preoperative RT plus surgery compared to surgery alone. This study was hobbled by several key limitations, as ARFS was a complex primary endpoint and the trial was not histotype-specific. Nevertheless, the STRASS trial provided some suggestive evidence regarding the possible benefit of preoperative RT in specific RPS histologic subtypes, including LPS and low-grade sarcoma subgroups. Further histotype-specific investigations are still required.
Conventional chemotherapy shows a low response in PLPS; therefore, new target therapies are urgently needed. Vascular endothelial growth factor (VEGF) expression was observed in 68% of PLS specimens, which is an excellent sign of the potential benefit of angiogenesis inhibitors (13). Angiogenesis inhibitors, including pazopanib, anlotinib, and regorafenib, have been approved for use in advanced and metastatic STS (5, 14, 15). Anlotinib is a multi-targeted tyrosine kinase inhibitor that selectively targets VEGFR-2, VEGFR-3, and VEGFR-4; FGFR-1, FGFR-2, FGFR-3, and FGFR-4; PDGFR; and c-Kit, contributing to reduced tumor growth and vasculature (16). A phase II clinical trial showed that anlotinib was the first TKI with antitumor activity in LPS patients that progressed after standard first-line therapy. The progression-free rate (PFR)12 weeks and the objective response rate (ORR) was 63% and 7.7% in LPS (17). However, there is no clear recommendation for locally advanced PLPS and whether angiogenesis inhibitors can be used in perioperative treatment.
A combination of RT and angiogenesis inhibitors has been demonstrated to be an effective therapy that enhances the sensitivity of tumor cells to radiation (6, 18). This effect can be explained by the fact that anti-VEGF normalizes tumor vasculature, resulting in increased tumor oxygenation and cytotoxicity to radiation (19). Combination treatment has been proven to increase the efficacy of RT in different malignancies. Several trials have evaluated the addition of bevacizumab, a humanized anti-VEGF monoclonal antibody, in the treatment of rectal cancer along with preoperative chemoradiotherapy (20–22). Higher pCR rates were observed in the bevacizumab group, ranging from 23.8% to 39.5%. Early clinical studies have also been conducted on STS. Sunitinib or bevacizumab was administered concurrently with preoperative RT in patients with STS originating from the extremities, retroperitoneum, and trunk (23–25). Remarkably, the pathological examination of tumor specimens revealed less than 10% viable tumor cells in approximately one-third of the patients after the combination treatment. Moreover, combination treatment did not lead to severe adverse events or affect the dose of RT. This implied that the addition of angiogenesis inhibitors to RT could increase RT efficacy without increasing toxicity.
In summary, to the best of our knowledge, this is the first case report of a pathologically complete response to the combination of perioperative RT and antiangiogenic agents in a patient with primary unresectable PLPS. Combined therapy provides a practical therapeutic approach to overcome the obstacles in PLPS that have no standard treatment. Further studies will help us better understand the underlying molecular mechanisms of PLPS and establish an optimal treatment strategy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CZ and WSL collected the clinical information, diagnostic information, therapeutic information, and images of the patients. BW provided the radiotherapy information. YZ and HT provided the surgical information and images. NZ reviewed the pathological sections and took pathological photos. CZ wrote the manuscript. CZ and WSL revised the manuscript. YHZ proofread the manuscript. YHZ and WQL were responsible for the study’s conception and design. XG, ZW, RZ, and YY took part in the management and follow-up of the patient. All authors contributed to the article and approved the submitted version.
This study was supported by the Shanghai Sailing Program (19YF1407100).
We appreciate our patient and her family, as well as the colleagues at Shanghai Zhongshan Hospital who supported us in the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol (2018) 36(2):151–9. doi: 10.1200/JCO.2017.74.9598
2. Gebhard S, Coindre JM, Michels JJ, Terrier P, Bertrand G, Trassard M, et al. Pleomorphic liposarcoma: Clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: A study from the French federation of cancer centers sarcoma group. Am J Surg Pathol (2002) 26(5):601–16. doi: 10.1097/00000478-200205000-00006
3. Baldini EH, Wang D, Haas RL, Catton CN, Indelicato DJ, Kirsch DG, et al. Treatment guidelines for preoperative radiation therapy for retroperitoneal sarcoma: Preliminary consensus of an international expert panel. Int J Radiat Oncol Biol Phys (2015) 92(3):602–12. doi: 10.1016/j.ijrobp.2015.02.013
4. Bonvalot S, Gronchi A, Le Pechoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (Eortc-62092: Strass): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2020) 21(10):1366–77. doi: 10.1016/S1470-2045(20)30446-0
5. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res (2018) 24(21):5233–8. doi: 10.1158/1078-0432.CCR-17-3766
6. Spalek MJ, Kozak K, Czarnecka AM, Bartnik E, Borkowska A, Rutkowski P. Neoadjuvant treatment options in soft tissue sarcomas. Cancers (Basel) (2020) 12(8):2061. doi: 10.3390/cancers12082061
7. Anderson WJ, Jo VY. Pleomorphic liposarcoma: Updates and current differential diagnosis. Semin Diagn Pathol (2019) 36(2):122–8. doi: 10.1053/j.semdp.2019.02.007
8. Hornick JL, Bosenberg MW, Mentzel T, McMenamin ME, Oliveira AM, Fletcher CD. Pleomorphic liposarcoma: Clinicopathologic analysis of 57 cases. Am J Surg Pathol (2004) 28(10):1257–67. doi: 10.1097/01.pas.0000135524.73447.4a
9. Albertsmeier M, Rauch A, Roeder F, Hasenhutl S, Pratschke S, Kirschneck M, et al. External beam radiation therapy for resectable soft tissue sarcoma: A systematic review and meta-analysis. Ann Surg Oncol (2018) 25(3):754–67. doi: 10.1245/s10434-017-6081-2
10. Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol (2006) 13(4):508–17. doi: 10.1245/ASO.2006.05.035
11. Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: A case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol (2016) 17(7):966–75. doi: 10.1016/S1470-2045(16)30050-X
12. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet (2002) 359(9325):2235–41. doi: 10.1016/S0140-6736(02)09292-9
13. Ghadimi MP, Liu P, Peng T, Bolshakov S, Young ED, Torres KE, et al. Pleomorphic liposarcoma: Clinical observations and molecular variables. Cancer (2011) 117(23):5359–69. doi: 10.1002/cncr.26195
14. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (Palette): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2012) 379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5
15. Berry V, Basson L, Bogart E, Mir O, Blay JY, Italiano A, et al. Regosarc: Regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma-a quality-adjusted time without symptoms of progression or toxicity analysis. Cancer (2017) 123(12):2294–302. doi: 10.1002/cncr.30661
16. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol (2016) 9(1):105. doi: 10.1186/s13045-016-0332-8
17. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schoffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase ii study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (Eortc study 62043). J Clin Oncol (2009) 27(19):3126–32. doi: 10.1200/JCO.2008.21.3223
18. Rani V, Prabhu A. Combining angiogenesis inhibitors with radiation: Advances and challenges in cancer treatment. Curr Pharm Des (2021) 27(7):919–31. doi: 10.2174/1381612826666201002145454
19. Mazeron R, Azria D, Deutsch E. [Angiogenesis inhibitors and radiation therapy: From biology to clinical practice]. Cancer Radiother (2009) 13(6-7):568–73. doi: 10.1016/j.canrad.2009.06.015
20. Borg C, Mantion G, Boudghene F, Mornex F, Ghiringhelli F, Adenis A, et al. Efficacy and safety of two neoadjuvant strategies with bevacizumab in mri-defined locally advanced T3 resectable rectal cancer: Final results of a randomized, noncomparative phase 2 inova study. Clin Colorectal Cancer (2019) 18(3):200–8.e1. doi: 10.1016/j.clcc.2019.04.006
21. Yu X, Wang QX, Xiao WW, Chang H, Zeng ZF, Lu ZH, et al. Neoadjuvant oxaliplatin and capecitabine combined with bevacizumab plus radiotherapy for locally advanced rectal cancer: Results of a single-institute phase ii study. Cancer Commun (Lond) (2018) 38(1):24. doi: 10.1186/s40880-018-0294-z
22. Nogue M, Salud A, Vicente P, Arrivi A, Roca JM, Losa F, et al. Addition of bevacizumab to xelox induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: The avacross study. Oncologist (2011) 16(5):614–20. doi: 10.1634/theoncologist.2010-0285
23. Jakob J, Simeonova A, Kasper B, Ronellenfitsch U, Rauch G, Wenz F, et al. Combined sunitinib and radiation therapy for preoperative treatment of soft tissue sarcoma: Results of a phase I trial of the German interdisciplinary sarcoma group (Gisg-03). Radiat Oncol (2016) 11:77. doi: 10.1186/s13014-016-0654-2
24. Jakob J, Simeonova A, Kasper B, Ronellenfitsch U, Wenz F, Hohenberger P. Combined radiation therapy and sunitinib for preoperative treatment of soft tissue sarcoma. Ann Surg Oncol (2015) 22(9):2839–45. doi: 10.1245/s10434-015-4680-3
Keywords: pleomorphic liposarcoma, preoperative radiotherapy, angiogenesis inhibitor, pathological complete response, case report
Citation: Zhang C, Liu W, Wang B, Zhu N, Guo X, Wang Z, Zhuang R, You Y, Zhang Y, Tong H, Lu W and Zhou Y (2023) Case report: Pathological complete response to perioperative treatment of radiotherapy combined with angiogenesis inhibitor in a patient with pleomorphic liposarcoma. Front. Oncol. 13:925233. doi: 10.3389/fonc.2023.925233
Received: 21 April 2022; Accepted: 09 January 2023;
Published: 27 January 2023.
Edited by:
Wenyin Shi, Thomas Jefferson University, United StatesReviewed by:
Guido Scoccianti, Careggi University Hospital, ItalyCopyright © 2023 Zhang, Liu, Wang, Zhu, Guo, Wang, Zhuang, You, Zhang, Tong, Lu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhong Zhou, emhvdS55dWhvbmdAenMtaG9zcGl0YWwuc2guY24=; Weiqi Lu, bHUud2VpcWlAenMtaG9zcGl0YWwuc2guY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.