- 1Department of Radiology, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital and Jiangsu Institute of Cancer Research, Nanjing, China
- 2Department of Intervention, The Second Hospital of Nanjing, Nanjing, China
- 3Department of Radiology, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research and The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China
Purpose: To evaluate the characteristic of blood supply of liver portal vein tumor thrombus (PVTT) using perfusion indexes and spectral parameters.
Methods: Between July 2020 and December 2022, the study enrolled 25 liver cancer patients completed with PVTT (male=20, female=5; age 41-74 years (59.48 ± 9.12)) from the Interventional Department of Jiangsu Cancer Hospital. There were 11 cases of type III PVTT, 12 of type II PVTT, and 2 of type I PVTT (Cheng’s classification). All patients underwent spectral perfusion scans through dual-layer spectral detector computed tomography. The PVTTs were divided into proximal and distal groups based on the distance between the tumor thrombus and the main portal vein. The perfusion analysis was performed on the 120-kVp conventional images to generate hepatic perfusion index (HPI). The spectral based images (SBIs) during the artery and venous peak phases were extracted from the perfusion data. The iodine map and 40&100-keV virtual monoenergetic image (VMI) were generated from SBI data. HPI, iodine concentration (IC), CT value at 40 and 100-keV, and spectral slope (40-100keV) of the primary lesion, proximal and distal PVTT, and liver parenchyma were measured and compared. The correlation between the primary lesion and proximal and distal PVTT was analyzed.
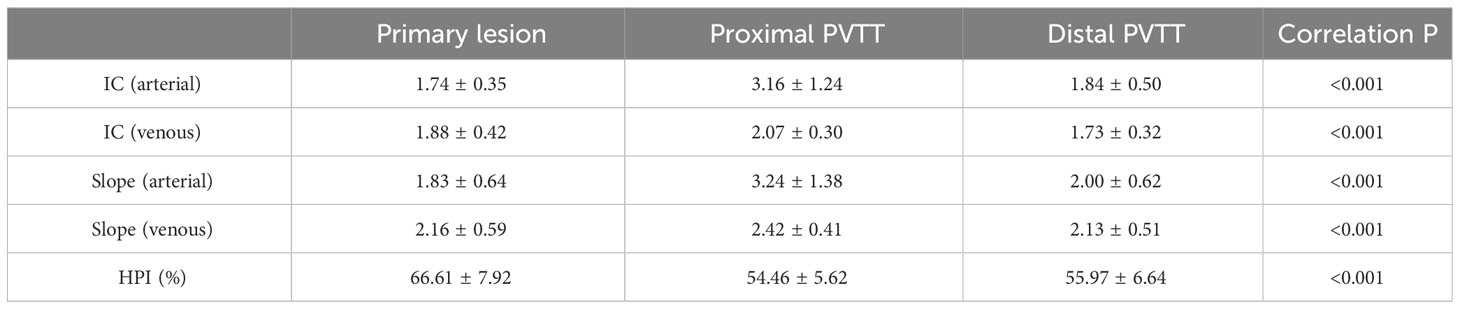
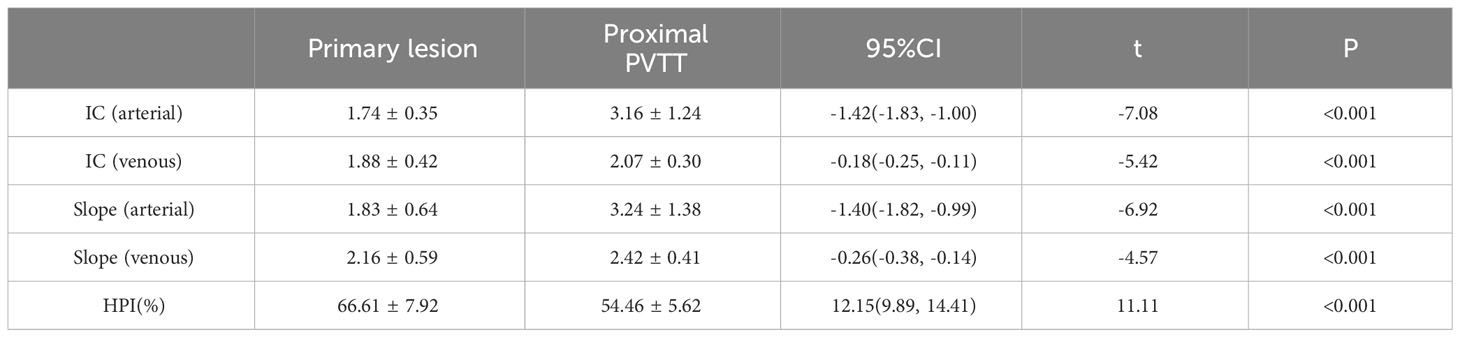
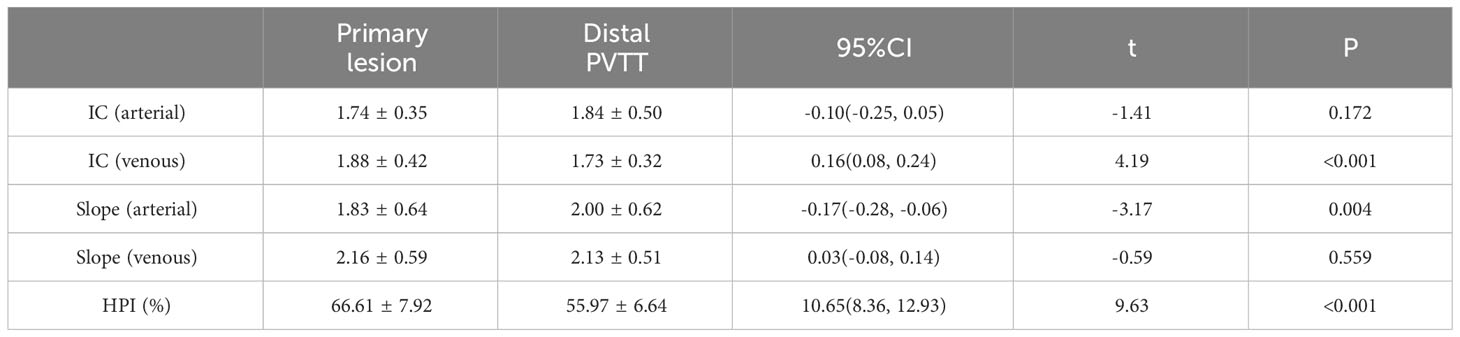
Results: The IC and spectral slope during the arterial and venous peak phases and HPI of the primary lesion, proximal PVTT, and distal PVTT were highly correlated (P<0.001). The differences between the IC and spectral slope during the arterial and venous peak phases and HPI of the primary lesion, proximal PVTT were statistically significant (P<0.001). The differences between the IC during venous peak phase and HPI of primary lesion, distal PVTT were statistically significant (P<0.001), and there was no statistically significant difference in arterial phase IC, arterial and venous phase spectral slopes.
Conclusion: The IC, slope, and HPI of the distal and proximal PVTT were highly correlated with the primary lesion, indicating that PVTT was similar to the primary lesion in the liver that they were both mainly supplied by the hepatic artery. However, there was still significant heterogeneity between the proximal PVTT and the primary lesion, while the difference in the distal PVTT was relatively small.
1 Introduction
Portal vein tumor thrombus (PVTT) is the most common form of vascular invasion, and its incidence ranges from 44% to 62.2% at the time of HCC diagnosis in China (1). The median survival time of HCC patients complicated with PVTT is about 2.7 months if they only receive supportive treatment (2). The local treatment of PVTT includes various methods such as transarterial chemoembolization (TACE) and iodine particle implantation surgery, and the choice of treatment plan is closely related to the blood supply of the primary lesion and PVTT. Dual-layer spectral detector CT (SDCT) has high application value in liver cancer, which can evaluate the properties and hemodynamics of primary lesions, PVTT, and liver parenchyma with multiple parameters (3). Perfusion combined with spectral detector CT can comprehensively or semi-quantitatively evaluate the blood supply of PVTT. Through energy spectrum images and quantitative analysis methods such as virtual monoenergetic image (VMI), iodine map and spectral slope, various related information of PVTT and primary lesions can be analyzed. This study used SDCT to evaluate the blood supply of PVTT, aiming to provide a basis for selecting the best treatment method for PVTT patients.
2 Materials and methods
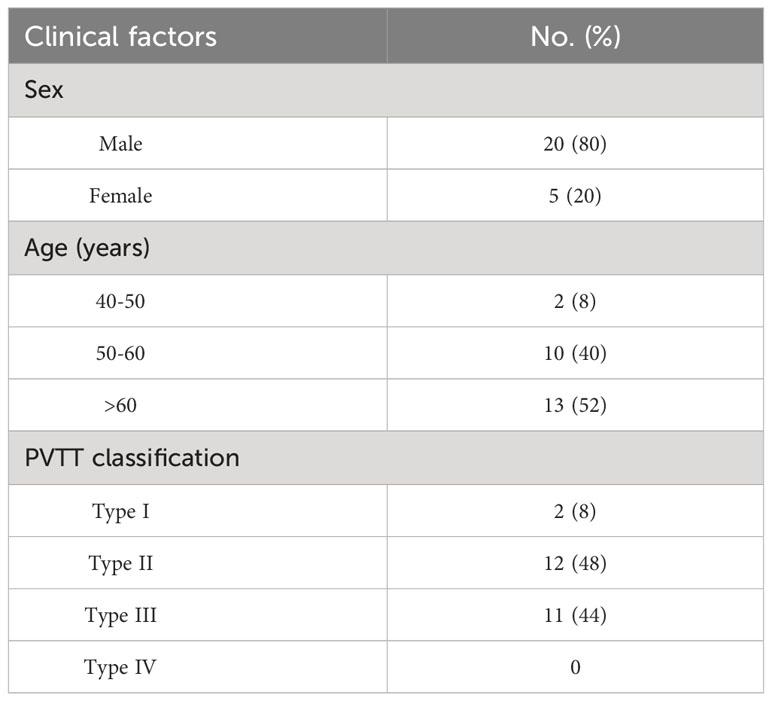
2.1 Characteristics of patient and tumors
Between July 2020 and December 2022, the study enrolled 25 liver cancer patients completed with PVTT [male=20, female=5; age 41-74 (59.48 ± 9.12)] from the Interventional Department of Jiangsu Cancer Hospital (Table 1). This retrospective study was approved by the institutional review board. All patients provided written informed consent before study participation according to the institutional. The inclusion criteria were as follows: 1: patients were histopathologically diagnosed with locally advanced HCC complicated with PVTT, or the patients had three diagnostic criteria: cirrhosis, typical imaging manifestations of HCC and elevated AFP; 2: patients showed adequate renal function with a serum creatinine level of no more than 2.0 mg/dL (177 umol/L); 3: patients had no history of iodine allergy. 4: patients had not received any treatment related to HCC.
2.2 CT scanning parameter
A clinical SDCT scanner (IQon, Philips Healthcare, Best, The Netherlands) was applied in this research, and 25 patients were scanned in a head-first, supine position. A body weight-adapted volume of a non-ionic, iodinated contrast agent (iohexol 350 mg/mL) was administered intravenously via the peripheral vein at a mean flow of 3.5 mL/s, followed by a 30 mL of saline flush. The perfusion scanning range was centered around the lesion, covering a total of 8cm and included five cycles of cyclic scanning. Phantom scans were performed five times through the 100 mAs of fixed tube current. The additional scan parameters used in patients and phantom scans were collimation at 64 × 0.625 mm, rotation time at 0.5s, pitch 0.671, and tube voltage at 120 keV.
2.3 Region of interest (ROI) design and image reconstruction
During the arterial and venous peak phases of the CT scanning (Figure 1), the circular ROIs of at least 500 mm² were placed on the primary lesion, proximal and distal PVTT, and liver parenchyma. To minimize any measurement error from different ROIs, we took the average value of three measurements. The ROIs of liver parenchyma and primary lesion were measured at the same level. ROIs did not include nonrepresentative structures like blood vessels, bile ducts, and lymph nodes. The perfusion analysis was performed on the 120-kVp conventional images to generate perfusion results (like hepatic perfusion index (HPI) (Figure 1).The spectral based images (SBIs) at the artery and venous phases were extracted from perfusion data. The iodine map and 40-100 keV VMI were generated from SBI, at a reconstructed slice thickness of 1 mm and an increment of 1 mm. The iodine concentration (IC), CT value at 40 and 100-keV, and spectral slope (40-100keV) of the primary lesion, proximal and distal PVTT, and liver parenchyma were measured (Figure 2).
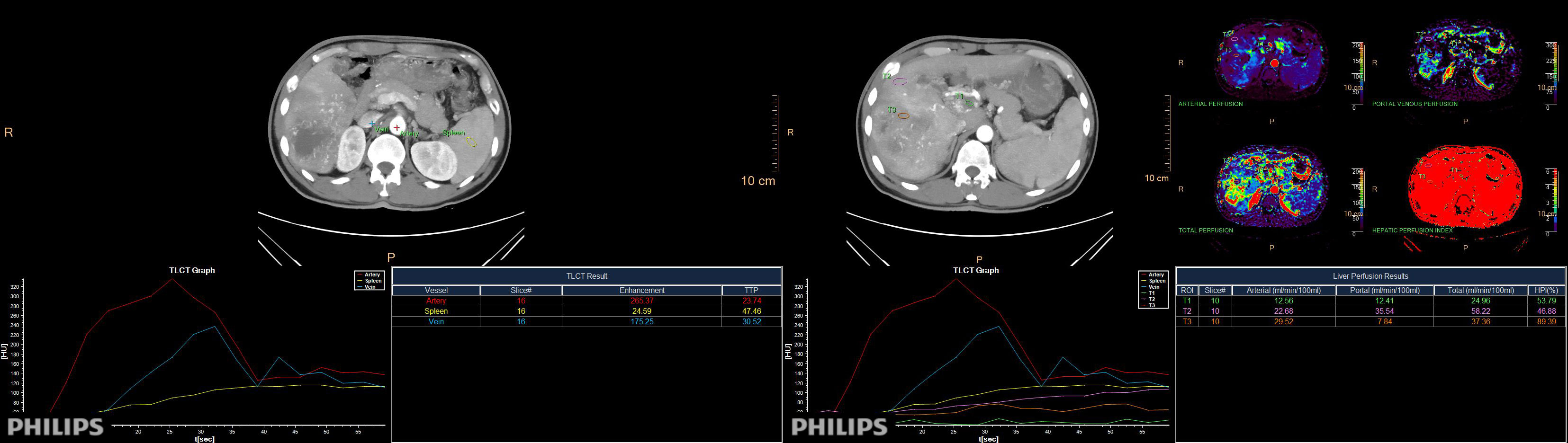
Figure 1 The peak time curve and perfusion analysis diagram of arteries, veins, and spleen. Directly obtain the HPI value of the region of interest through measurement.
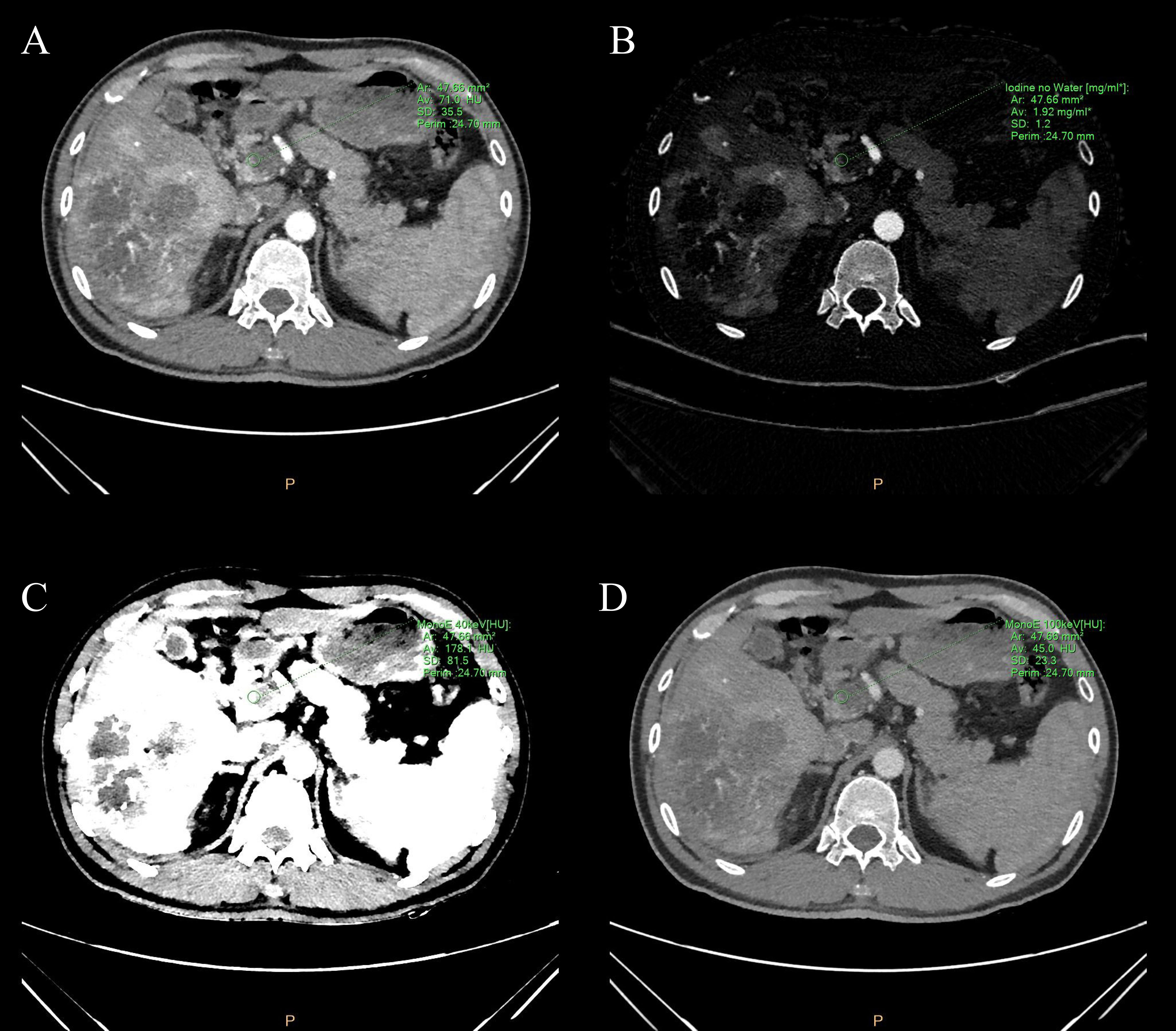
Figure 2 (A) Conventional image, (B) iodine map image, and (C, D) 40&100-keV virtual monoenergetic images of the portal vein tumor thrombus during the peak arterial phase. The green circle represents the measured ROI in the portal vein tumor thrombus. ROI is plotted by using spectral CT post-processing software. The green letters around are some spectral parameters.
2.4 Statistical analysis
The SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. The measurement data was described as mean ± standard deviation, and the K-S test was used to evaluate whether the measurable variables exhibit a normal distribution. A scatter plot was created to observe whether its distribution was linear. Pearson correlation analysis was used to evaluate the correlation between each group of data for normal distribution variables. Paired samples were subjected to paired sample T-test, and the mean difference between groups was analyzed. The statistical significance was defined as P value of no more than 0.05.
3 Results
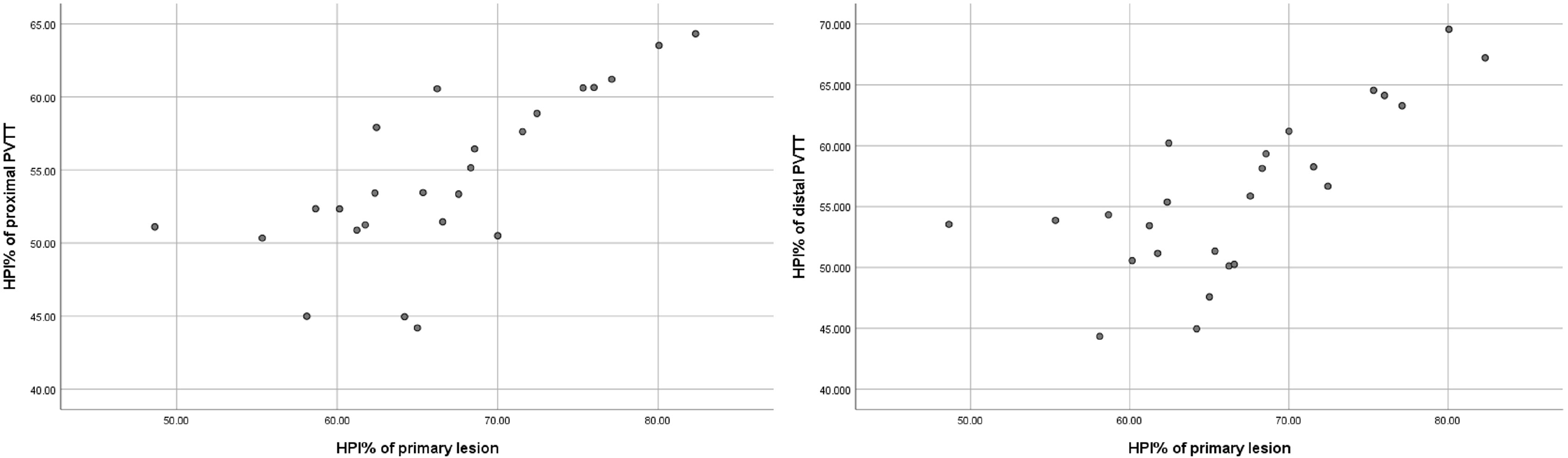
The IC and spectral slope during the arterial and venous peak phases and HPI of the primary lesion, proximal PVTT, and distal PVTT were highly correlated (P<0.001) (Table 2; Figures 3, 4). The differences between the IC and spectral slope during the arterial and venous peak phases and HPI of the primary lesion, proximal PVTT were statistically significant (P<0.001) (Table 3). The differences between the IC during venous peak phase and HPI of primary lesion, distal PVTT were statistically significant (P<0.001), and there was no statistically significant difference in arterial phase IC, arterial and venous phase spectral slopes (Table 4).
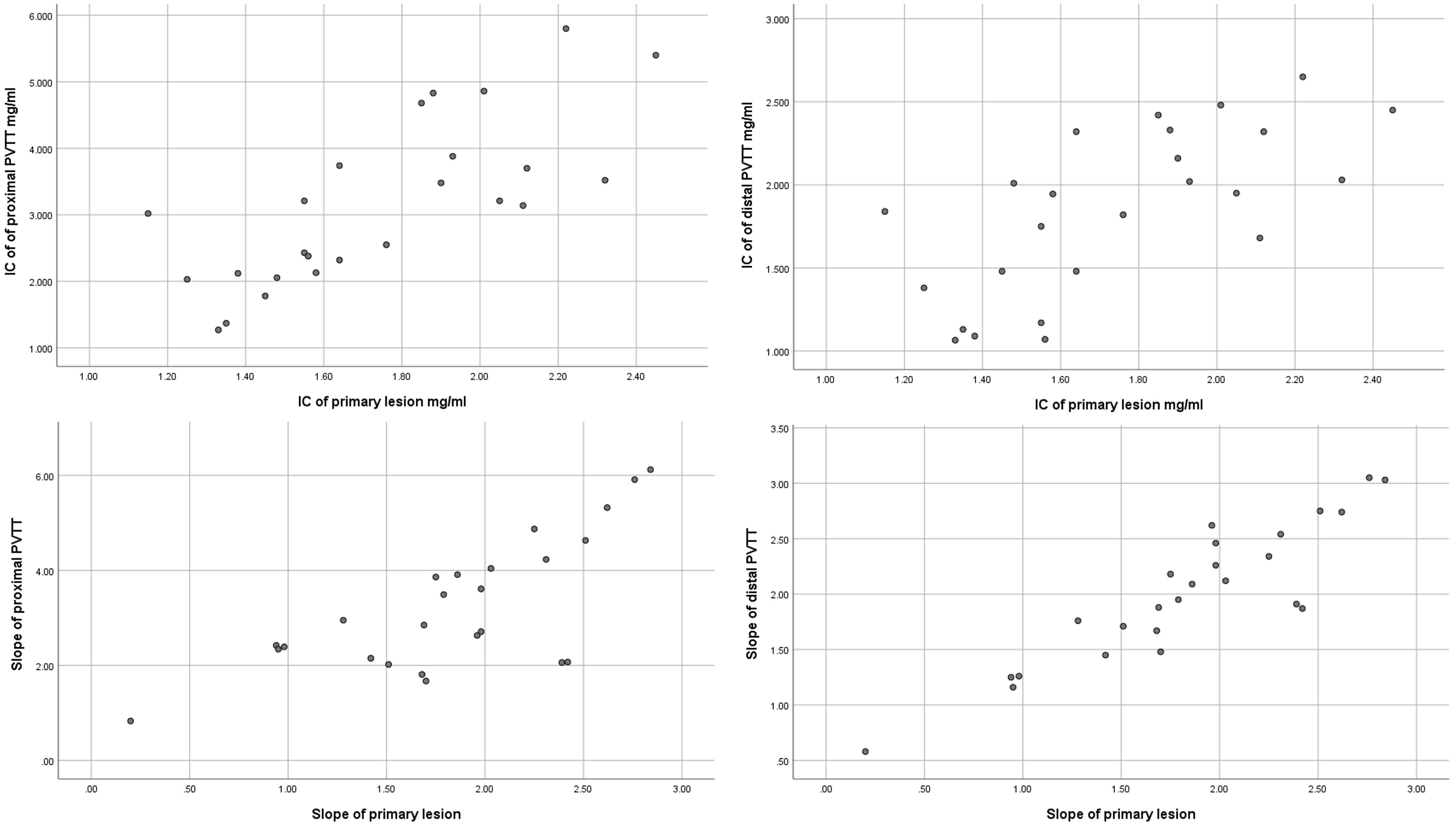
Figure 4 The scatter plot of IC from the primary lesion and proximal and distal PVTT during the peak arterial phase. The scatter plot of slope from the primary lesion and proximal and distal PVTT during the peak arterial phase.
4 Discussion
4.1 The treatment methods of PVTT
The primary causes of PVTT development include gene mutation, active replication of HBV, and direct invasion of the portal vein by tumor (4–6). The Barcelona Clinic Liver Cancer guidelines classify HCC accompanied by PVTT as advanced stage (BCLC C stage), and such patients are only eligible for palliative systemic treatment (7) Despite the varying burden of HCC faced by various countries, more proactive management methods are proposed for advanced HCC cases (8–10), including local, locoregional and systemic therapies. Local therapies include surgery (liver resection and transplantation) and radiotherapy the 3-dimensional conformal radiotherapy (3D-CRT), external beam radiotherapy (EBRT)) (10).Locoregional therapies include transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and hepatic artery infusion chemotherapy (HAIC) (11). Systemic therapies include immunotherapy with nivolumab, pembrolizumab and others, and targeted therapy with sorafenib, regorafenib, Lenvatinib etc (12). The use of drugs combined with other methods like radiation therapy (RT), TACE, TARE, and HAIC can effectively control HCC development. However, the main treatment method has always been a focus of clinical discussion, and various treatment methods through hepatic artery perfusion need to clarify whether the target lesion has hepatic artery blood supply and the degree of hepatic artery blood supply, which will directly affect the efficacy of TACE for lesion treatment and whether other methods need to be combined.
4.2 The application of CT in PVTT evaluation
Perfusion CT can objectively and quantitatively evaluate the blood perfusion of the tumor and its adjacent tissues (13). SDCT can offer energy spectrum images and quantitative analysis methods in liver diseases, such as virtual mono energy images (VMIs), virtual plain scans, iodine density maps, atomic number maps, and energy spectrum curves; it can further provide valuable information for disease localization and qualitative diagnosis (14). Moreover, it can provide more tissue characteristics beyond a conventional CT scanner (15). The function of spectral slop (40-100 keV) is similar to spectral attenuation curves, it can plot the CT numbers of tissues at different energy levels of VMIs. Although hepatic arteries are not the main blood supply to the liver, they are the dominant blood supply to liver tumors due to neoplastic angiogenesis (16), which is the basis for the TACE. Our research showed that the blood supply of PVTT was similar to that of liver cancer lesions, and both were primarily supplied by arteries, which was of great significance for selecting the optimal treatment method for advanced HCC patients complicated with PVTT. Moreover, this study found that the mean HPI value was significantly higher in the primary lesion than in the liver parenchyma, and the high HPI values could explain the reduction of the portal perfusion inflow and a simultaneous increase in arterial inflow in the primary lesion, which further validated the reliability of the selection of control criteria in this study. The difference in mean values between the primary lesion and proximal PVTT was statistically significant, while there was a statistically significant difference in IC values and HPI between the primary lesion and distal PVTT during the venous peak period. There was no statistically significant difference in mean values between the remaining items. PVTT might obstruct the portal vein blood flow of the tumor (17) and the corresponding segment of liver parenchyma to influence perfusion parameters. Nevertheless, the blood supply of the distal PVTT was less affected by the main portal vein, so the values of IC and spectral slope from the distal PVTT were similar.
4.3 Selection of Treatment plans guided by PVTT blood supply evaluation
According to the guidelines for HCC in China, patients with liver cancer combined with PVTT can apply TACE, systemic therapy, radiotherapy, chemotherapy, or surgical resection according to the situation. Surgical resection is widely recognized as a curative treatment measure, but it can only be targeted at patients with PVTT I-II who have good liver function. Various guidelines agreed that TACE was the preferred and effective palliative treatment for advanced liver cancer (18, 19). Chung et al (20) enrolled 83(66.4%) of the 125 advanced HCC patients complicated with PVTT who were treated with TACE and 42 (33.6%) who received supportive care. They found that repeated TACE had more significant survival benefits than supportive care in treating patients with Child-Pugh class A HCC. With the diversification of treatment methods, Li et al. (21) conducted a meta-analysis that included seven studies with 1,018 patients, in which 602 patients received TACE and I125 irradiation stent placement and 416 underwent TACE and stent placement without endovascular brachytherapy (EVBT).The study found that I125 irradiation stent could improve the cumulative stent patency rate in 6 and 12months and the survival rate in 12 and 24months, indicating that the I125 radiation stent was more effective in treating HCC with PVTT and TACE combined with EVBT may become an alternative treatment method for HCC with PVTT. Kim et al. found that HCC patients complicated with PVTT who received TACE with RT had a longer median time to progression and OS than those who got TACE or sorafenib (P < 0.001) (22).
Based on the above studies, it was found that HCC combined with PVTT still needed to be combined with other different treatment methods on the basis of TACE, which was basically consistent with the results of this study. This study showed that PVTT was similar to the primary lesion which was mainly supplied by the hepatic artery, and TACE was effective. However, the efficacy of TACE in treating PVTT was not as good as that of primary lesions. As a result, the author believed that although PVTT was mainly supplied by the hepatic artery, there was still significant heterogeneity between proximal PVTT and primary lesions. Most parameters of distal PVTT were not statistically different from primary lesions, but the difference in HPI was still significant. Therefore, TACE alone was not effective in treating PVTT. Therefore, considering the blood supply characteristics of PVTT, a comprehensive treatment plan combined with TACE should become the mainstream treatment method for patients with advanced hepatocellular carcinoma accompanied by portal vein tumor thrombus. Before selecting the treatment plan, performing spectral perfusion analysis on PVTT to evaluate its hepatic artery blood supply and the degree of difference with liver cancer and choosing an appropriate combination plan may have high reference value for improving the treatment efficacy.
In summary, both PVTT and primary lesions were supplied by the hepatic artery, but the degree of blood supply and tumor tissue still had certain heterogeneity compared to the liver cancer. A comprehensive treatment plan based on TACE combined with various methods should become the mainstream treatment method for patients with advanced hepatocellular carcinoma and portal vein cancer thrombus.
4.4 Limitation
This study used a clear proportion of blood supply to primary liver cancer lesions as a reference standard, and the evaluation method mainly relied on imaging, which was relatively single. In the later stage, DSA angiography results will be considered to further clarify the conclusions of this study. If part of patients have pathological results as support, the conclusion will be more clear. Due to the fact that the patients collected in this study are mainly with advanced liver cancer and the inclusion criteria are relatively strict, the sample size is relatively small, and the results have certain limitations. In the later stage, the sample size will be further expanded to make the conclusions more accurate.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jiangsu Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Methodology, Project administration, Writing – original draft, Writing – review & editing. CP: Data curation, Methodology, Writing – original draft. FD: Formal Analysis, Investigation, Writing – review & editing. LS: Investigation, Writing – review & editing. KL: Investigation, Software, Writing – review & editing. WQ: Conceptualization, Methodology, Writing – review & editing. ZK: Data curation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Roentgen special fund for image research of Jiangsu Medical Association (SYH-32011500008 2021003) and The Jiangsu Cancer Hospital Young Talents Plan (Jiangsu China).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun JX, Guo RP, Bi XY, Wu MC, Tang ZY, Lau WY, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China (2021 edition). Liver Cancer (2022) 11(4):315–28. doi: 10.1159/000523997
2. Tao ZW, Cheng BQ, Zhou T, Gao YJ. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat Dis Int (2022) 21(2):134–44. doi: 10.1016/j.hbpd.2021.12.004
3. Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging (2013) 12(3):530–47. doi: 10.1102/1470-7330.2012.0044
4. Fan XX, Li YR, Yi X, Chen GQ, Jin SX, Dai YL, et al. Epigenome-wide DNA methylation profiling of portal vein tumor thrombosis (PVTT) tissues in hepatocellular carcinoma patients. Neoplasia (2020) 22(11):630–43. doi: 10.1016/j.neo.2020.09.007
5. Chen C, Chen DP, Gu YY, Hu LH, Wang D, Lin JH, et al. Vascular invasion in hepatitis B virus-related hepatocellular carcinoma with underlying cirrhosis: possible associations with ascites and hepatitis B viral factors? Tumour Biol (2015) 36(8):6255–63. doi: 10.1007/s13277-015-3311-8
6. Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology (2019) 156(6):1582–1599.e1581. doi: 10.1053/j.gastro.2019.01.265
7. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
8. Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw (2019) 17(4):302–10. doi: 10.6004/jnccn.2019.0019
9. Yau T, Tang VYF, Yao TJ, Fan ST, Lo CM, Poon RTP. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology (2014) 146(7):1691–1700.e1693. doi: 10.1053/j.gastro.2014.02.032
10. Leung TWT, Tang AMY, Zee B, Lau WY, Lai PBS, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer (2002) 94(6):1760–9. doi: 10.1002/cncr.10384
11. Chen CP. Role of external beam radiotherapy in hepatocellular carcinoma. Clinics liver Dis (2020) 24(4):701–17. doi: 10.1016/j.cld.2020.07.006
12. Galle PR, Finn RS, Qin SK, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(7):991–1001. doi: 10.1016/s1470-2045(21)00151-0
13. Ippolito D, Sironi S, Pozzi M, Antolini L, Invernizzi F, Ratti L, et al. Perfusion CT in cirrhotic patients with early stage hepatocellular carcinoma: assessment of tumor-related vascularization. Eur J Radiol (2010) 73(1):148–52. doi: 10.1016/j.ejrad.2008.10.014
14. Chellini D, Kinman K. Dual-energy CT principles and applications. Radiol Technol (2020) 91(6):561ct–76ct.
15. Rajiah P, Parakh A, Kay F, Baruah D, Kambadakone AR, Leng S. Update on multienergy CT: physics, principles, and applications. Radiographics (2020) 40(5):1284–308. doi: 10.1148/rg.2020200038
16. Morse MA, Sun WJ, Kim R, He AR, Abada PB, Mynderse M, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res (2019) 25(3):912–20. doi: 10.1158/1078-0432.Ccr-18-1254
17. Li H, Liu J, Chen M, Li Hang, Long LL. Therapeutic evaluation of radiotherapy with contrast-enhanced ultrasound in non-resectable hepatocellular carcinoma patients with portal vein tumor thrombosis. Med Sci Monit (2018) 24:8183–9. doi: 10.12659/msm.911073
18. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer (2021) 10(3):181–223. doi: 10.1159/000514174
19. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
20. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology (2011) 258(2):627–34. doi: 10.1148/radiol.10101058
21. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. I125 irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: A meta-analysis. Cancer Radiother (2021) 25(4):340–9. doi: 10.1016/j.canrad.2020.12.003
22. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol (2015) 26(3):320–329.e326. doi: 10.1016/j.jvir.2014.10.019
Keywords: PVTT, hepatocellular carcinoma (HCC), spectral computed tomography, spectral based image, virtual monoenergetic images
Citation: Pan C, Dai F, Sheng L, Li K, Qiao W, Kang Z and Zhang X (2024) Clinical application of spectral CT perfusion scanning in evaluating the blood supply source of portal vein tumor thrombus in hepatocellular carcinoma. Front. Oncol. 13:1348679. doi: 10.3389/fonc.2023.1348679
Received: 03 December 2023; Accepted: 27 December 2023;
Published: 17 January 2024.
Edited by:
Francisco Tustumi, University of São Paulo, BrazilCopyright © 2024 Pan, Dai, Sheng, Li, Qiao, Kang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuming Zhang, emhhbmd4aXVtaW5nMzYwQDE2My5jb20=; Zheng Kang, MTM2NjEwMTAwMkBxcS5jb20=
†These authors have contributed equally to this work
 Chunhan Pan
Chunhan Pan Feng Dai2†
Feng Dai2† Xiuming Zhang
Xiuming Zhang